Novel Echocardiographic Measurements of Right Ventricular–Pulmonary Artery Coupling in Predicting the Prognosis of Precapillary Pulmonary Hypertension
Abstract
1. Introduction
2. Methods Section
2.1. Echocardiography
2.2. Assessment of Disease Progression
3. Statistical Analysis
4. Results
4.1. General Results
4.2. Echocardiography
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. ESC/ERS Scientific Document Group. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm. Circ. 2021, 11, 204589402097730. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Jansa, P.; Kim, N.H.; Naeije, R.; Simonneau, G. Chronic thromboembolic pulmonary hypertension: Role of medical therapy. Eur. Respir. J. 2013, 41, 985–990. [Google Scholar] [CrossRef]
- Bousseau, S.; Sobrano Fais, R.; Gu, S.; Frump, A.; Lahm, T. Pathophysiology and new advances in pulmonary hypertension. BMJ Med. 2023, 23, e000137. [Google Scholar] [CrossRef]
- Gall, H.; Felix, J.F.; Schneck, F.K.; Milger, K.; Sommer, N.; Voswinckel, R.; Franco, O.H.; Hofman, A.; Schermuly, R.T.; Weissmann, N.; et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J. Heart Lung Transpl. 2017, 36, 957–967. [Google Scholar] [CrossRef]
- Lang, I.M.; Pesavento, R.; Bonderman, D.; Yuan, J.X. Risk factors and basic mechanisms of chronic thromboembolic pulmonary hypertension: A current understanding. Eur. Respir. J. 2013, 41, 462–468. [Google Scholar] [CrossRef]
- Simons, J.E.; Mann, E.B.; Pierozynski, A. Assessment of Risk of Disease Progression in Pulmonary Arterial Hypertension: Insights from an International Survey of Clinical Practice. Adv. Ther. 2019, 36, 2351–2363. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Owens, B.; Hayes, R.; Wen, S. A Systematic Review of Echocardiographic Parameters for the Screening of Pulmonary Hypertension: What Are the Odds? Cureus 2022, 14, e32185. [Google Scholar] [CrossRef]
- Topyła-Putowska, W.; Tomaszewski, M.; Wysokiński, A.; Tomaszewski, A. Echocardiography in Pulmonary Arterial Hypertension: Comprehensive Evaluation and Technical Considerations. J. Clin. Med. 2021, 10, 3229. [Google Scholar] [CrossRef]
