Schizophrenia and Glutathione: A Challenging Story
Abstract
:1. Introduction
2. Oxidative Stress and Glutathione
3. Glutathione in Schizophrenia
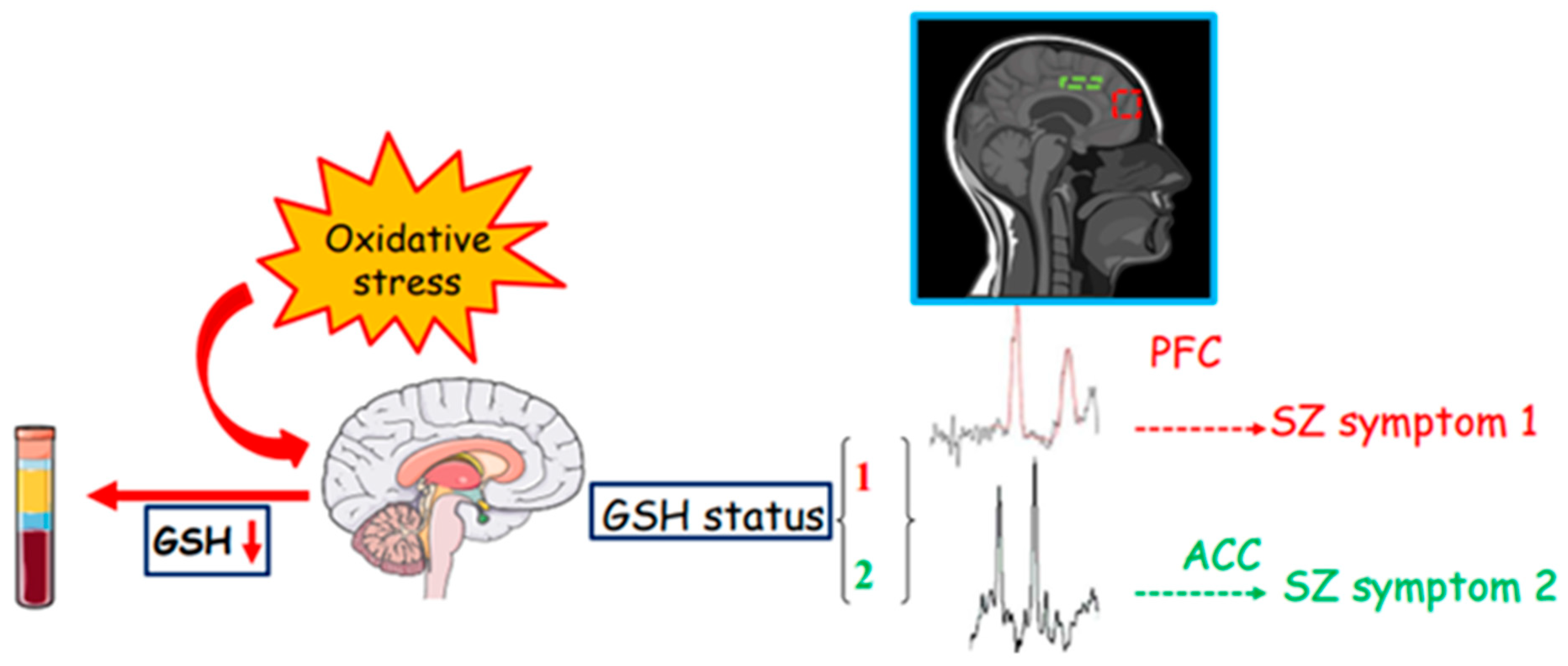
4. From the Periphery to the Center
5. N-Acetylcysteine: A Support to GSH Implication in SZ
6. NMDAR Hypofunction and Its Link to Glutathione in SZ
7. Glutathione as a Biomarker for Schizophrenia
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 73–486. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef]
- Van Os, J.; Kapur, S. Schizophrenia. Lancet 2009, 374, 635–645. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kulhara, P. What is schizophrenia: A neurodevelopmental or neurodegenerative disorder or a combination of both? A critical analysis. Indian J. Psychiatry 2010, 52, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.H.; Monin, A.; Dwir, D.; O’Donnell, P.; Cuenod, M.; Do, K.Q. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: A “central hub” in schizophrenia pathophysiology? Schizophr. Res. 2016, 176, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Keshavan, M.S. Antioxidants, redox signaling, and pathophysiology in schizophrenia: An integrative view. Antioxid. Redox Signal. 2011, 15, 2011–2035. [Google Scholar] [CrossRef]
- Cuenod, M.; Steullet, P.; Cabungcal, J.H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in vicious circles: A perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry 2022, 27, 1886–1897. [Google Scholar] [CrossRef]
- Do, K.Q.; Trabesinger, A.H.; Kirsten-Krüger, M.; Lauer, C.J.; Dydak, U.; Hell, D.; Holsboer, F.; Boesiger, P.; Cuénod, M. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur. J. Neurosci. 2000, 12, 3721–3728. [Google Scholar] [CrossRef]
- Gawryluk, J.W.; Wang, J.F.; Andreazza, A.C.; Shao, L.; Young, L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011, 14, 123–130. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Yang, K.; Marsman, A.; Pradhan, S.; Wang, M.; Ward, R.E.; Bonekamp, S.; Ambinder, E.B.; Higgs, C.P.; Kim, P.K.; et al. A multimodal approach to studying the relationship between peripheral glutathione, brain glutamate, and cognition in health and in schizophrenia. Mol. Psychiatry 2021, 26, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Kang, Y.; Viswanath, V.; Jha, N.; Qiao, X.; Mo, J.Q.; Andersen, J.K. Brain gamma-glutamyl cysteine synthetase (GCS) mRNA expression patterns correlate with regional-specific enzyme activities and glutathione levels. J. Neurosci. Res. 1999, 58, 436–441. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Olas, B.; Głowacki, R.; Bald, E. Oxidative/nitrative modifications of plasma proteins and thiols from patients with schizophrenia. Neuropsychobiology 2009, 59, 1–7. [Google Scholar] [CrossRef]
- Raffa, M.; Mechri, A.; Othman, L.B.; Fendri, C.; Gaha, L.; Kerkeni, A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1178–1183. [Google Scholar] [CrossRef]
- Raffa, M.; Barhoumi, S.; Atig, F.; Fendri, C.; Kerkeni, A.; Mechri, A. Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 371–375. [Google Scholar] [CrossRef]
