Compatibility Assessment of Novel Orodispersible Film Vehicle for Personalized Medicine with Selected Active Pharmaceutical Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation and Preparation of the Films
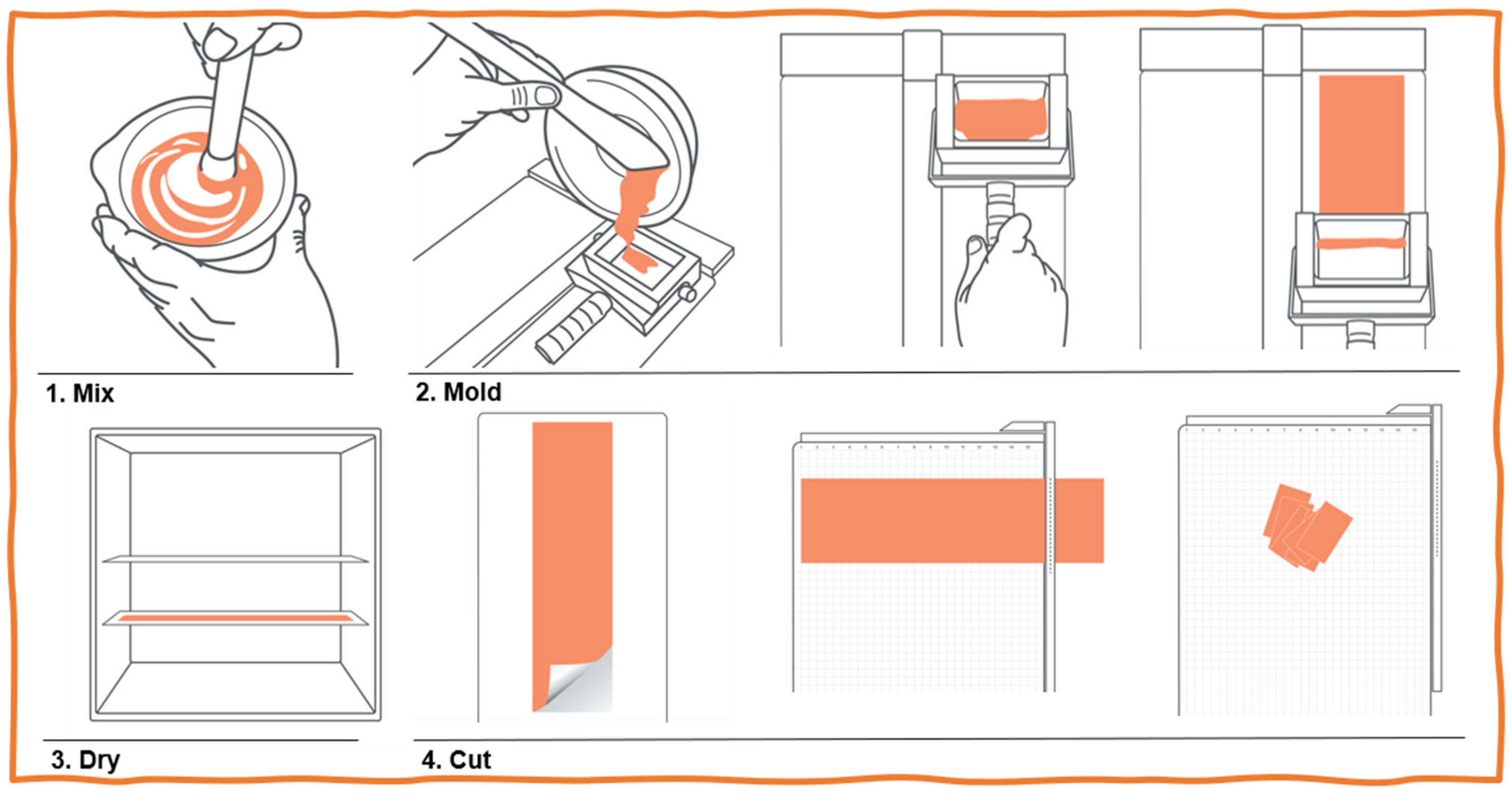
- All APIs were sieved, crushed in a mortar, and then carefully mixed with the OrphylloTM until a paste was obtained.