- He, Q.; Lin, Y.; Zhu, Y.; Gao, L.; Ji, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Usefulness of Right Ventricle-Pulmonary Artery Coupling in Cardiovascular Disease. J. Clin. Med. 2023, 12, 2526. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): Endorsed by: Association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- D’Alto, M.; Bossone, E.; Opotowsky, A.R.; Ghio, S.; Rudski, L.G.; Naeije, R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. Int. J. Cardiol. 2018, 263, 177–183. [Google Scholar] [CrossRef] [PubMed]
- More, K.; Soni, R.; Gupta, S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin. Fetal Neonatal Med. 2022, 27, 101366. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Klersy, C.; Magrini, G.; D’Armini, A.M.; Scelsi, L.; Raineri, C.; Pasotti, M.; Serio, A.; Campana, C.; Viganò, M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2010, 140, 272–278. [Google Scholar] [CrossRef]
- Ghio, S.; Pica, S.; Klersy, C.; Guzzafame, E.; Scelsi, L.; Raineri, C.; Turco, A.; Schirinzi, S.; Visconti, L.O. Prognostic value of TAPSE after therapy optimisation in patients with pulmonary arterial hypertension is independent of the haemodynamic effects of therapy. Open Heart 2016, 3, e000408. [Google Scholar] [CrossRef]
- Forfia, P.R.; Fisher, M.R.; Mathai, S.C.; Housten-Harris, T.; Hemnes, A.R.; Borlaug, B.A.; Chamera, E.; Corretti, M.C.; Champi-on, H.C.; Abraham, T.P.; et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2006, 174, 1034–4101. [Google Scholar] [CrossRef]
- Schmid, E.; Hilberath, J.N.; Blumenstock, G.; Shekar, P.S.; Kling, S.; Shernan, S.K.; Rosenberger, P.; Nowak-Machen, M. Tricuspid annular plane systolic excursion (TAPSE) predicts poor outcome in patients undergoing acute pulmonary embolectomy. Heart Lung Vessel. 2015, 7, 151–158. [Google Scholar]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography-A comprehensive review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Kleczynski, P.; Dziewierz, A.; Wiktorowicz, A.; Bagienski, M.; Rzeszutko, L.; Sorysz, D.; Trebacz, J.; Sobczynski, R.; Tomala, M.; Dudek, D. Prognostic value of tricuspid regurgitation velocity and probability of pulmonary hypertension in patients undergoing transcatheter aortic valve implantation. Int. J. Cardiovasc. Imaging 2017, 33, 1931–1938. [Google Scholar] [CrossRef]
