Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods
Abstract
:1. Introduction
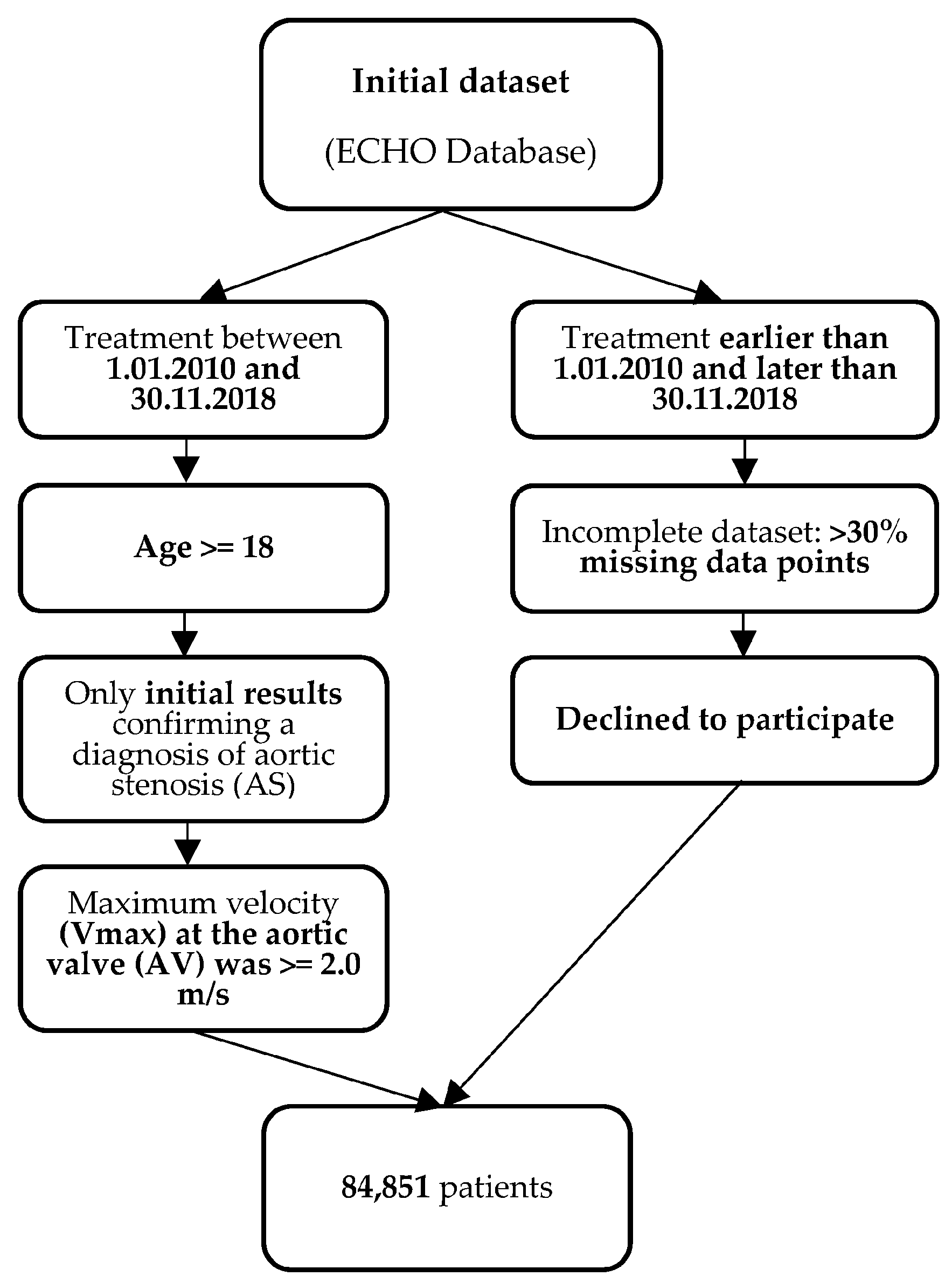
2. Materials and Methods
2.1. Study Cohort
2.1.1. Inclusion Criteria
- Patients from the ECHO database who initiated treatment between 1 January 2010 and 30 November 2018;
- For patients who underwent multiple ECHO examinations during this period, only the initial results confirming a diagnosis of aortic stenosis (AS) were considered for the study. ECHO examinations were primarily conducted in the following clinical scenarios: suspected cardiac etiology based on symptoms, signs, or other tests, as well as evaluation and follow-up of individuals with cardiovascular disease;
- The age of the patients was equal to or greater than 18 years;
- Patients were included in the study if the maximum velocity (Vmax) at the aortic valve (AV) was equal to or greater than 2.0 m/s, based on the definition of AS outlined in the 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease [6].
