The PATIENT Approach: A New Bundle for the Management of Chronic Pain
Abstract
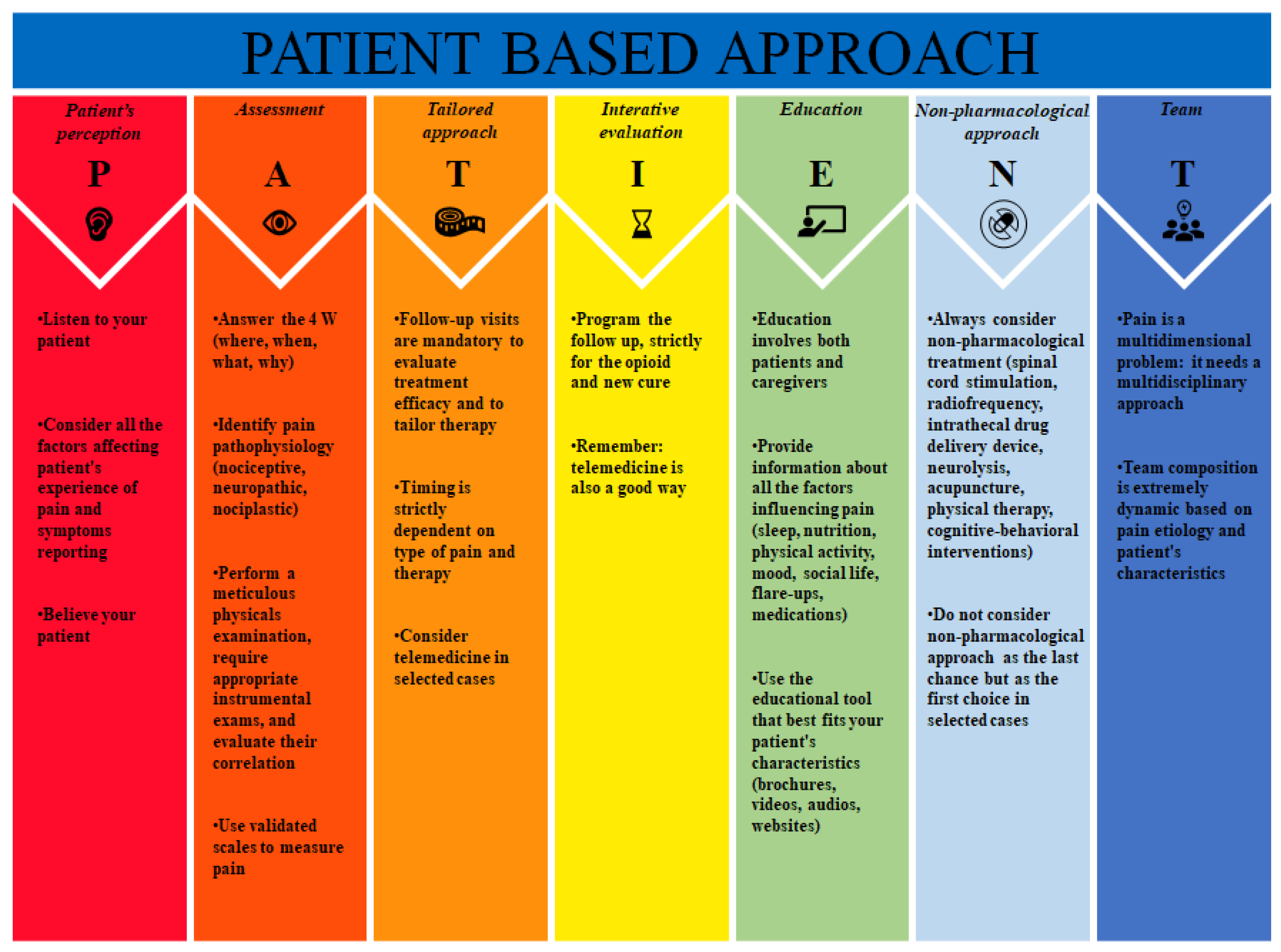
:1. Introduction
2. Materials and Methods
3. PATIENT Approach
3.1. Patient’s Perception
3.2. Assessment
- -
- Where: the localization of pain;
- -
- When: the moment the pain started and the periodicity and frequency of its worsening;
- -
- What: what are the sensations (e.g., burning, stabbing, itching);
- -
- Why: possible causes of the pain that the patient can identify (e.g., trauma, surgery, job).