- Yogeswaran, A.; Richter, M.J.; Sommer, N.; Ghofrani, H.A.; Seeger, W.; Tello, K.; Werner, S.; Khodr, T.; Henning, G. Advanced risk stratification of intermediate risk group in pulmonary arterial hypertension. Pulm. Circ. 2020, 10, 2045894020961739. [Google Scholar] [CrossRef] [PubMed]
- Tsarova, K.; Morgan, A.E.; Melendres-Groves, L.; Ibrahim, M.M.; Ma, C.L.; Pan, I.Z.; Hatton, N.D.; Beck, E.M.; Ferrel, M.N.; Selzman, C.H.; et al. Imaging in Pulmonary Vascular Disease-Understanding Right Ventricle-Pulmonary Artery Coupling. Compr. Physiol. 2022, 12, 3705–3730. [Google Scholar] [CrossRef] [PubMed]
- Boulate, D.; Amsallem, M.; Kuznetsova, T.; Zamanian, R.T.; Fadel, E.; Mercier, O.; Haddad, F. Echocardiographic evaluations of right ventriculo-arterial coupling in experimental and clinical pulmonary hypertension. Physiol. Rep. 2019, 7, e14322. [Google Scholar] [CrossRef] [PubMed]
- Aubert, R.; Venner, C.; Huttin, O.; Haine, D.; Filippetti, L.; Guillaumot, A.; Mandry, D.; Marie, P.Y.; Juilliere, Y.; Chabot, F.; et al. Three-Dimensional Echocardiography for the Assessment of Right Ventriculo-Arterial Coupling. J. Am. Soc. Echocardiogr. 2018, 31, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Çolak, A.; Kumral, Z.; Kış, M.; Şentürk, B.; Sezgin, D.; Ömeroğlu Şimşek, G.; Sevinç, C.; Akdeniz, B. The Usefulness of the TAPSE/sPAP Ratio for Predicting Survival in Medically Treated Chronic Thromboembolic Pulmonary Hypertension. Turk. Kardiyol. Dern. Ars. 2023, 51, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, A.; Rauwolf, T.; Groscheck, T.; Kropf, S.; Luani, B.; Tanev, I.; Hansen, M.; Meißler, S.; Steendijk, P.; Braun-Dullaeus, R.C. Pressure-volume loop validation of TAPSE/PASP for right ventricular arterial coupling in heart failure with pulmonary hypertension. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 168–176. [Google Scholar] [CrossRef]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U.; et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef]
- Topyła-Putowska, W.; Tomaszewski, M.; Wojtkowska, A.; Styczeń, A.; Wysokiński, A. Tricuspid Regurgitation Velocity/Tricuspid Annular Plane Systolic Excursion (TRV/TAPSE) Ratio as a Novel Indicator of Disease Severity and Prognosis in Patients with Precapillary Pulmonary Hypertension. Diseases 2023, 11, 117. [Google Scholar] [CrossRef]