2.1.2. Exclusion Criteria
- Patients whose treatment started before 1 January 2010 or ended after 30 November 2018;
- Patients who had incomplete datasets;
- Patients who declined to participate in the study.
2.2. Statistical Methods
2.3. Data Preprocessing
2.4. Classification Model Grid Search and Features Importance
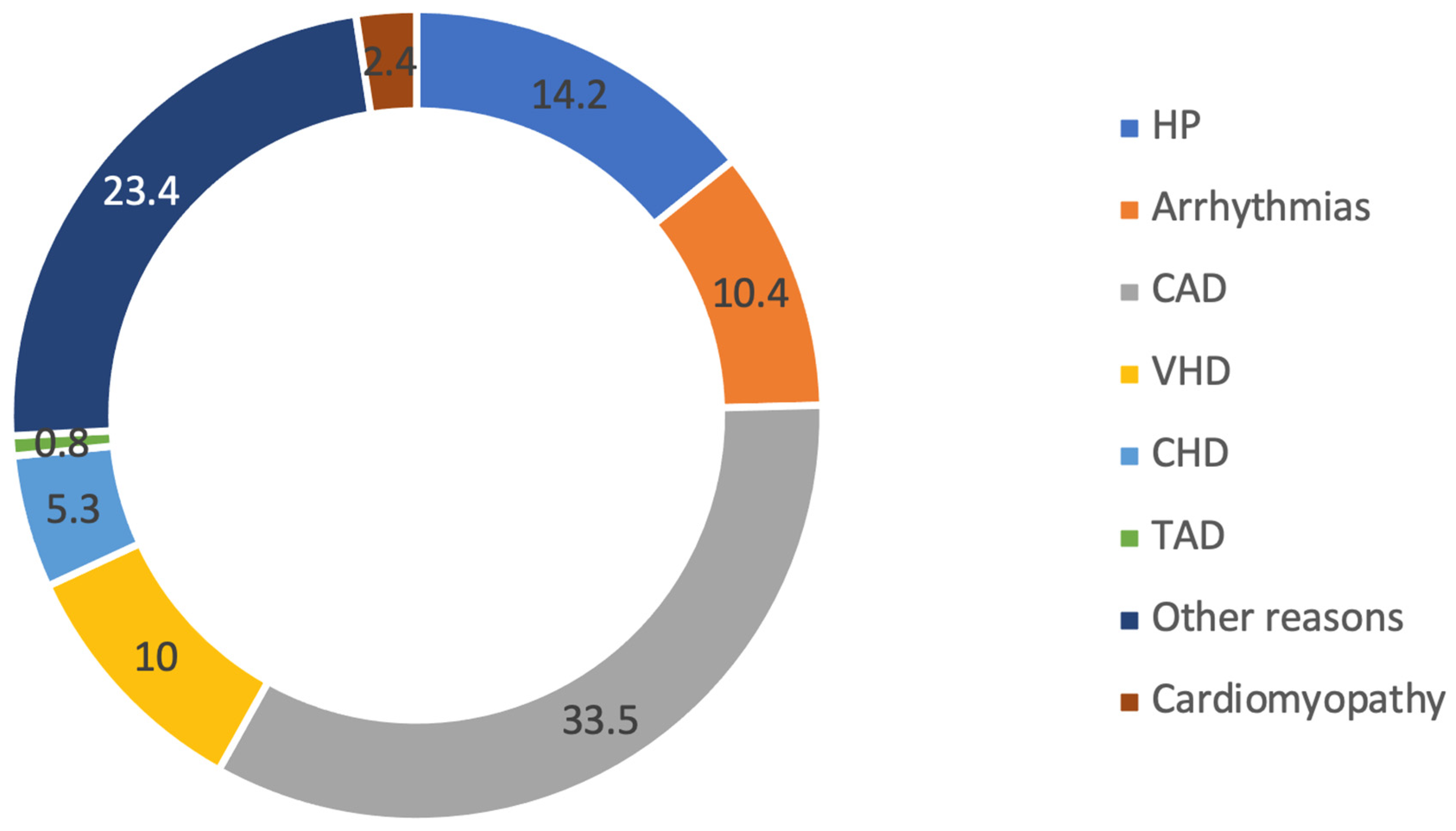
3. Results
4. Discussion
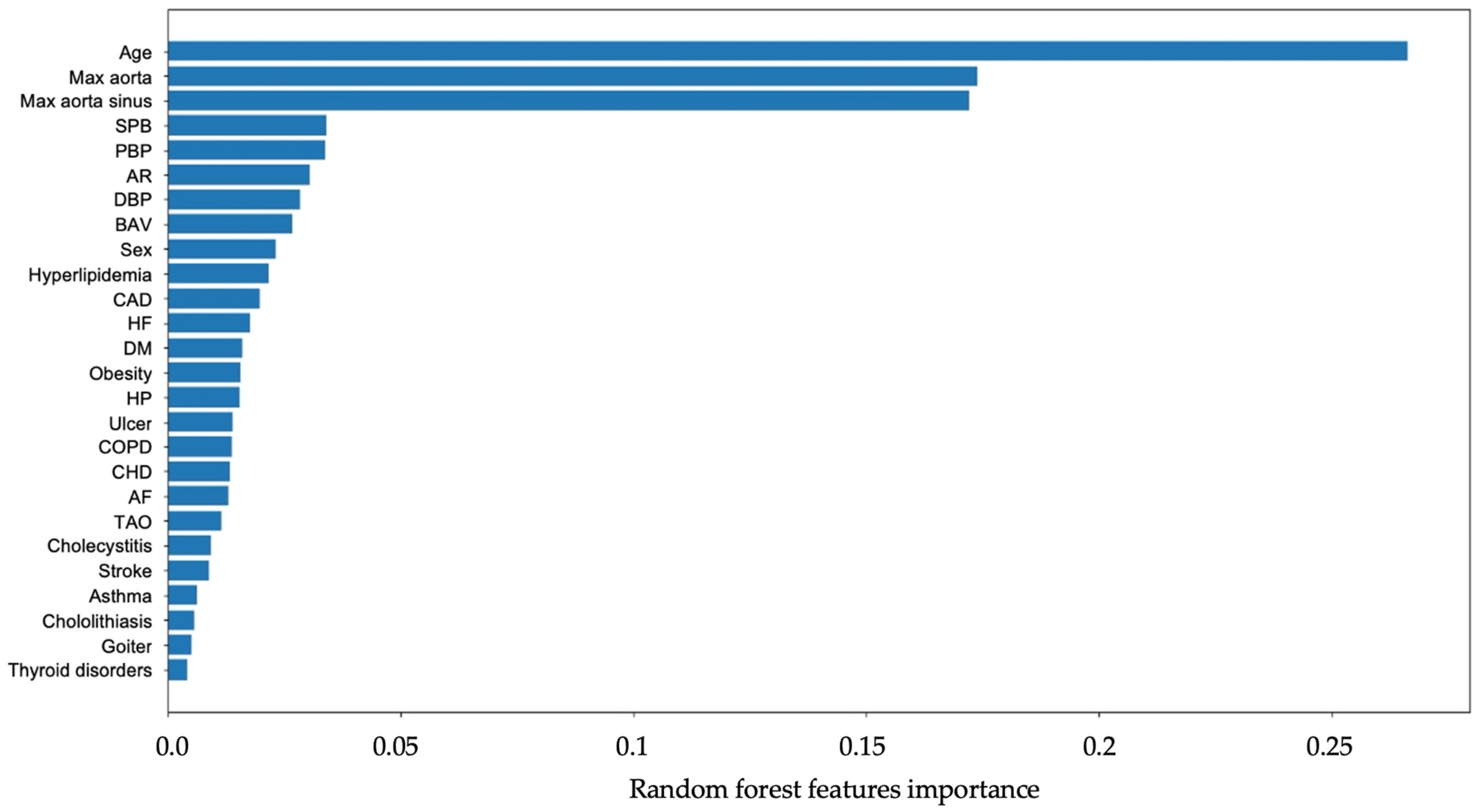
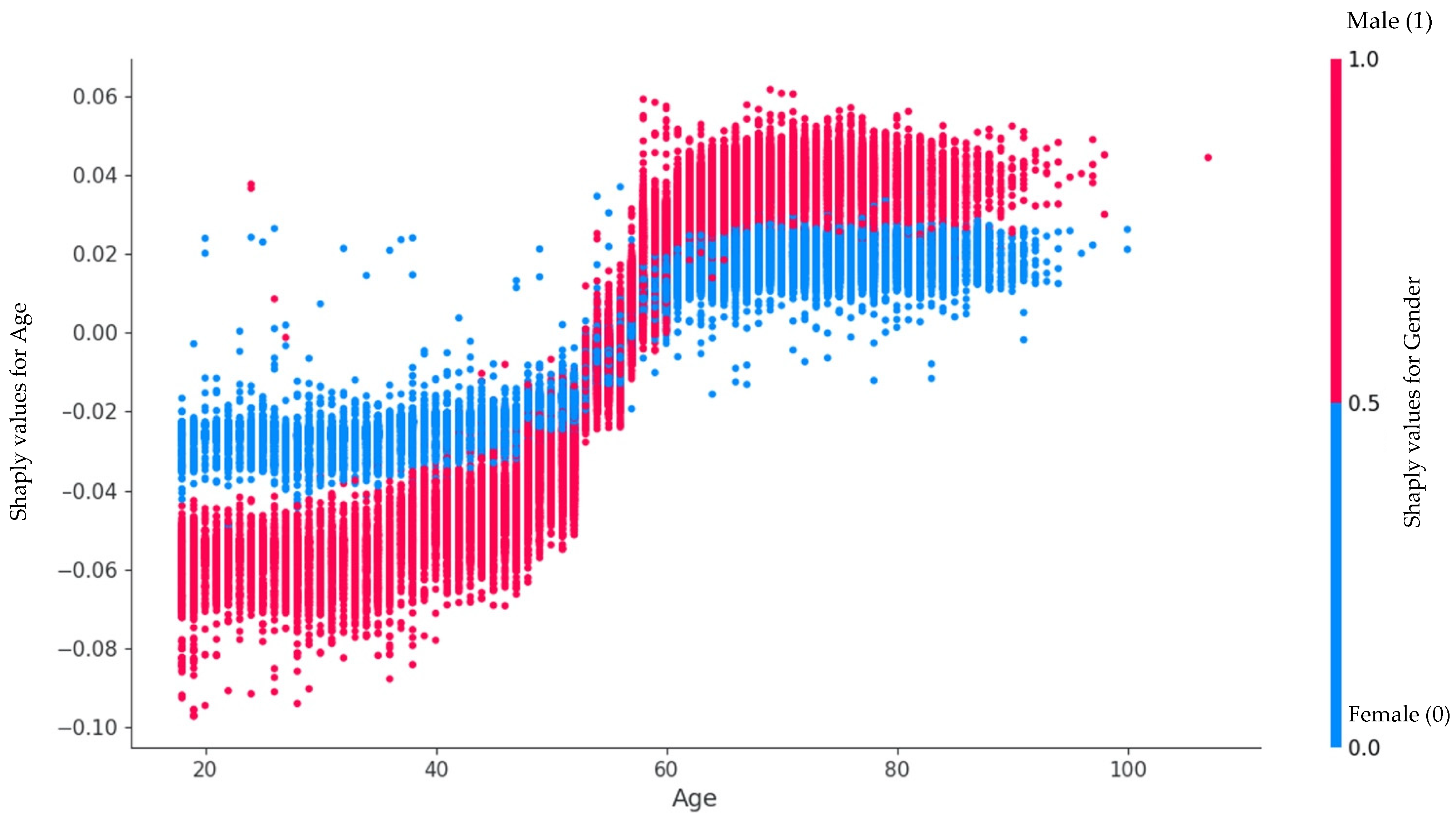
4.1. Features Importance
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irtyuga, O.; Kopanitsa, G.; Kostareva, A.; Metsker, O.; Uspensky, V.; Mikhail, G.; Faggian, G.; Sefieva, G.; Derevitskii, I.; Malashicheva, A.; et al. Application of Machine Learning Methods to Analyze Occurrence and Clinical Features of Ascending Aortic Dilatation in Patients with and without Bicuspid Aortic Valve. J. Pers. Med. 2022, 12, 794. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, Regional, and National Burden of Calcific Aortic Valve and Degenerative Mitral Valve Diseases, 1990–2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef]
- Nightingale, A.K. Aortic sclerosis: Not an innocent murmur but a marker of increased cardiovascular risk. Heart 2005, 91, 1389–1393. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef]
- Della Corte, A.; Bancone, C.; Quarto, C.; Dialetto, G.; Covino, F.E.; Scardone, M.; Caianiello, G.; Cotrufo, M. Predictors of ascending aortic dilatation with bicuspid aortic valve: A wide spectrum of disease expression. Eur. J. Cardiothorac. Surg. 2007, 31, 397–404. [Google Scholar] [CrossRef]
- Agnese, V.; Pasta, S.; Michelena, H.I.; Minà, C.; Romano, G.M.; Carerj, S.; Zito, C.; Maalouf, J.F.; Foley, T.A.; Raffa, G.; et al. Patterns of ascending aortic dilatation and predictors of surgical replacement of the aorta: A comparison of bicuspid and tricuspid aortic valve patients over eight years of follow-up. J. Mol. Cell Cardiol. 2020, 135, 31–39, Erratum in J. Mol. Cell. Cardiol. 2020, 143, 159. [Google Scholar] [CrossRef]