- Raffa, M.; Bel Hadj Youssef, I.; Ben Othman, L.; Fendri, C.; Mechri, A. Concentrations plasmatiques des glutathions et leurs corrélations avec les caractéristiques cliniques et thérapeutiques des patients atteints de schizophrénie [Plasmatic glutathione levels and their relationships with clinical and therapeutic features in patients with schizophrenia]. Encephale 2021, 47, 10–14. (In French) [Google Scholar] [CrossRef]
- Ruiz-Litago, F.; Seco, J.; Echevarría, E.; Martínez-Cengotitabengoa, M.; Gil, J.; Irazusta, J.; González-Pinto, A.M. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: A 12-months follow-up study. Psychiatry Res. 2012, 200, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, L.G.; Tanaka, T.; Hayes, L.N.; Kim, M.; Lee, B.J.; Matsuda, T.; Nucifora, F.C., Jr.; Sedlak, T.; Mojtabai, R.; Eaton, W.; et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl. Psychiatry 2017, 7, e1215. [Google Scholar] [CrossRef] [PubMed]
- Guidara, W.; Messedi, M.; Naifar, M.; Maalej, M.; Grayaa, S.; Omri, S.; Ben Thabet, J.; Maalej, M.; Charfi, N.; Ayadi, F. Predictive value of oxidative stress biomarkers in drug-free patients with schizophrenia and schizo-affective disorder. Psychiatry Res. 2020, 293, 113467. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.A.; Smirnova, L.P.; Shchigoreva, Y.G.; Semke, A.V.; Bokhan, N.A. Serum Glutathione in Patients with Schizophrenia in Dynamics of Antipsychotic Therapy. Bull. Exp. Biol. Med. 2015, 160, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.; Stefanović, A.; Milovanović, S.; Ðorđević, B.; Kotur-Stevuljević, J.; Ivanišević, J.; Miljković, M.; Spasić, S. Associations of oxidative stress status parameters with traditional cardiovascular disease risk factors in patients with schizophrenia. Scand. J. Clin. Lab. Investig. 2014, 74, 184–191. [Google Scholar] [CrossRef]
- Fukushima, T.; Iizuka, H.; Yokota, A.; Suzuki, T.; Ohno, C.; Kono, Y.; Nishikiori, M.; Seki, A.; Ichiba, H.; Watanabe, Y.; et al. Quantitative analyses of schizophrenia-associated metabolites in serum: Serum D-lactate levels are negatively correlated with gamma-glutamylcysteine in medicated schizophrenia patients. PLoS ONE 2014, 9, e101652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Liencres, C.; Tas, C.; Brown, E.C.; Erdin, S.; Onur, E.; Cubukcoglu, Z.; Aydemir, O.; Esen-Danaci, A.; Brüne, M. Oxidative stress in schizophrenia: A case-control study on the effects on social cognition and neurocognition. BMC Psychiatry 2014, 14, 268. [Google Scholar] [CrossRef]
- Tsai, M.C.; Liou, C.W.; Lin, T.K.; Lin, I.M.; Huang, T.L. Changes in oxidative stress markers in patients with schizophrenia: The effect of antipsychotic drugs. Psychiatry Res. 2013, 209, 284–290. [Google Scholar] [CrossRef]
- Tao, Q.; Miao, Y.; Li, H.; Yuan, X.; Huang, X.; Wang, Y.; Andreassen, O.A.; Fan, X.; Yang, Y.; Song, X. Insulin Resistance and Oxidative Stress: In Relation to Cognitive Function and Psychopathology in Drug-Naïve, First-Episode Drug-Free Schizophrenia. Front. Psychiatry 2020, 11, 537280. [Google Scholar] [CrossRef]
- Cruz, B.F.; de Campos-Carli, S.M.; de Oliveira, A.M.; de Brito, C.B.; Garcia, Z.M.; do Nascimento Arifa, R.D.; de Souza, D.D.G.; Teixeira, A.L.; Salgado, J.V. Investigating potential associations between neurocognition/social cognition and oxidative stress in schizophrenia. Psychiatry Res. 2021, 298, 113832. [Google Scholar] [CrossRef]