- Pestelli, G.; Fiorencis, A.; Trevisan, F.; Luisi, G.A.; Smarrazzo, V.; Mele, D. New measures of right ventricle-pulmonary artery coupling in heart failure: An all-cause mortality echocardiographic study. Int. J. Cardiol. 2021, 329, 234–241. [Google Scholar] [CrossRef]
- Dzikowska-Diduch, O.; Kurnicka, K.; Lichodziejewska, B.; Zdończyk, O.; Dąbrowska, D.; Roik, M.; Pacho, S.; Bielecki, M.; Pruszczyk, P. A Novel Doppler TRPG/AcT Index Improves Echocardiographic Diagnosis of Pulmonary Hypertension after Pulmonary Embolism. J. Clin. Med. 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Boucly, A.; Weatherald, J.; Savale, L.; Jaïs, X.; Cottin, V.; Prevot, G.; Picard, F.; de Groote, P.; Jevnikar, M.; Bergot, E.; et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur. Respir. J. 2017, 50, 1700889. [Google Scholar] [CrossRef] [PubMed]
- Farber, H.W.; Miller, D.P.; Poms, A.D.; Badesch, D.B.; Frost, A.E.; Muros-Le Rouzic, E.; Romero, A.J.; Benton, W.W.; Elliott, C.G.; McGoon, M.D.; et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest 2015, 148, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.; Channick, R.N.; Delcroix, M.; Galiè, N.; Ghofrani, H.A.; Jansa, P.; Le Brun, F.O.; Mehta, S.; Perchenet, L.; Pulido, T.; et al. Association between six-minute walk distance and long-term outcomes in patients with pulmonary arterial hypertension: Data from the randomized SERAPHIN trial. PLoS ONE 2018, 13, e0193226. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Grimminger, F.; Grünig, E.; Huang, Y.; Jansa, P.; Jing, Z.C.; Kilpatrick, D.; Langleben, D.; Rosenkranz, S.; Menezes, F.; et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: Data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir. Med. 2016, 4, 361–371. [Google Scholar] [CrossRef]
- Jutras-Beaudoin, N.; Toro, V.; Lajoie, A.C.; Breuils-Bonnet, S.; Paulin, R.; Potus, F. Neutrophil-Lymphocyte Ratio as an Independent Predictor of Survival in Pulmonary Arterial Hypertension: An Exploratory Study. CJC Open 2021, 4, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Özpelit, E.; Akdeniz, B.; Özpelit, M.E.; Tas, S.; Bozkurt, S.; Tertemiz, K.C.; Sevinç, C.; Badak, Ö. Prognostic value of neutrophil-to-lymphocyte ratio in pulmonary arterial hypertension. J. Int. Med. Res. 2015, 43, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Minatsuki, S.; Hatano, M.; Saito, A.; Yagi, H.; Shimbo, M.; Soma, K.; Fujiwara, T.; Itoh, H.; Konoeda, C.; et al. The ratio of TAPSE to PASP predicts prognosis in lung transplant candidates with pulmonary arterial hypertension. Sci. Rep. 2023, 13, 3758. [Google Scholar] [CrossRef]