- Côté, N.; Clavel, M.A. Sex Differences in the Pathophysiology, Diagnosis, and Management of Aortic Stenosis. Cardiol. Clin. 2020, 38, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, A.C.; AlBadri, A.; Wei, J.; Nelson, M.D.; Li, D.; Makkar, R.; Merz, C.N.B. Sex differences in aortic stenosis: Identification of knowledge gaps for sex-specific personalized medicine. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 21, 100197. [Google Scholar] [CrossRef] [PubMed]
- Tribouilloy, C.; Bohbot, Y.; Rusinaru, D.; Belkhir, K.; Diouf, M.; Altes, A.; Delpierre, Q.; Serbout, S.; Kubala, M.; Levy, F.; et al. Excess Mortality and Undertreatment of Women With Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e018816. [Google Scholar] [CrossRef] [PubMed]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Aggarwal, S.R.; Swett, K.R.; Kirtane, A.J.; Kodali, S.K.; Nazif, T.M.; Pu, M.; Dadhania, R.; Kaplan, R.C.; Rodriguez, C.J. Burden of Valvular Heart Diseases in Hispanic/Latino Individuals in the United States: The Echocardiographic Study of Latinos. Mayo Clin. Proc. 2019, 94, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Kontogeorgos, S.; Thunström, E.; Basic, C.; Hansson, P.O.; Zhong, Y.; Ergatoudes, C.; Morales, D.; Mandalenakis, Z.; Rosengren, A.; Caidahl, K.; et al. Prevalence and risk factors of aortic stenosis and aortic sclerosis: A 21-year follow-up of middle-aged men. Scand. Cardiovasc. J. 2020, 54, 115–123. [Google Scholar] [CrossRef]
- Rosenhek, R. Mild and moderate aortic stenosis Natural history and risk stratification by echocardiography. Eur. Heart J. 2004, 25, 199–205. [Google Scholar] [CrossRef]