- Raffa, M.; Atig, F.; Mhalla, A.; Kerkeni, A.; Mechri, A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 2011, 11, 124. [Google Scholar] [CrossRef]
- Altuntas, I.; Aksoy, H.; Coskun, I.; Cayköylü, A.; Akçay, F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin. Chem. Lab. Med. 2000, 38, 1277–1281. [Google Scholar] [CrossRef]
- Pavlović, D. Oxidative stress as marker of positive symptoms in schizophrenia. Facta Univ. 2002, 9, 157–161. [Google Scholar]
- Lavoie, S.; Berger, M.; Schlögelhofer, M.; Schäfer, M.R.; Rice, S.; Kim, S.W.; Hesse, J.; McGorry, P.D.; Smesny, S.; Amminger, G.P. Erythrocyte glutathione levels as long-term predictor of transition to psychosis. Transl. Psychiatry 2017, 7, e1064. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Leonard, S.; Reddy, R. Altered glutathione redox state in schizophrenia. Dis. Markers 2006, 22, 83–93. [Google Scholar] [CrossRef]
- Samuelsson, M.; Skogh, E.; Lundberg, K.; Vrethem, M.; Öllinger, K. Taurine and glutathione in plasma and cerebrospinal fluid in olanzapine treated patients with schizophrenia. Psychiatry Res. 2013, 210, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Langbein, K.; Hesse, J.; Gussew, A.; Milleit, B.; Lavoie, S.; Amminger, G.P.; Gaser, C.; Wagner, G.; Reichenbach, J.R.; Hipler, U.C.; et al. Disturbed glutathione antioxidative defense is associated with structural brain changes in neuroleptic-naïve first-episode psychosis patients. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cengotitabengoa, M.; Mac-Dowell, K.S.; Leza, J.C.; Micó, J.A.; Fernandez, M.; Echevarría, E.; Sanjuan, J.; Elorza, J.; González-Pinto, A. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr. Res. 2012, 137, 66–72. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P., Jr.; Mody, V.C., Jr.; Reed, R.L.; Brown, L.A. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Mills, B.J.; Lang, C.A. Differential distribution of free and bound glutathione and cyst(e)ine in human blood. Biochem. Pharmacol. 1996, 52, 401–406. [Google Scholar] [CrossRef]
- Ballesteros, A.; Jiang, P.; Summerfelt, A.; Du, X.; Chiappelli, J.; O’Donnell, P.; Kochunov, P.; Hong, L.E. No evidence of exogenous origin for the abnormal glutathione redox state in schizophrenia. Schizophr. Res. 2013, 146, 184–189. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Zhao, G.; Kong, D.; Liu, L.; Yuan, S.; Chen, W.; Feng, C.; Li, Z. Comparative Analysis of the Pre- and Post-Medication Effects of Antipsychotic Agents on the Blood-Based Oxidative Stress Biomarkers in Patients with Schizophrenia: A Meta-Analysis. Curr. Neuropharmacol. 2023, 21, 340–352. [Google Scholar] [CrossRef]
- Wang, A.M.; Pradhan, S.; Coughlin, J.M.; Trivedi, A.; DuBois, S.L.; Crawford, J.L.; Sedlak, T.W.; Nucifora, F.C., Jr.; Nestadt, G.; Nucifora, L.G.; et al. Assessing Brain Metabolism With 7-T Proton Magnetic Resonance Spectroscopy in Patients With First-Episode Psychosis. JAMA Psychiatry 2019, 76, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, T.; Hafizi, S.; Andreazza, A.C.; Kiang, M.; Bagby, R.M.; Navas, E.; Laksono, I.; Truong, P.; Gerritsen, C.; Prce, I.; et al. Glutathione, the Major Redox Regulator, in the Prefrontal Cortex of Individuals at Clinical High Risk for Psychosis. Int. J. Neuropsychopharmacol. 2018, 21, 311–318. [Google Scholar] [CrossRef]