3.3. Tailored Approach
3.4. Iterative Evaluation
3.5. Education
3.6. Non-Pharmacological Approach
3.6.1. Spinal Cord Stimulation
3.6.2. Radiofrequency
3.6.3. Other Non-Pharmacological Approaches
3.7. Team
4. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Kawai, A.T.; Wollan, P.; Yawn, B.P. Adverse Impacts of Chronic Pain on Health-Related Quality of Life, Work Productivity, Depression and Anxiety in a Community-Based Study. Fam. Pract. 2017, 34, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Mackey, S. Future Directions for Pain Management: Lessons from the Institute of Medicine Pain Report and the National Pain Strategy. Hand Clin. 2016, 32, 91–98. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of Chronic Pain: Domains, Methods, and Mechanisms. J. Pain 2016, 17, T10–T20. [Google Scholar] [CrossRef]
- Campbell, J.N. APS 1995 Presidential Address. Pain Forum. 1996, 5, 85–88. [Google Scholar] [CrossRef]
- Gibson, S.J.; Helme, R.D. Age-Related Differences in Pain Perception and Report. Clin. Geriatr. Med. 2001, 17, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age Changes in Pain Perception: A Systematic-Review and Meta-Analysis of Age Effects on Pain and Tolerance Thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113. [Google Scholar] [CrossRef]
- Ahmed, Y.; Popovic, M.; Wan, B.A.; Lam, M.; Lam, H.; Ganesh, V.; Milakovic, M.; DeAngelis, C.; Malek, L.; Chow, E. Does Gender Affect Self-Perceived Pain in Cancer Patients?—A Meta-Analysis. Ann. Palliat. Med. 2017, 6, S177–S184. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Maixner, W. Gender Differences in the Responses to Noxious Stimuli. Pain Forum 1995, 4, 209–221. [Google Scholar] [CrossRef]
- Racine, M.; Tousignant-Laflamme, Y.; Kloda, L.A.; Dion, D.; Dupuis, G.; Choinière, M. A Systematic Literature Review of 10 Years of Research on Sex/Gender and Experimental Pain Perception—Part 1: Are There Really Differences between Women and Men? Pain 2012, 153, 602–618. [Google Scholar] [CrossRef]
- Riley, J.L.; Robinson, M.E.; Wise, E.A.; Myers, C.D.; Fillingim, R.B. Sex Differences in the Perception of Noxious Experimental Stimuli: A Meta-Analysis. Pain 1998, 74, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Atzeni, F.; Bazzichi, L.; Beretta, G.; Costantini, E.; Sacerdote, P.; Tassorelli, C. Pain in Women: A Perspective Review on a Relevant Clinical Issue That Deserves Prioritization. Pain Ther. 2021, 10, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; Edwards, R.R.; Powell, T. The Relationship of Sex and Clinical Pain to Experimental Pain Responses. Pain 1999, 83, 419–425. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Edwards, R.R.; Powell, T. Sex-Dependent Effects of Reported Familial Pain History on Recent Pain Complaints and Experimental Pain Responses. Pain 2000, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Coghill, R.C.; McHaffie, J.G.; Yen, Y.-F. Neural Correlates of Interindividual Differences in the Subjective Experience of Pain. Proc. Natl. Acad. Sci. USA 2003, 100, 8538–8542. [Google Scholar] [CrossRef]
- Stroemel-Scheder, C.; Kundermann, B.; Lautenbacher, S. The Effects of Recovery Sleep on Pain Perception: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 113, 408–425. [Google Scholar] [CrossRef]
- Torensma, B.; Thomassen, I.; van Velzen, M.; In ’t Veld, B.A. Pain Experience and Perception in the Obese Subject Systematic Review (Revised Version). Obes. Surg. 2016, 26, 631–639. [Google Scholar] [CrossRef]