- Kopanitsa, G. Integration of Hospital Information and Clinical Decision Support Systems to Enable the Reuse of Electronic Health Record Data. Methods Inf. Med. 2017, 56, 238–247. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Prokhorenkova, L.; Gusev, G.; Vorobev, A.; Dorogush, A.V.; Gulin, A. CatBoost: Unbiased boosting with categorical features. In Advances in Neural Information Processing Systems, Proceedings of the 32nd International Conference on Neural Information Processing Systems, Montréal, QC, Canada, 3–8 December 2017; Curran Associates, Inc.: Red Hook, NY, USA, 2018; pp. 6639–6640. [Google Scholar]
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.I. A unified approach to interpreting model predictions. In Advances in Neural Information Processing Systems, Proceedings of the 31st Annual Conference on Neural Information Processing Systems (NIPS 2017), Long Beach, CA, USA, 4–9 December 2017; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 4768–4777. [Google Scholar]
- Stewart, S.; Chan, Y.-K.; Playford, D.; Strange, G.A. Incident aortic stenosis in 49 449 men and 42 229 women investigated with routine echocardiography. Heart 2022, 108, 875–881. [Google Scholar] [CrossRef]
- Büttner, P.; Feistner, L.; Lurz, P.; Thiele, H.; Hutcheson, J.D.; Schlotter, F. Dissecting Calcific Aortic Valve Disease—The Role, Etiology, and Drivers of Valvular Fibrosis. Front. Cardiovasc. Med. 2021, 8, 660797. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.K.; Regeer, M.V.; Ng, A.C.; McCormack, L.; Poh, K.K.; Yeo, T.C.; Shanks, M.; Parent, S.; Enache, R.; Popescu, B.A.; et al. Sex Differences in Phenotypes of Bicuspid Aortic Valve and Aortopathy: Insights From a Large Multicenter, International Registry. Circ. Cardiovasc. Imaging 2017, 10, e005155. [Google Scholar] [CrossRef]