- Mahadik, S.P.; Mukherjee, S.; Scheffer, R.; Correnti, E.E.; Mahadik, J.S. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol. Psychiatry 1998, 43, 674–679. [Google Scholar] [CrossRef]
- Pazvantoglu, O.; Selek, S.; Okay, I.T.; Sengul, C.; Karabekiroglu, K.; Dilbaz, N.; Erel, O. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin. Neurosci. 2009, 63, 693–700. [Google Scholar] [CrossRef]
- Micó, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef]
- Huang, T.T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef]
- Fraguas, D.; Gonzalez-Pinto, A.; Micó, J.A.; Reig, S.; Parellada, M.; Martínez-Cengotitabengoa, M.; Castro-Fornieles, J.; Rapado-Castro, M.; Baeza, I.; Janssen, J.; et al. Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr. Res. 2012, 137, 58–65. [Google Scholar] [CrossRef]
- Juchnowicz, D.; Dzikowski, M.; Rog, J.; Waszkiewicz, N.; Karakuła, K.H.; Zalewska, A.; Maciejczyk, M.; Karakula-Juchnowicz, H. Pro/Antioxidant State as a Potential Biomarker of Schizophrenia. J. Clin. Med. 2021, 10, 4156. [Google Scholar] [CrossRef]
- Ballesteros, A.; Summerfelt, A.; Du, X.; Jiang, P.; Chiappelli, J.; Tagamets, M.; O’Donnell, P.; Kochunov, P.; Hong, L.E. Electrophysiological intermediate biomarkers for oxidative stress in schizophrenia. Clin. Neurophysiol. 2013, 124, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, D.; Obata, T.; Shirayama, Y.; Nonaka, H.; Kanazawa, Y.; Yoshitome, E.; Takanashi, J.; Matsuda, T.; Shimizu, E.; Ikehira, H.; et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: A 3T 1H-MRS study. PLoS ONE 2008, 3, e1944. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, S.; Noda, Y.; Tarumi, R.; Mimura, Y.; Yoshida, K.; Iwata, Y.; Elsalhy, M.; Kuromiya, M.; Kurose, S.; Masuda, F.; et al. Glutathione levels and activities of glutathione metabolism enzymes in patients with schizophrenia: A systematic review and meta-analysis. J. Psychopharmacol. 2019, 33, 1199–1214. [Google Scholar] [CrossRef] [PubMed]
- Dadheech, G.; Sharma, P.; Gautam, S. Oxidative Stress-Induced Response of Some Endogenous Antioxidants in Schizophrenia. Indian J. Clin. Biochem. 2012, 27, 278–283. [Google Scholar] [CrossRef]
- Martínez-Cengotitabengoa, M.; Micó, J.A.; Arango, C.; Castro-Fornieles, J.; Graell, M.; Payá, B.; Leza, J.C.; Zorrilla, I.; Parellada, M.; López, M.P.; et al. Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr. Res. 2014, 156, 23–29. [Google Scholar] [CrossRef]
- Wood, S.J.; Berger, G.E.; Wellard, R.M.; Proffitt, T.M.; McConchie, M.; Berk, M.; McGorry, P.D.; Pantelis, C. Medial temporal lobe glutathione concentration in first episode psychosis: A 1H-MRS investigation. Neurobiol. Dis. 2009, 33, 354–357. [Google Scholar] [CrossRef]
- Reyes-Madrigal, F.; León-Ortiz, P.; Mao, X.; Mora-Durán, R.; Shungu, D.C.; de la Fuente-Sandoval, C. Striatal Glutathione in First-episode Psychosis Patients Measured In Vivo with Proton Magnetic Resonance Spectroscopy. Arch. Med. Res. 2019, 50, 207–213. [Google Scholar] [CrossRef]
- Das, T.K.; Javadzadeh, A.; Dey, A.; Sabesan, P.; Théberge, J.; Radua, J.; Palaniyappan, L. Antioxidant defense in schizophrenia and bipolar disorder: A meta-analysis of MRS studies of anterior cingulate glutathione. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 94–102. [Google Scholar] [CrossRef]