- Price, R.C.; Asenjo, J.F.; Christou, N.V.; Backman, S.B.; Schweinhardt, P. The Role of Excess Subcutaneous Fat in Pain and Sensory Sensitivity in Obesity. Eur. J. Pain 2013, 17, 1316–1326. [Google Scholar] [CrossRef]
- Merskey, H. Logic, Truth and Language in Concepts of Pain. Qual. Life Res. 1994, 3 (Suppl. 1), S69–S76. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Dworkin, R.H.; Turk, D.C.; Wyrwich, K.W.; Beaton, D.; Cleeland, C.S.; Farrar, J.T.; Haythornthwaite, J.A.; Jensen, M.P.; Kerns, R.D.; Ader, D.N.; et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J. Pain 2008, 9, 105–121. [Google Scholar] [CrossRef]
- Glowacki, D. Effective Pain Management and Improvements in Patients’ Outcomes and Satisfaction. Crit. Care Nurse 2015, 35, 33–41. [Google Scholar] [CrossRef]
- Balagué, F.; Mannion, A.F.; Pellisé, F.; Cedraschi, C. Non-Specific Low Back Pain. Lancet 2012, 379, 482–491. [Google Scholar] [CrossRef]
- Breivik, H.; Borchgrevink, P.C.; Allen, S.M.; Rosseland, L.A.; Romundstad, L.; Hals, E.K.B.; Kvarstein, G.; Stubhaug, A. Assessment of Pain. Br. J. Anaesth. 2008, 101, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Burbank, K.M.; Stevenson, J.H.; Czarnecki, G.R.; Dorfman, J. Chronic Shoulder Pain: Part I. Evaluation and Diagnosis. Am. Fam. Physician 2008, 77, 453–460. [Google Scholar] [PubMed]
- Morone, N.E.; Weiner, D.K. Pain as the Fifth Vital Sign: Exposing the Vital Need for Pain Education. Clin. Ther. 2013, 35, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Breivik, E.K.; Björnsson, G.A.; Skovlund, E. A Comparison of Pain Rating Scales by Sampling from Clinical Trial Data. Clin. J. Pain 2000, 16, 22–28. [Google Scholar] [CrossRef]
- Attal, N.; Perrot, S.; Fermanian, J.; Bouhassira, D. The Neuropathic Components of Chronic Low Back Pain: A Prospective Multicenter Study Using the DN4 Questionnaire. J. Pain 2011, 12, 1080–1087. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Cuomo, A.; Bimonte, S.; Forte, C.A.; Botti, G.; Cascella, M. Multimodal Approaches and Tailored Therapies for Pain Management: The Trolley Analgesic Model. J. Pain Res. 2019, 12, 711–714. [Google Scholar] [CrossRef]
- Gelot, S.; Nakhla, E. Opioid Dosing in Renal and Hepatic Impairment. U.S. Pharm. 2014, 39, 34–38. [Google Scholar]
- Knotkova, H.; Fine, P.G.; Portenoy, R.K. Opioid Rotation: The Science and the Limitations of the Equianalgesic Dose Table. J. Pain Symptom Manag. 2009, 38, 426–439. [Google Scholar] [CrossRef]
- Moseley, G.L. Evidence for a Direct Relationship between Cognitive and Physical Change during an Education Intervention in People with Chronic Low Back Pain. Eur. J. Pain 2004, 8, 39–45. [Google Scholar] [CrossRef]
- Attal, N. Pharmacological Treatments of Neuropathic Pain: The Latest Recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef]
- Fine, P.G.; Portenoy, R.K. Ad Hoc Expert Panel on Evidence Review and Guidelines for Opioid Rotation. Establishing “Best Practices” for Opioid Rotation: Conclusions of an Expert Panel. J. Pain Symptom Manag. 2009, 38, 418–425. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, L.; Zhou, Q. Opioid Analgesics-Related Pharmacokinetic Drug Interactions: From the Perspectives of Evidence Based on Randomized Controlled Trials and Clinical Risk Management. J. Pain Res. 2017, 10, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Ayad, S.; Khanna, A.K.; Iqbal, S.U.; Singla, N. Characterisation and Monitoring of Postoperative Respiratory Depression: Current Approaches and Future Considerations. Br. J. Anaesth. 2019, 123, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Sporer, K.A. The Serotonin Syndrome. Implicated Drugs, Pathophysiology and Management. Drug Saf. 1995, 13, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Jacox, A.; Carr, D.B.; Payne, R. New Clinical-Practice Guidelines for the Management of Pain in Patients with Cancer. N. Engl. J. Med. 1994, 330, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Olarte, J.M.N. Breakthrough Cancer Pain and Rational Drug Use. Support. Care Cancer 2017, 25, 11–17. [Google Scholar] [CrossRef]