- Palacios-Fernandez, S.; Salcedo, M.; Belinchon-Romero, I.; Gonzalez-Alcaide, G.; Ramos-Rincón, J.M. Epidemiological and Clinical Features in Very Old Men and Women (≥80 Years) Hospitalized with Aortic Stenosis in Spain, 2016–2019: Results from the Spanish Hospital Discharge Database. J. Clin. Med. 2022, 11, 5588. [Google Scholar] [CrossRef]
- Toyofuku, M.; Taniguchi, T.; Morimoto, T.; Yamaji, K.; Furukawa, Y.; Takahashi, K.; Tamura, T.; Shiomi, H.; Ando, K.; Kanamori, N.; et al. Sex Differences in Severe Aortic Stenosis-Clinical Presentation and Mortality. Circ. J. 2017, 81, 1213–1221. [Google Scholar] [CrossRef]
- Evangelista, A. Aortic Stenosis in Bicuspid and Tricuspid Valves: A Different Spectrum of the Disease With Clinical Implications. JACC Cardiovasc. Imaging 2021, 14, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Shen, M.; Tastet, L.; Capoulade, R.; Arsenault, M.; Bedard, E.; Clavel, M.A.; Pibarot, P. Effect of bicuspid aortic valve phenotype on progression of aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Svensson, L.G.; Griffin, B.P.; Desai, M.Y. Contemporary natural history of bicuspid aortic valve disease: A systematic review. Heart 2017, 103, 1323–1330. [Google Scholar] [CrossRef]
- Butcher, S.C.; Fortuni, F.; Kong, W.; Vollema, E.M.; Prevedello, F.; Perry, R.; Ng, A.C.T.; Poh, K.K.; Almeida, A.G.; González-Gómez, A.; et al. Prognostic implications of left atrial dilation in aortic regurgitation due to bicuspid aortic valve. Heart 2022, 108, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, T.; Gillette, B.; Pakhomov, A.; Kahoun, J.; Mayer, H.; Burghaus, R.; Lippert, J.; Saxena, M. Multiscale classification of heart failure phenotypes by unsupervised clustering of unstructured electronic medical record data. Sci. Rep. 2020, 10, 21340. [Google Scholar] [CrossRef]