- Kumar, J.; Liddle, E.B.; Fernandes, C.C.; Palaniyappan, L.; Hall, E.L.; Robson, S.E.; Simmonite, M.; Fiesal, J.; Katshu, M.Z.; Qureshi, A.; et al. Glutathione and glutamate in schizophrenia: A 7T MRS study. Mol. Psychiatry 2020, 25, 873–882. [Google Scholar] [CrossRef]
- Terpstra, M.; Vaughan, T.J.; Ugurbil, K.; Lim, K.O.; Schulz, S.C.; Gruetter, R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: Application to schizophrenia. MAGMA 2005, 18, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Monin, A.; Baumann, P.S.; Griffa, A.; Xin, L.; Mekle, R.; Fournier, M.; Butticaz, C.; Klaey, M.; Cabungcal, J.H.; Steullet, P.; et al. Glutathione deficit impairs myelin maturation: Relevance for white matter integrity in schizophrenia patients. Mol. Psychiatry 2015, 20, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Dempster, K.; Jeon, P.; MacKinley, M.; Williamson, P.; Théberge, J.; Palaniyappan, L. Early treatment response in first episode psychosis: A 7-T magnetic resonance spectroscopic study of glutathione and glutamate. Mol. Psychiatry 2020, 25, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.D.; Williams, S.R. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal. Biochem. 2017, 529, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Palaniyappan, L.; Park, M.T.M.; Jeon, P.; Limongi, R.; Yang, K.; Sawa, A.; Théberge, J. Is There a Glutathione Centered Redox Dysregulation Subtype of Schizophrenia? Antioxidants 2021, 10, 1703. [Google Scholar] [CrossRef]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N- acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef]
- Choy, K.H.; Dean, O.; Berk, M.; Bush, A.I.; van den Buuse, M. Effects of N-acetyl-cysteine treatment on glutathione depletion and a short-term spatial memory deficit in 2-cyclohexene-1-one-treated rats. Eur. J. Pharmacol. 2010, 649, 224–228. [Google Scholar] [CrossRef]
- Monte, A.S.; da Silva, F.E.R.; Lima, C.N.C.; Vasconcelos, G.S.; Gomes, N.S.; Miyajima, F.; Vasconcelos, S.M.M.; Gama, C.S.; Seeman, M.V.; de Lucena, D.F.; et al. Sex influences in the preventive effects of N-acetylcysteine in a two-hit animal model of schizophrenia. J. Psychopharmacol. 2020, 34, 125–136. [Google Scholar] [CrossRef]
- Conus, P.; Seidman, L.J.; Fournier, M.; Xin, L.; Cleusix, M.; Baumann, P.S.; Ferrari, C.; Cousins, A.; Alameda, L.; Gholam-Rezaee, M.; et al. N-acetylcysteine in a Double-Blind Randomized Placebo-Controlled Trial: Toward Biomarker-Guided Treatment in Early Psychosis. Schizophr. Bull. 2018, 44, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Retsa, C.; Knebel, J.F.; Geiser, E.; Ferrari, C.; Jenni, R.; Fournier, M.; Alameda, L.; Baumann, P.S.; Clarke, S.; Conus, P.; et al. Treatment in early psychosis with N-acetyl-cysteine for 6months improves low-level auditory processing: Pilot study. Schizophr. Res. 2018, 191, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Liffick, E.; Hummer, T.A.; Vohs, J.L.; Yang, Z.; Mehdiyoun, N.F.; Visco, A.C.; Metzler, E.; Zhang, Y.; Francis, M.M. Effects of 12-month, double-blind N-acetyl cysteine on symptoms, cognition and brain morphology in early phase schizophrenia spectrum disorders. Schizophr. Res. 2018, 199, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Copolov, D.; Dean, O.; Lu, K.; Jeavons, S.; Schapkaitz, I.; Anderson-Hunt, M.; Judd, F.; Katz, F.; Katz, P.; et al. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol. Psychiatry 2008, 64, 361–368. [Google Scholar] [CrossRef]