- This mixture was laminated/spread onto glass plates using a laminating apparatus (FagronLab).
- The glass plates were transferred to a film dryer (FagronLab) previously equilibrated at 40 °C for 40 min.
- After completely drying, films were cut with a guillotine (paper trimmer) into squares of 3 × 3 cm, packed into individual laminated matte aluminum sachets, and stored at noncontrolled room temperature to mimic real-use conditions (15–30 °C).
2.2. Compatibility Study
2.3. Disintegration
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rathi, V.; Senthil, V.; Kammili, L.; Hans, R. A brief review on oral film technology. Int. J. Res. Ayur. Pharm. 2011, 2, 1138–1147. [Google Scholar]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in Orodispersible Films for Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, M.B.H.; Riaz, T.; Jamshaid, M.; Bashir, I.; Zulfiqar, S. Oral Films: A Comprehensive Review. Int. Curr. Pharm. J. 2016, 5, 111–117. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, A.; Brandão, M.A.F.; Raposo, F.J.; Polonini, H.C.; Raposo, N.R.B. Orodispersible Films for Compounding Pharmacies. Int. J. Pharm. Compd. 2017, 21, 454–461. [Google Scholar]
- de Souza Apolinário, R.; Chaves, M.D.G.A.M.C.; Gonçalves, H.R.M.; Martins, I.C.F.; de Paula, R.M.; Granato, A.P.A.G.; Polonini, H.C.; Brandão, M.A.F.B.; Ferreira, A.d.O.F.; Raposo, N.R.B.; et al. Compounded Orodispersible Films with Natural Ingredients for Halitosis: A Clinical Experience. Int. J. Pharm. Compd. 2018, 22, 512–515. [Google Scholar]
- British Pharmacopoeia Commission. Oromucosal Preparations. In British Pharmacopoeia 2021; British Pharmacopoeia Commission: London, UK, 2021. [Google Scholar]
- Council of Europe. Oromucosal Preparations. In European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2021. [Google Scholar]
- USP—United States Pharmacopeia. <1151> Pharmaceutical Dosage Forms. 2023. Available online: https://online.uspnf.com/uspnf/document/1_GUID-431F93A9-1FEC-42AE-8556-AA5B604B2E36_8_en-US?source=Quick%20Search&highlight=1151 (accessed on 20 October 2023).
- Visser, J.C.; Wibier, L.; Kiefer, O.; Orlu, M.; Breitkreutz, J.; Woerdenbag, H.J.; Taxis, K. A Pediatrics Utilization Study in The Netherlands to Identify Active Pharmaceutical Ingredients Suitable for Inkjet Printing on Orodispersible Films. Pharmaceutics 2020, 12, 164. [Google Scholar] [CrossRef]
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V. Orodispersible Thin Film: A New Patient-Centered Innovation. J. Drug Deliv. Sci. Technol. 2020, 59, 101843. [Google Scholar] [CrossRef]
- Yafout, M.; Elhorr, H.; Ousaid, A.; El Otmani, I.S.; Khayati, Y. Orodispersible Films as a Solution to Drug Acceptability Issues: A Short Review. Asian J. Res. Med. Pharm. Sci. 2021, 10, 36–41. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, G.K.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized Orodispersible Films by Hot Melt Ram Extrusion 3D Printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Wibier, L.; Mekhaeil, M.; Woerdenbag, H.J.; Taxis, K. Orodispersible Films as a Personalized Dosage Form for Nursing Home Residents, an Exploratory Study. Int. J. Clin. Pharm. 2020, 42, 436–444. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of Plasticizers on the Physico-Mechanical Properties of Pullulan Based Pharmaceutical Oral Films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D Printing of Multilayered Orodispersible Films with In-Process Drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.S.; Aljaeid, B.M.; Mokhtar, M.; Shehata, T.M. Taste-Masked Orodispersible Tablets of Cyclosporine Self-Nanoemulsion Lyophilized with Dry Silica. Pharm. Dev. Technol. 2015, 20, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Jani, R.; Patel, D. Hot Melt Extrusion: An Industrially Feasible Approach for Casting Orodispersible Film. Asian J. Pharm. Sci. 2015, 10, 292–305. [Google Scholar] [CrossRef]
- Medicines & Healthcare Products Regulatory Agency. British Pharmacopoeia; Medicines & Healthcare Products Regulatory Agency: London, UK, 2023. [Google Scholar]
- USP—United States Pharmacopeia. <795> Pharmaceutical Compounding—Nonsterile Preparations. 2023. Available online: https://online.uspnf.com/uspnf/document/1_GUID-98DCB48D-DC23-4A63-AD2E-01CA8979FB7E_5_en-US?source=Search%20Results&highlight=795 (accessed on 20 October 2023).