- Harris, A.W.; Pibarot, P.; Otto, C.M. Aortic Stenosis. Cardiol. Clin. 2020, 38, 55–63. [Google Scholar] [CrossRef] [PubMed]


| Features | BAV, n = 983 Median; Quartiles | TAV, n = 39,703 Median; Quartiles | ||||
|---|---|---|---|---|---|---|
| With AS, n = 536 | Without AS, n = 447 | p | With AS, n = 4423 | Without AS, n = 35,280 | p | |
| Age, years (median and bounds) | 50 (34; 60) | 29 (21; 46) | <0.0001 | 66 (57; 74) | 57 (46; 65) | <0.0001 |
| Aortic diameter at the sinus of the Valsalva, mm | 37 (34; 41) | 37 (33; 41) | <0.05 | 36 (34; 39) | 36 (33; 39) | <0.0001 |
| Aortic diameter at the proximal ascending aorta, mm | 39 (35; 44) | 24.8 (21.8; 27.8) | <0.0001 | 37 (34; 40) | 34 (31; 37) | <0.0001 |
| BMI, kg/m2 | 26.3 (23.9; 30) | 24.8 (21.8; 27.8) | <0.0001 | 27.3 (24.5; 30.3) | 27.4 (24.5; 30.6) | 0.48 |
| AS dpmax, mmHg | 29 (20; 50) | 10 (7; 12) | <0.0001 | 30 (20; 53) | 6 (5; 8) | <0.0001 |
| EF LV (%) | 63.8 (57.4; 69) | 64.2 (59.5; 69.7) | 0.04 | 62.4 (53.4; 68) | 60.9 (51.5; 67) | <0.0001 |
| SBP office, mmHg | 135 (124; 142) | 130 (120; 140) | 0.12 | 140 (129; 150) | 130 (120; 140) | <0.0001 |
| DBP office, mmHg | 80 (75; 85) | 80 (80; 85) | 0.86 | 80 (80; 88) | 80 (80; 87) | 0.46 |
| AR, n (%) | 123 (22.95) | 118 (26.46) | 0.20 | 778 (17.59) | 1286 (3.65) | <0.0001 |
| Hypertension, n (%) | 316 (58.95) | 268 (59.96) | 0. 5 | 2803 (63.37) | 25,532 (72.37) | <0.001 |
| Diabetes mellitus, n (%) | 31 (5.78) | 17 (3.80) | 0.15 | 451 (10.20) | 3204 (9.08) | 0. 2 |
| CAD, n (%) | 127 (23.69) | 46 (10.29) | <0.001 | 1818 (41.10) | 14,222 (40.31) | 0.31 |
| COPD, n (%) | 58 (10.82) | 23 (5.15) | 0.001 | 460 (10.40) | 3687 (10.45) | 0.92 |
| Asthma, n (%) | 22 (4.10) | 9 (2.01) | 0.06 | 88 (1.99) | 732(2.07) | 0.71 |
| Obesity, (BMI > 30), n (%) | 61 (11.3) | 19 (4.25) | <0.0001 | 394 (8.9) | 3005 (8.52) | 0.15 |