- Vega-Loza, A.; Van, C.; M Moreno, A.; Aleman, F. Gene Therapies to Reduce Chronic Pain: Are We There Yet? Pain Manag. 2020, 10, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-Based Medicines for Chronic Neuropathic Pain in Adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.L.; Grant, A.R.; Moseley, G.L. Low Back Pain and the Social Determinants of Health: A Systematic Review and Narrative Synthesis. Pain 2020, 161, 2476–2493. [Google Scholar] [CrossRef]
- Emerick, T.; Alter, B.; Jarquin, S.; Brancolini, S.; Bernstein, C.; Luong, K.; Morrisseyand, S.; Wasan, A. Telemedicine for Chronic Pain in the COVID-19 Era and Beyond. Pain Med. 2020, 21, 1743–1748. [Google Scholar] [CrossRef]
- Cascella, M.; Marinangeli, F.; Vittori, A.; Scala, C.; Piccinini, M.; Braga, A.; Miceli, L.; Vellucci, R. Open Issues and Practical Suggestions for Telemedicine in Chronic Pain. Int. J. Environ. Res. Public Health 2021, 18, 12416. [Google Scholar] [CrossRef]
- Brain, K.; Burrows, T.L.; Rollo, M.E.; Chai, L.K.; Clarke, E.D.; Hayes, C.; Hodson, F.J.; Collins, C.E. A Systematic Review and Meta-Analysis of Nutrition Interventions for Chronic Noncancer Pain. J. Hum. Nutr. Diet. 2019, 32, 198–225. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.B.; Drummond, P.D. Sleep Problems Are Associated with Chronic Pain Over and Above Mutual Associations with Depression and Catastrophizing. Clin. J. Pain 2016, 32, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef]
- Rantonen, J.; Vehtari, A.; Karppinen, J.; Luoto, S.; Viikari-Juntura, E.; Hupli, M.; Malmivaara, A.; Taimela, S. Face-to-Face Information Combined with a Booklet versus a Booklet Alone for Treatment of Mild Low-Back Pain: A Randomized Controlled Trial. Scand J. Work Environ. Health 2014, 40, 156–166. [Google Scholar] [CrossRef]
- Abu Abed, M.; Himmel, W.; Vormfelde, S.; Koschack, J. Video-Assisted Patient Education to Modify Behavior: A Systematic Review. Patient Educ. Couns. 2014, 97, 16–22. [Google Scholar] [CrossRef]
- Chi, N.-C.; Barani, E.; Fu, Y.-K.; Nakad, L.; Gilbertson-White, S.; Herr, K.; Saeidzadeh, S. Interventions to Support Family Caregivers in Pain Management: A Systematic Review. J. Pain Symptom Manag. 2020, 60, 630–656.e31. [Google Scholar] [CrossRef]
- Kumar, K.; Rizvi, S. Cost-Effectiveness of Spinal Cord Stimulation Therapy in Management of Chronic Pain. Pain Med. 2013, 14, 1631–1649. [Google Scholar] [CrossRef] [PubMed]
- Echeverria-Villalobos, M.; Mitchell, J.; Fiorda-Diaz, J.; Weaver, T. Effects of Dorsal Column Spinal Cord Stimulation on Neuroinflammation: Revisiting Molecular Mechanisms and Clinical Outcomes on Chronic Lumbar/Leg Pain and Failed Back Surgery Syndrome. J. Pain Res. 2021, 14, 2337–2345. [Google Scholar] [CrossRef]
- Chakravarthy, K.; Fishman, M.A.; Zuidema, X.; Hunter, C.W.; Levy, R. Mechanism of Action in Burst Spinal Cord Stimulation: Review and Recent Advances. Pain Med. 2019, 20, S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal Cord Stimulation: Clinical Efficacy and Potential Mechanisms. Pain Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, K.; Cheng, J.; Patijn, J.; Van Kleef, M.; Lataster, A.; Mekhail, N.; Van Zundert, J. Lumbosacral Radicular Pain. Pain Pract. 2010, 10, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Aryal, V.; Poudel, S.; Zulfiqar, F.; Shrestha, T.; Singh, A.; Shah, S.A.; Soomro, U.; Choudhari, J.; Quinonez, J.; Ruxmohan, S.; et al. Updates on the Role of Spinal Cord Stimulation in the Management of Non-Surgical Chronic Lower Back Pain. Cureus 2021, 13, e18928. [Google Scholar] [CrossRef]