- Polonini, H.C.; Silva, S.L.; Loures, S.; Almy, R.; Balland, A.; Brandão, M.A.F.; Ferreira, A.O. Compatibility of Proton Pump Inhibitors in a Preservative-Free Suspending Vehicle. Eur. J. Hosp. Pharm. 2018, 25, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.O.; Polonini, H.C.; Silva, S.L.; Patrício, F.B.; Brandão, M.A.F.; Raposo, N.R.B. Feasibility of Amlodipine Besylate, Chloroquine Phosphate, Dapsone, Phenytoin, Pyridoxine Hydrochloride, Sulfadiazine, Sulfasalazine, Tetracycline Hydrochloride, Trimethoprim and Zonisamide in SyrSpend® SF PH4 Oral Suspensions. J. Pharm. Biomed. Anal. 2016, 118, 105–112. [Google Scholar] [CrossRef]
- British Pharmacopoeia. Appendix XII A. Disintegration; British Pharmacopoeia: London, UK, 2023. [Google Scholar]
- USP—United States Pharmacopeia. <701> Disintegration. 2023. Available online: https://online.uspnf.com/uspnf/document/2_GUID-6B930D76-6026-4693-844B-FB9C90728D4F_30201_en-US?source=Search%20Results&highlight=701 (accessed on 20 October 2023).
- Raposo, F.J.; Polonini, H.C.; Ferreira, A.; Raposo, N.R.B.; Brandão, M.A.F. Technological Device for Manufacturing Transdermal Films: Possible Applications to the Individualized Treatment for Erectile Dysfunction. AAPS PharmSciTech 2017, 18, 2824–2831. [Google Scholar] [CrossRef]
- GRAS Notice Inventory. FDA. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 12 April 2023).
- El-Setouhy, D.A.; El-Malak, N.S.A. Formulation of a Novel Tianeptine Sodium Orodispersible Film. AAPS PharmSciTech 2010, 11, 1018–1025. [Google Scholar] [CrossRef]
- Scarpa, M.; Paudel, A.; Kloprogge, F.; Hsiao, W.K.; Bresciani, M.; Gaisford, S.; Orlu, M. Key Acceptability Attributes of Orodispersible Films. Eur. J. Pharm. Biopharm. 2018, 125, 131–140. [Google Scholar] [CrossRef]
- Saab, M.; Mehanna, M.M. Disintegration Time of Orally Dissolving Films: Various Methodologies and in-Vitro/in-Vivo Correlation. Die Pharm.—Int. J. Pharm. Sci. 2019, 74, 227–230. [Google Scholar]
- Steiner, D.; Finke, J.H.; Kwade, A. Model-Based Description of Disintegration Time and Dissolution Rate of Nanoparticle-Loaded Orodispersible Films. Eur. J. Pharm. Sci. 2019, 132, 18–26. [Google Scholar] [CrossRef] [PubMed]
| API | Quantity per Film | |
|---|---|---|
| API | OrPhylloTM (Base, Reconstituted) | |
| 5-Hydroxytryptophan (5-HTP) | 50 mg | qs 1 film |
| Bromopride | 5 mg | qs 1 film |
| Coenzyme Q10 | 20 mg | qs 1 film |
| Melatonin | 3 mg | qs 1 film |
| Resveratrol | 5 mg | qs 1 film |
| Tadalafil | 10 mg | qs 1 film |
| Vitamin B12 | 1 mg | qs 1 film |
| Vitamin D3 | 2000 UI | qs 1 film |