| Hyperlipidemia, n (%) | 135 (25.19) | 56 (12.53) | <0.0001 | 1170 (26.45) | 9061 (25.68) | 0.27 |
| Heart failure, n (%) | 320 (59.70) | 156 (34.90) | <0.0001 | 2332 (52.72) | 14,770 (41.87) | <0.001 |
| Variables | BAV, n = 541 Median; Quartiles | TAV, n = 43,613 Median; Quartiles | ||||
|---|---|---|---|---|---|---|
| With AS, n = 365 | Without AS, n = 185 | p | With AS, n = 5928 | Without AS, n = 40,925 | p | |
| Age, years (median and bounds) | 49 (31; 61) | 31 (26; 49); | <0.0001 | 71 (62; 77) | 58 (41; 68); | <0.0001 |
| Aortic diameter at the sinus of the Valsalva, mm | 32 (30; 35) | 32 (29; 36) | 0.89 | 32 (30; 35) | 32 (30; 34) | <0.0001 |
| Aortic diameter at the proximal ascending aorta, mm | 36 (32; 40) | 33 (29; 39) | <0.0001 | 34 (31; 37) | 31 (28; 34) | <0.0001 |
| BMI, kg/m2 | 25.6 (22.6; 29.9) | 24.6 (21.7; 26.9) | 0.01 | 28.8 (25.2; 32.6) | 27.1 (23.5; 31.2) | <0.0001 |
| AS dpmax, mmHg | 32 (22; 56) | 10 (8; 13) | <0.0001 | 31 (20; 60) | 7 (5; 9) | <0.0001 |
| EF LV (%) | 66.9 (61.8; 71.4) | 66 (61; 70) | 0.15 | 65.9 (60.7; 70) | 65.7 (60.6; 70) | 0.34 |
| SBP office, mmHg | 120 (120; 140) | 120 (110; 127.5) | 0.13 | 140 (130; 150) | 130 (120; 140) | <0.0001 |
| DBP office, mmHg | 80 (70; 80) | 80 (70; 80) | 0.61 | 80 (80; 90) | 80 (75; 85) | <0.0001 |
| AR, n (%) | 64 (17.53) | 27 (14.59) | 0.38 | 814 (13.74) | 1284 (3.14) | <0.0001 |
| Hypertension, n (%) | 177 (48.5) | 99 (53.5) | 0. 1 | 3589 (60.5) | 26,923 (65.78) | 0.3 |
| Diabetes mellitus, n (%) | 20 (5.48) | 9 (4.86) | 0.76 | 824 (13.9) | 3870 (9.45) | 0.1 |
| CAD, n (%) | 58 (15.89) | 18 (9.73) | 0.05 | 2183 (36.83) | 9968 (26.45) | <0.0001 |
| COPD, n (%) | 15 (4.11) | 5 (2.70) | 0.40 | 426 (7.19) | 2144 (5.69) | 0.5 |
| Asthma, n (%) | 10 (2.74) | 5 (2.70) | 0.98 | 208 (3.51) | 1133 (3.01) | 0.8 |
| Obesity, (BMI > 30), n (%) | 47 (12.8) | 5 (2.70) | 0.0002 | 862 (14.54) | 4026 (9.83) | <0.0001 |