- Dones, I.; Levi, V. Spinal Cord Stimulation for Neuropathic Pain: Current Trends and Future Applications. Brain Sci. 2018, 8, 138. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. Spinal Cord Stimulation versus Conventional Medical Management for Neuropathic Pain: A Multicentre Randomised Controlled Trial in Patients with Failed Back Surgery Syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef] [PubMed]
- North, R.B.; Kidd, D.H.; Farrokhi, F.; Piantadosi, S.A. Spinal Cord Stimulation versus Repeated Lumbosacral Spine Surgery for Chronic Pain: A Randomized, Controlled Trial. Neurosurgery 2005, 56, 98–107. [Google Scholar] [CrossRef]
- Pollard, E.M.; Lamer, T.J.; Moeschler, S.M.; Gazelka, H.M.; Hooten, W.M.; Bendel, M.A.; Warner, N.S.; Murad, M.H. The Effect of Spinal Cord Stimulation on Pain Medication Reduction in Intractable Spine and Limb Pain: A Systematic Review of Randomized Controlled Trials and Meta-Analysis. J. Pain Res. 2019, 12, 1311–1324. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-KHz High-Frequency and Traditional Low-Frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: 24-Month Results From a Multicenter, Randomized, Controlled Pivotal Trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation Technol. Neural Interface 2018, 21, 56–66. [Google Scholar] [CrossRef]
- Odonkor, C.; Kwak, R.; Ting, K.; Hao, D.; Collins, B.; Ahmed, S. Fantastic Four: Age, Spinal Cord Stimulator Waveform, Pain Localization and History of Spine Surgery Influence the Odds of Successful Spinal Cord Stimulator Trial. Pain Physician 2020, 23, E19–E30. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chang, M.C. Efficacy of Pulsed Radiofrequency in Controlling Pain Caused by Spinal Disorders: A Narrative Review. Ann. Palliat. Med. 2020, 9, 3528–3536. [Google Scholar] [CrossRef]
- Erdine, S.; Bilir, A.; Cosman, E.R.; Cosman, E.R. Ultrastructural Changes in Axons Following Exposure to Pulsed Radiofrequency Fields. Pain Pract. 2009, 9, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Iwasaka, H.; Takeshima, N.; Noguchi, T. Mechanisms of Analgesic Action of Pulsed Radiofrequency on Adjuvant-Induced Pain in the Rat: Roles of Descending Adrenergic and Serotonergic Systems. Eur. J. Pain 2009, 13, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. Efficacy of Pulsed Radiofrequency Stimulation in Patients with Peripheral Neuropathic Pain: A Narrative Review. Pain Physician 2018, 21, E225–E234. [Google Scholar] [CrossRef]
- Ma, K.; Fan, Y.; Yi, J.; Huang, X.; Liu, X.; Cheng, Z.; Huang, C.; Wang, Y. Efficacy of Pulsed Radiofrequency in the Treatment of Thoracic Postherpetic Neuralgia from the Angulus Costae: A Randomized, Double-Blinded, Controlled Trial. Pain Physician 2013, 16, 15–25. [Google Scholar]
- Pi, Z.B.; Lin, H.; He, G.D.; Cai, Z.; Xu, X.Z. Randomized and Controlled Prospective Trials of Ultrasound-Guided Spinal Nerve Posterior Ramus Pulsed Radiofrequency Treatment for Lower Back Post-Herpetic Neuralgia. Clin. Ter. 2015, 166, e301–e305. [Google Scholar] [CrossRef]
- Kim, E.D.; Lee, Y.I.; Park, H.J. Comparison of Efficacy of Continuous Epidural Block and Pulsed Radiofrequency to the Dorsal Root Ganglion for Management of Pain Persisting beyond the Acute Phase of Herpes Zoster. PLoS ONE 2017, 12, e0183559. [Google Scholar] [CrossRef]