- Sepehrmanesh, Z.; Heidary, M.; Akasheh, N.; Akbari, H.; Heidary, M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: A double-blind, randomized clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 289–296. [Google Scholar] [CrossRef]
- Rapado-Castro, M.; Dodd, S.; Bush, A.I.; Malhi, G.S.; Skvarc, D.R.; On, Z.X.; Berk, M.; Dean, O.M. Cognitive effects of adjunctive N-acetyl cysteine in psychosis. Psychol. Med. 2017, 47, 866–876. [Google Scholar] [CrossRef]
- Farokhnia, M.; Azarkolah, A.; Adinehfar, F.; Khodaie-Ardakani, M.R.; Hosseini, S.M.; Yekehtaz, H.; Tabrizi, M.; Rezaei, F.; Salehi, B.; Sadeghi, S.M.; et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: A randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 2013, 36, 185–192. [Google Scholar] [CrossRef]
- Yolland, C.O.; Hanratty, D.; Neill, E.; Rossell, S.L.; Berk, M.; Dean, O.M.; Castle, D.J.; Tan, E.J.; Phillipou, A.; Harris, A.W.; et al. Meta-analysis of randomised controlled trials with N-acetylcysteine in the treatment of schizophrenia. Aust. N. Z. J. Psychiatry 2020, 54, 453–466. [Google Scholar] [CrossRef]
- Mullier, E.; Roine, T.; Griffa, A.; Xin, L.; Baumann, P.S.; Klauser, P.; Cleusix, M.; Jenni, R.; Alemàn-Gómez, Y.; Gruetter, R.; et al. N-Acetyl-Cysteine Supplementation Improves Functional Connectivity Within the Cingulate Cortex in Early Psychosis: A Pilot Study. Int. J. Neuropsychopharmacol. 2019, 22, 478–487. [Google Scholar] [CrossRef]
- Klauser, P.; Xin, L.; Fournier, M.; Griffa, A.; Cleusix, M.; Jenni, R.; Cuenod, M.; Gruetter, R.; Hagmann, P.; Conus, P.; et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: A double-blind randomized placebo-controlled trial. Transl. Psychiatry 2018, 8, 220. [Google Scholar] [CrossRef]
- Hardingham, G.E.; Do, K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016, 17, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Neijt, H.C.; Cuénod, M.; Do, K.Q. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: Relevance to schizophrenia. Neuroscience 2006, 137, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Choi, Y.B.; Takahashi, H.; Zhang, D.; Li, W.; Godzik, A.; Bankston, L.A. Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Köhr, G.; Eckardt, S.; Lüddens, H.; Monyer, H.; Seeburg, P.H. NMDA receptor channels: Subunit-specific potentiation by reducing agents. Neuron 1994, 12, 1031–1040. [Google Scholar] [CrossRef]
- Do, K.Q.; Cabungcal, J.H.; Frank, A.; Steullet, P.; Cuenod, M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009, 19, 220–230. [Google Scholar] [CrossRef]
- Zhuo, D.Y.; Wu, Y.L.; Yao, W.X.; Cao, Y.; Wu, C.F.; Tanaka, M. Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol. Biochem. Behav. 2007, 86, 1–7. [Google Scholar] [CrossRef]
- Radonjić, N.V.; Knezević, I.D.; Vilimanovich, U.; Kravić-Stevović, T.; Marina, L.V.; Nikolić, T.; Todorović, V.; Bumbasirević, V.; Petronijević, N.D. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Neuropharmacology 2010, 58, 739–745. [Google Scholar] [CrossRef]