| API | Mobile Phase Composition | Work Concentration (μg/mL) * | Column | Flow (mL/min) | UV Detection Wavelength (nm) |
|---|---|---|---|---|---|
| 5-HTP | Methanol 3% in 0.05 M phosphate buffer | 50.0; 20 μL injection | C18, 4.6 mm × 25 cm, at 25 °C | 1.5 | 275 |
| Bromopride | Phosphate buffer pH 7.0 and acetonitrile (60:40, v/v) | 80.0, in water and acetonitrile (3:2); 20 μL injection | L11, 4.6 mm × 25 cm, at 25 °C | 1.0 | 310 |
| Coenzyme Q10 | Methanol and ethanol (65:35, v/v). | 1000.0, in ethanol; 10 μL injection | C18, 4.6 mm × 15 cm, at 35 °C | 1.0 | 275 |
| Melatonin | Acetonitrile and phosphate buffer pH 3.5 (25:75, v/v) | 30.0; 20 μL injection | C18, 4.6 mm × 15 cm, at 25 °C | 1.0 | 222 |
| Resveratrol | Acetonitrile and water (55:45, v/v) | 50.0; 20 μL injection | C18, 4.6 mm × 25 cm, at 25 °C | 1.4 | 307 |
| Tadalafil | Acetonitrile, water, and trifluoroacetic acid (35:65:1, v/v/v) | 100.0, in water; 20 μL injection | C18, 4.6 mm × 15 cm, at 35 °C | 1.0 | 285 |
| Vitamin B12 | Methanol and water (7:13, v/v) | 5.0, in water; 100 μL injection | C18, 4.6 mm × 15 cm, at 25 °C | 0.5 | 361 |
| Vitamin D3 | Water and methanol (3:97, v/v) | 5.0, in methanol 90%; 100 μL injection | C18, 4.6 mm × 10 cm; at 25 °C | 1.0 | 264 |
| API | HCl | NaOH | UV | Heat | H2O2 |
|---|---|---|---|---|---|
| %d * | %d * | %d * | %d * | %d * | |
| 5-HTP | −14.18 | −13.03 | −13.63 | −4.17 | −2.96 |
| Bromopride | −5.90 | −15.35 | −8.78 | 3.65 | −5.67 |
| Coenzyme Q10 | −98.45 | ND | ND | −29.69 | 38.77 |
| Melatonin | −93.03 | 63.19 | −44.51 | −4.17 | 2.01 |
| Resveratrol | −81.38 | −91.31 | 1.82 | −12.88 | ND |
| Tadalafil | 13.72 | 9.98 | 13.29 | 9.68 | 54.57 |
| Vitamin B12 | −12.59 | −96.40 | −1.86 | −1.70 | 12.44 |
| Vitamin D3 | −2.48 | −4.99 | −51.88 | −46.89 | 65.19 |
| Elapsed Time (Days) | % Recovery (Mean ± Standard Deviation; n = 6) |
|---|---|
| Room Temperature (15–30 °C) | |
| 5-HTP—50 mg/oral film | |
| t = 0 | 100.00 ± 2.99 |
| t = 30 | 101.34 ± 2.10 |
| t = 60 | 101.16 ± 3.33 |
| t = 90 | 97.06 ± 1.78 |
| t = 120 | 98.70 ± 1.43 |
| t = 150 | 101.95 ± 2.92 |
| Bromopride—5 mg/oral film | |
| t = 0 | 100.00 ± 2.82 |
| t = 30 | 95.42 ± 5.99 |
| t = 60 | 97.36 ± 2.01 |
| t = 90 | 97.53 ± 2.20 |
| t = 120 | 96.65 ± 2.03 |
| t = 150 | 97.03 ± 1.87 |
| t = 180 | 109.72 ± 5.10 |
| Coenzyme Q10—20 mg/oral film | |
| t = 0 | 100.00 ± 3.83 |
| t = 7 | 100.79 ± 5.54 |
| t = 14 | 103.95 ± 2.38 |
| t = 30 | 102.31 ± 3.14 |
| t = 60 | 106.15 ± 4.51 |
| t = 90 | 102.61 ± 2.38 |
| t = 120 | 103.17 ± 4.84 |
| t = 150 | 104.37 ± 4.87 |
| t = 180 | 104.81 ± 2.94 |