- Kapural, L.; Deering, J.P. A Technological Overview of Cooled Radiofrequency Ablation and Its Effectiveness in the Management of Chronic Knee Pain. Pain Manag. 2020, 10, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, G.; Dupoiron, D.; Seegers, V.; Lebrec, N.; Boré, F.; Dubois, P.-Y.; Leblanc, D.; Delorme, T.; Jubier-Hamon, S. Intrathecal Drug Delivery Systems for Refractory Pancreatic Cancer Pain: Observational Follow-up Study Over an 11-Year Period in a Comprehensive Cancer Center. Anesth. Analg. 2018, 126, 2038–2046. [Google Scholar] [CrossRef]
- Capozza, M.A.; Triarico, S.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Ruggiero, A. Narrative Review of Intrathecal Drug Delivery (IDD): Indications, Devices and Potential Complications. Ann. Transl. Med. 2021, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Pope, J.E.; Hayek, S.M.; Bux, A.; Buchser, E.; Eldabe, S.; De Andrés, J.A.; Erdek, M.; Patin, D.; Grider, J.S.; et al. The Polyanalgesic Consensus Conference (PACC): Recommendations on Intrathecal Drug Infusion Systems Best Practices and Guidelines. Neuromodulation 2017, 20, 96–132. [Google Scholar] [CrossRef]
- Deer, T.R.; Smith, H.S.; Burton, A.W.; Pope, J.E.; Doleys, D.M.; Levy, R.M.; Staats, P.S.; Wallace, M.S.; Webster, L.R.; Rauck, R.L.; et al. Comprehensive Consensus Based Guidelines on Intrathecal Drug Delivery Systems in the Treatment of Pain Caused by Cancer Pain. Pain Physician 2011, 14, E283–E312. [Google Scholar] [CrossRef]
- Eide, P.K.; Stubhaug, A. Relief of Trigeminal Neuralgia after Percutaneous Retrogasserian Glycerol Rhizolysis Is Dependent on Normalization of Abnormal Temporal Summation of Pain, without General Impairment of Sensory Perception. Neurosurgery 1998, 43, 462–474. [Google Scholar] [CrossRef]
- Resnick, D.K.; Levy, E.I.; Jannetta, P.J. Microvascular Decompression for Pediatric Onset Trigeminal Neuralgia. Neurosurgery 1998, 43, 804–808. [Google Scholar] [CrossRef]
- Sharma, R.; Phalak, M.; Katiyar, V.; Borkar, S.; Kale, S.S.; Mahapatra, A.K. Microvascular Decompression versus Stereotactic Radiosurgery as Primary Treatment Modality for Trigeminal Neuralgia: A Systematic Review and Meta-Analysis of Prospective Comparative Trials. Neurol. India 2018, 66, 688–694. [Google Scholar] [CrossRef]
- Wang, J.Y.; Bender, M.T.; Bettegowda, C. Percutaneous Procedures for the Treatment of Trigeminal Neuralgia. Neurosurg. Clin. N. Am. 2016, 27, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Xu-Hui, W.; Chun, Z.; Guang-Jian, S.; Min-Hui, X.; Guang-Xin, C.; Yong-Wen, Z.; Lun-Shan, X. Long-Term Outcomes of Percutaneous Retrogasserian Glycerol Rhizotomy in 3370 Patients with Trigeminal Neuralgia. Turk. Neurosurg. 2011, 21, 48–52. [Google Scholar] [PubMed]
- Pradel, W.; Hlawitschka, M.; Eckelt, U.; Herzog, R.; Koch, K. Cryosurgical Treatment of Genuine Trigeminal Neuralgia. Br. J. Oral. Maxillofac. Surg. 2002, 40, 244–247. [Google Scholar] [CrossRef]
- He, T.; Zhu, W.; Du, S.-Q.; Yang, J.-W.; Li, F.; Yang, B.-F.; Shi, G.-X.; Liu, C.-Z. Neural Mechanisms of Acupuncture as Revealed by FMRI Studies. Auton. Neurosci. 2015, 190, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Irnich, D.; Witt, C.M.; Linde, K. Acupuncture Trialists’ Collaboration. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J. Pain 2018, 19, 455–474. [Google Scholar] [CrossRef]