- Da Silva, F.C.; do Carmo de Oliveira Cito, M.; da Silva, M.I.; Moura, B.A.; de Aquino Neto, M.R.; Feitosa, M.L.; de Castro Chaves, R.; Macedo, D.S.; de Vasconcelos, S.M.; de França Fonteles, M.M.; et al. Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res. Bull. 2010, 83, 9–15. [Google Scholar] [CrossRef]
- Baxter, P.S.; Bell, K.F.; Hasel, P.; Kaindl, A.M.; Fricker, M.; Thomson, D.; Cregan, S.P.; Gillingwater, T.H.; Hardingham, G.E. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2015, 6, 6761, Erratum in Nat. Commun. 2017, 8, 16158. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Rosse, R.B.; Schwartz, B.L.; Mastropaolo, J. A revised excitotoxic hypothesis of schizophrenia: Therapeutic implications. Clin. Neuropharmacol. 2001, 24, 43–49. [Google Scholar] [CrossRef]
- Yang, Y.; Dieter, M.Z.; Chen, Y.; Shertzer, H.G.; Nebert, D.W.; Dalton, T.P. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(-/-) knockout mouse. Novel model system for a severely compromised oxidative stress response. J. Biol. Chem. 2002, 277, 49446–49452. [Google Scholar] [CrossRef] [PubMed]
- Tosic, M.; Ott, J.; Barral, S.; Bovet, P.; Deppen, P.; Gheorghita, F.; Matthey, M.L.; Parnas, J.; Preisig, M.; Saraga, M.; et al. Schizophrenia and oxidative stress: Glutamate cysteine ligase modifier as a susceptibility gene. Am. J. Hum. Genet. 2006, 79, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Steullet, P.; Cabungcal, J.H.; Kulak, A.; Kraftsik, R.; Chen, Y.; Dalton, T.P.; Cuenod, M.; Do, K.Q. Redox dysregulation affects the ventral but not dorsal hippocampus: Impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J. Neurosci. 2010, 30, 2547–2558. [Google Scholar] [CrossRef]
- Back, S.A.; Gan, X.; Li, Y.; Rosenberg, P.A.; Volpe, J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998, 18, 6241–6253. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Ladi, E.; Mayer-Proschel, M.; Noble, M. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc. Natl. Acad. Sci. USA 2000, 97, 10032–10037. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Neural synchrony in brain disorders: Relevance for cognitive dysfunctions and pathophysiology. Neuron 2006, 52, 155–168. [Google Scholar] [CrossRef]
- Kannan, R.; Kuhlenkamp, J.F.; Jeandidier, E.; Trinh, H.; Ookhtens, M.; Kaplowitz, N. Evidence for carrier-mediated transport of glutathione across the blood-brain barrier in the rat. J. Clin. Investig. 1990, 85, 2009–2013. [Google Scholar] [CrossRef]
- Cabungcal, J.H.; Nicolas, D.; Kraftsik, R.; Cuénod, M.; Do, K.Q.; Hornung, J.P. Glutathione deficit during development induces anomalies in the rat anterior cingulate GABAergic neurons: Relevance to schizophrenia. Neurobiol. Dis. 2006, 22, 624–637. [Google Scholar] [CrossRef]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef]
- Thompson, P.M.; Egbufoama, S.; Vawter, M.P. SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 411–417. [Google Scholar] [CrossRef]
- Thompson, P.M.; Kelley, M.; Yao, J.; Tsai, G.; van Kammen, D.P. Elevated cerebrospinal fluid SNAP-25 in schizophrenia. Biol. Psychiatry 2003, 53, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
| Study Population | Sample | GSH Status | Note/SZ Symptoms | Reference |
|---|---|---|---|---|