| Melatonin—3 mg/oral film | |
| t = 0 | 100.00 ± 1.80 |
| t = 30 | 100.50 ± 2.91 |
| t = 60 | 97.96 ± 2.50 |
| t = 90 | 97.21 ± 3.53 |
| t = 120 | 100.56 ± 4.60 |
| t = 150 | 101.30 ± 4.96 |
| t = 180 | 93.56 ± 2.63 |
| Tadalafil—10 mg/oral film | |
| t = 0 | 100.00 ± 2.52 |
| t = 7 | 99.28 ± 4.38 |
| t = 14 | 102.47 ± 2.73 |
| t = 30 | 96.46 ± 1.36 |
| t = 60 | 95.67 ± 2.76 |
| t = 90 | 95.83 ± 1.08 |
| t = 120 | 98.16 ± 0.93 |
| t = 150 | 92.23 ± 4.83 |
| t = 180 | 91.47 ± 2.63 |
| Resveratrol—5 mg/oral film | |
| t = 0 | 100.00 ± 3.16 |
| t = 7 | 100.72 ± 5.90 |
| t = 14 | 101.29 ± 4.21 |
| t = 30 | 100.56 ± 1.01 |
| t = 60 | 99.21 ± 1.53 |
| t = 90 | 96.37 ± 2.26 |
| t = 120 | 96.21 ± 3.31 |
| t = 150 | 98.24 ± 1.79 |
| t = 180 | 99.04 ± 3.67 |
| Vitamin B12—1 mg /oral film | |
| t = 0 | 100.00 ± 4.03 |
| t = 7 | 102.40 ± 2.30 |
| t = 14 | 101.78 ± 1.26 |
| t = 30 | 101.32 ± 3.06 |
| t = 60 | 102.17 ± 1.55 |
| t = 90 | 100.71 ± 1.68 |
| t = 120 | 99.69 ± 2.41 |
| t = 150 | 99.85 ± 2.69 |
| t = 180 | 95.47 ± 1.96 |
| Vitamin D3—2000 UI/oral film | |
| t = 0 | 100.00 ± 4.17 |
| t = 7 | 102.16 ± 4.68 |
| t = 14 | 102.96 ± 4.61 |
| t = 30 | 102.85 ± 4.18 |
| t = 60 | 100.70 ± 4.26 |
| t = 90 | 102.27 ± 3.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polonini, H.C.; Ferreira, A.O.; Raposo, N.R.B.; da Silva, P.J.L.C.; Brandão, M.A.F. Compatibility Assessment of Novel Orodispersible Film Vehicle for Personalized Medicine with Selected Active Pharmaceutical Ingredients. J. Pers. Med. 2023, 13, 1565. https://doi.org/10.3390/jpm13111565
Polonini HC, Ferreira AO, Raposo NRB, da Silva PJLC, Brandão MAF. Compatibility Assessment of Novel Orodispersible Film Vehicle for Personalized Medicine with Selected Active Pharmaceutical Ingredients. Journal of Personalized Medicine. 2023; 13(11):1565. https://doi.org/10.3390/jpm13111565
Chicago/Turabian StylePolonini, Hudson C., Anderson O. Ferreira, Nádia R. B. Raposo, Paulo José L. C. da Silva, and Marcos Antônio F. Brandão. 2023. "Compatibility Assessment of Novel Orodispersible Film Vehicle for Personalized Medicine with Selected Active Pharmaceutical Ingredients" Journal of Personalized Medicine 13, no. 11: 1565. https://doi.org/10.3390/jpm13111565
APA StylePolonini, H. C., Ferreira, A. O., Raposo, N. R. B., da Silva, P. J. L. C., & Brandão, M. A. F. (2023). Compatibility Assessment of Novel Orodispersible Film Vehicle for Personalized Medicine with Selected Active Pharmaceutical Ingredients. Journal of Personalized Medicine, 13(11), 1565. https://doi.org/10.3390/jpm13111565