- Corbett, M.S.; Rice, S.J.C.; Madurasinghe, V.; Slack, R.; Fayter, D.A.; Harden, M.; Sutton, A.J.; Macpherson, H.; Woolacott, N.F. Acupuncture and Other Physical Treatments for the Relief of Pain Due to Osteoarthritis of the Knee: Network Meta-Analysis. Osteoarthr. Cartil. 2013, 21, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Deare, J.C.; Zheng, Z.; Xue, C.C.L.; Liu, J.P.; Shang, J.; Scott, S.W.; Littlejohn, G. Acupuncture for Treating Fibromyalgia. Cochrane Database Syst. Rev. 2013, 2013, CD007070. [Google Scholar] [CrossRef]
- Kelly, R.B.; Willis, J. Acupuncture for Pain. Am. Fam. Physician 2019, 100, 89–96. [Google Scholar]
- Ettinger, W.H.; Burns, R.; Messier, S.P.; Applegate, W.; Rejeski, W.J.; Morgan, T.; Shumaker, S.; Berry, M.J.; O’Toole, M.; Monu, J.; et al. A Randomized Trial Comparing Aerobic Exercise and Resistance Exercise with a Health Education Program in Older Adults with Knee Osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 1997, 277, 25–31. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Torres, R.T.; Duarte, J.A.; Gonçalves, R.S. Non-Pharmacological and Non-Surgical Interventions for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Acta Reumatol. Port 2019, 44, 173–217. [Google Scholar]
- Cifu, D.X. Braddom’s Physical Medicine and Rehabilitation; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780323625395. [Google Scholar]
- Bradt, J.; Dileo, C.; Magill, L.; Teague, A. Music Interventions for Improving Psychological and Physical Outcomes in Cancer Patients. Cochrane Database Syst. Rev. 2016, 8, CD006911. [Google Scholar] [CrossRef]
- Gauthier, K.; Dulong, C.; Argáez, C. Multidisciplinary Programs for Chronic Non-Malignant Pain Cite As: Multidisciplinary Treatment Programs for Patients with Chronic Non-Malignant Pain: A Review of Clinical Effectiveness, Cost-Effectiveness, and Guidelines. In Summary with Critical Appraisal; National Institutes of Health: Bethesda, MD, USA, 2017. [Google Scholar]
- Marra, A.; Hayhurst, C.J.; Hughes, C.G.; Merengoni, A.; Bellelli, G.; Pandharipande, P.; Morandi, A. Avoiding Inappropriate Medication Prescription in Older Intensive Care Survivors. JCOM 2018, 25, 67–83. [Google Scholar]
- Schwan, J.; Sclafani, J.; Tawfik, V.L. Chronic Pain Management in the Elderly. Anesthesiol. Clin. 2019, 37, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Bair, M.J.; Ang, D.; Wu, J.; Outcalt, S.D.; Sargent, C.; Kempf, C.; Froman, A.; Schmid, A.A.; Damush, T.M.; Yu, Z.; et al. Evaluation of Stepped Care for Chronic Pain (ESCAPE) in Veterans of the Iraq and Afghanistan Conflicts: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Petruzzo, A.; Giannetta, N.; Ruggiero, A.; Di Muzio, M.; Latina, R. Management of Chronic Musculoskeletal Pain in Veterans: A Systematic Review. Acta Biomed. 2021, 92, e2021011. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buonanno, P.; Marra, A.; Iacovazzo, C.; Vargas, M.; Nappi, S.; Squillacioti, F.; de Siena, A.U.; Servillo, G. The PATIENT Approach: A New Bundle for the Management of Chronic Pain. J. Pers. Med. 2023, 13, 1551. https://doi.org/10.3390/jpm13111551
Buonanno P, Marra A, Iacovazzo C, Vargas M, Nappi S, Squillacioti F, de Siena AU, Servillo G. The PATIENT Approach: A New Bundle for the Management of Chronic Pain. Journal of Personalized Medicine. 2023; 13(11):1551. https://doi.org/10.3390/jpm13111551
Chicago/Turabian StyleBuonanno, Pasquale, Annachiara Marra, Carmine Iacovazzo, Maria Vargas, Serena Nappi, Francesco Squillacioti, Andrea Uriel de Siena, and Giuseppe Servillo. 2023. "The PATIENT Approach: A New Bundle for the Management of Chronic Pain" Journal of Personalized Medicine 13, no. 11: 1551. https://doi.org/10.3390/jpm13111551