| Hyperlipidemia, n (%) | 94 (25.75) | 19 (10.27) | <0.0001 | 1661 (28.02) | 8888 (23.59) | <0.0001 |
| Heart failure, n (%) | 213 (58.36) | 60 (32.43) | <0.0001 | 3221 (54.34) | 14,120 (37.47) | <0.0001 |
| Precision | Recall | F1 Score | Accuracy | AUC | |
|---|---|---|---|---|---|
| ANN | 0.83 | 0.72 | 0.77 | 0.81 | 0.78 |
| SVM | 0.77 | 0.78 | 0.78 | 0.80 | 0.79 |
| Decision Tree | 0.79 | 0.81 | 0.78 | 0.82 | 0.79 |
| Random Forest | 0.79 | 0.81 | 0.80 | 0.83 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irtyuga, O.; Babakekhyan, M.; Kostareva, A.; Uspensky, V.; Gordeev, M.; Faggian, G.; Malashicheva, A.; Metsker, O.; Shlyakhto, E.; Kopanitsa, G. Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods. J. Pers. Med. 2023, 13, 1588. https://doi.org/10.3390/jpm13111588
Irtyuga O, Babakekhyan M, Kostareva A, Uspensky V, Gordeev M, Faggian G, Malashicheva A, Metsker O, Shlyakhto E, Kopanitsa G. Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods. Journal of Personalized Medicine. 2023; 13(11):1588. https://doi.org/10.3390/jpm13111588
Chicago/Turabian StyleIrtyuga, Olga, Mary Babakekhyan, Anna Kostareva, Vladimir Uspensky, Michail Gordeev, Giuseppe Faggian, Anna Malashicheva, Oleg Metsker, Evgeny Shlyakhto, and Georgy Kopanitsa. 2023. "Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods" Journal of Personalized Medicine 13, no. 11: 1588. https://doi.org/10.3390/jpm13111588
APA StyleIrtyuga, O., Babakekhyan, M., Kostareva, A., Uspensky, V., Gordeev, M., Faggian, G., Malashicheva, A., Metsker, O., Shlyakhto, E., & Kopanitsa, G. (2023). Analysis of Prevalence and Clinical Features of Aortic Stenosis in Patients with and without Bicuspid Aortic Valve Using Machine Learning Methods. Journal of Personalized Medicine, 13(11), 1588. https://doi.org/10.3390/jpm13111588