| SZ | Plasma | GSHr↓ | Dietrich-Mutsazalska et al. (2009) [17] | |
| SZ | Plasma | GSHt, GSHr↓ | Raffa et al. (2009) [18] | |
| SZ | Plasma | GSHt, GSHr, GSSG↓ | Raffa et al. (2012) [19] | |
| FEP | Plasma | GSHr↓ | Ruiz-Litago et al. (2012) [21] | |
| SZ | Plasma | GSHt↓ | PANSS↑ | Nucifora et al. (2017) [22] |
| SZ | Plasma | GSHr↓ | Guidara et al. (2020) [23] | |
| SZ | plasma, lymphocytes | GSHt↓ | Couglin et al. (2021) [11] | |
| SZ | Plasma | GSHt (/) | Samuelsson et al. (2013) [36] | |
| SZ | Serum | GSHr↓ | Ivanova et al. (2015) [24] | |
| SZ | Serum | GSHr↓ | Fukushima et al. (2014) [26] | |
| SZ | Serum | GSHr↓ | PANSS (/) | Gonzalez-Liencres et al. (2014) [27] |
| SZ | Serum | GSHr↓ | PANSS↑ | Tsai et al. (2013) [28] |
| FEP | Serum | GSSG↑ | PANSS (/) | Tao et al. (2020) [29] |
| SZ | Serum | GSHr↓ | Cruz et al. (2021) [30] | |
| SZ | Erythrocytes | GSHr↓ | Altuntas et al. (2000) [32] | |
| Risk of psychosis | Erythrocytes | GSHr↓ | Lavoie et al. (2017) [34] | |
| FEP | Erythrocytes | GSHt↓, GSHr↓, GSSG↑ | GSHt, GSHr positive correlation with positive symptoms | Raffa et al. (2011) [31] |
| FEP | Erythrocytes | GSHt (/), GSHr (/), GSSG (/) | Langbein et al. (2018) [37] | |
| FEP | Erythrocytes | GSHt↓ | Mico et al. (2011) [47] | |
| FEP | Erythrocytes | GSHt↓ | Fraguas et al. (2012) [49] | |
| FEP | Erythrocytes | GSHt (/) | Martinez-Cengotitabengoa et al. (2012) [38] | |
| FEP | erythrocytes | GSHr↓ | Martinez-Cengotitabengoa et al. (2014) [55] | |
| SZ | erythrocytes | GSHr↓ | Dadheech et al. (2012) [54] | |
| SZ | Prefrontal cortex | GSHt, GSHr, GSSG↓ | Gawryluk et al. (2011) [10] | |
| SZ | caudate | GSHr↓, GSSG↓, GSHr:GSSG↓ | Yao et al. (2006) [35] | |
| SZ | CSF | GSHr↓ | Do et al. (2000) [9] | |
| SZ | Whole blood | GSHr↓ GSSG↑ | Ballesteros et al. (2013) [41,51] | |
| FEP | Striatum | GSHr↓ | PANSS↓ positive correlation | Reyes-Madrigal et al. (2019) [57] |
| FEP | ACC, Thalamus DLPFC OFR CSO | GSHr↓ GSHr↓ GSHr (/) GSHr (/) GSHr (/) | correlation with severity of cognitive symptoms | Wang et al. (2019) [43] |
| SZ | Prefrontal cortex | GSHr↓ | Do et al. (2000) [9] | |
| SZ | ACC Insula visual cortex | GSHr↓ GSHr (/) GSHr(/) | Kumar et al. (2020) [59] | |
| FEP | Medial temporal lobe | GSHr↑ | Wood et al. (2009) [56] | |
| SZ | Posterior medial frontal cortex | GSHr (/) | Matsuzawa et al. (2008) [52] | |
| SZ | Medial prefrontal cortex | GSHr (/) | Monin et al. (2015) [61] | |
| SZ | ACC | GSHr (/) | Terpstra et al. (2005) [60] | |
| Risk of psychosis | mPFC | GSHr (/) | Da Silva et al. (2018) [44] | |
| FEP | Dorsal ACC | GSHr (/) | Dempster et al. (2020) [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carletti, B.; Banaj, N.; Piras, F.; Bossù, P. Schizophrenia and Glutathione: A Challenging Story. J. Pers. Med. 2023, 13, 1526. https://doi.org/10.3390/jpm13111526
Carletti B, Banaj N, Piras F, Bossù P. Schizophrenia and Glutathione: A Challenging Story. Journal of Personalized Medicine. 2023; 13(11):1526. https://doi.org/10.3390/jpm13111526
Chicago/Turabian StyleCarletti, Barbara, Nerisa Banaj, Fabrizio Piras, and Paola Bossù. 2023. "Schizophrenia and Glutathione: A Challenging Story" Journal of Personalized Medicine 13, no. 11: 1526. https://doi.org/10.3390/jpm13111526