- Fauvel, C.; Raitiere, O.; Boucly, A.; De Groote, P.; Renard, S.; Bertona, J.; Lamblin, N.; Artaud-Macari, E.; Viacroze, C.; Schleifer, D.; et al. Interest of TAPSE/sPAP ratio for noninvasive pulmonary arterial hypertension risk assessment. J. Heart Lung Transpl. 2022, 41, 1761–1772. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ohtani, T.; Nakatani, S.; Hayashi, K.; Yamaguchi, O.; Komuro, I.; Sakata, Y. Impact of Body Size on Inferior Vena Cava Parameters for Estimating Right Atrial Pressure: A Need for Standardization? J. Am. Soc. Echocardiogr. 2015, 28, 1420–1427. [Google Scholar] [CrossRef]
- Rich, J.D.; Shah, S.J.; Swamy, R.S.; Kamp, A.; Rich, S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: Implications for clinical practice. Chest 2011, 139, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.R.; Forfia, P.R.; Chamera, E.; Housten-Harris, T.; Champion, H.C.; Girgis, R.E.; Corretti, M.C.; Hassoun, P.M. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Mallery, J.A.; Gardin, J.M.; King, S.W.; Ey, S.; Henry, W.L. Effects of heart rate and pulmonary artery pressure on Doppler pulmonary artery acceleration time in experimental acute pulmonary hypertension. Chest 1991, 100, 470–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsuda, M.; Sekiguchi, T.; Sugishita, Y.; Kuwako, K.; Lida, K.; Ito, I. Reliability of non-invasive estimates of pulmonary hypertension by pulsed Doppler echocardiography. Br. Heart J. 1986, 56, 158–164. [Google Scholar] [CrossRef]
- Kazimierczyk, R.; Kazimierczyk, E.; Knapp, M.; Sobkowicz, B.; Malek, L.A.; Blaszczak, P.; Ptaszynska-Kopczynska, K.; Grzywna, R.; Kaminski, K.A. Echocardiographic Assessment of Right Ventricular–Arterial Coupling in Predicting Prognosis of Pulmonary Arterial Hypertension Patients. J. Clin. Med. 2021, 10, 2995. [Google Scholar] [CrossRef]
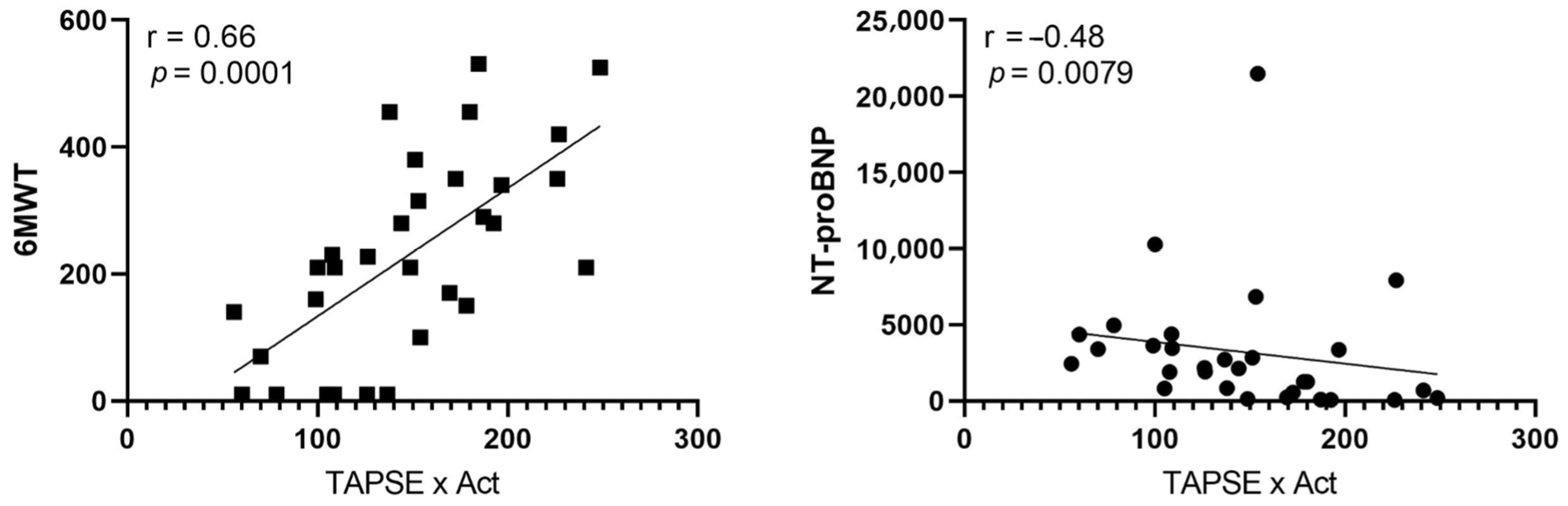

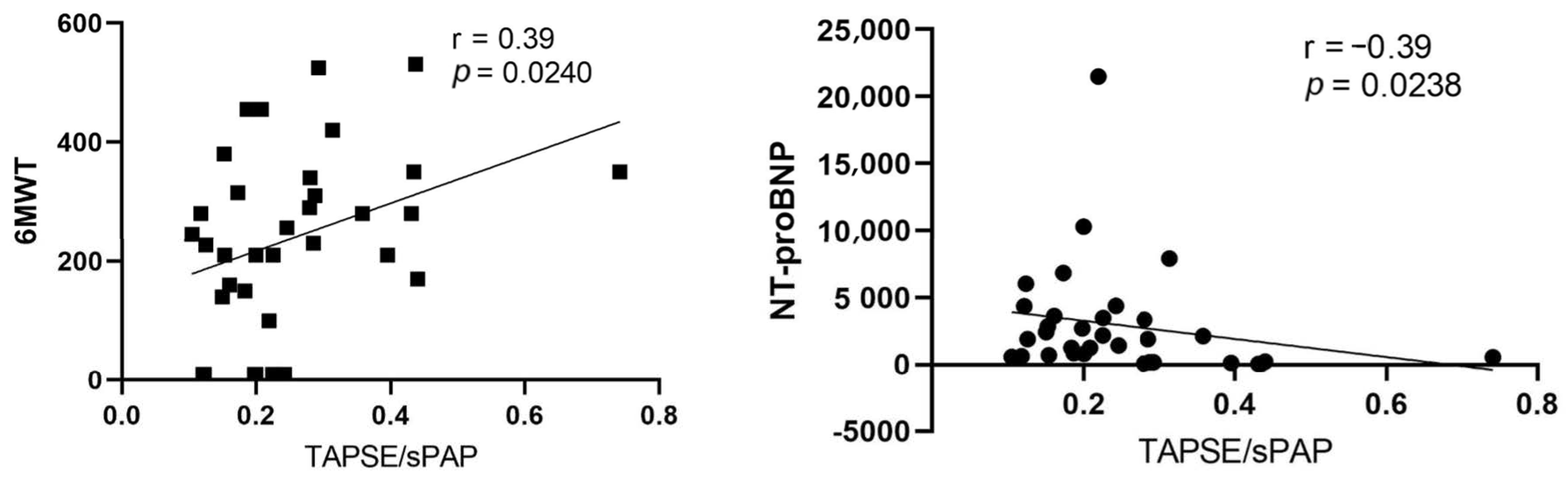
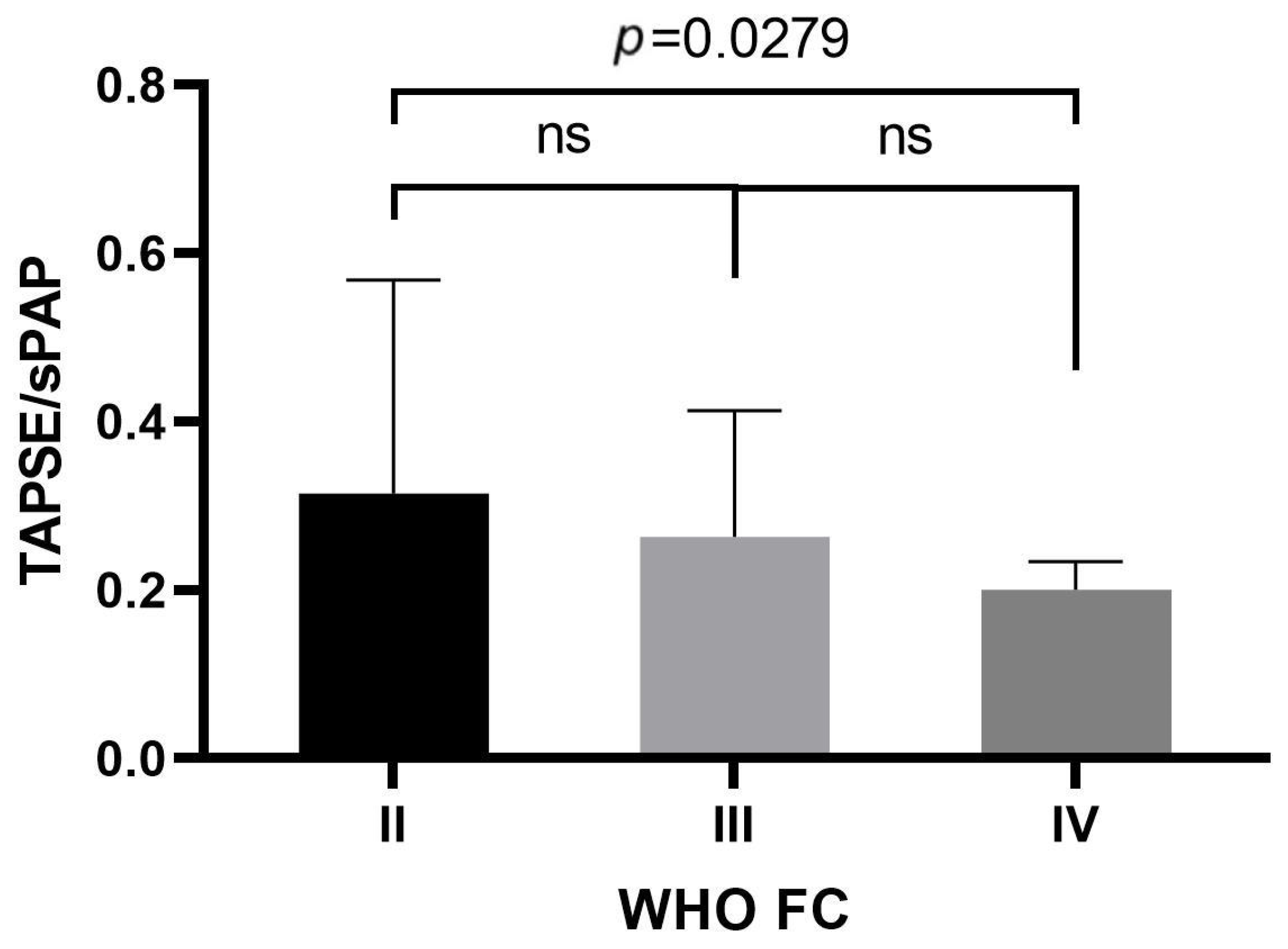
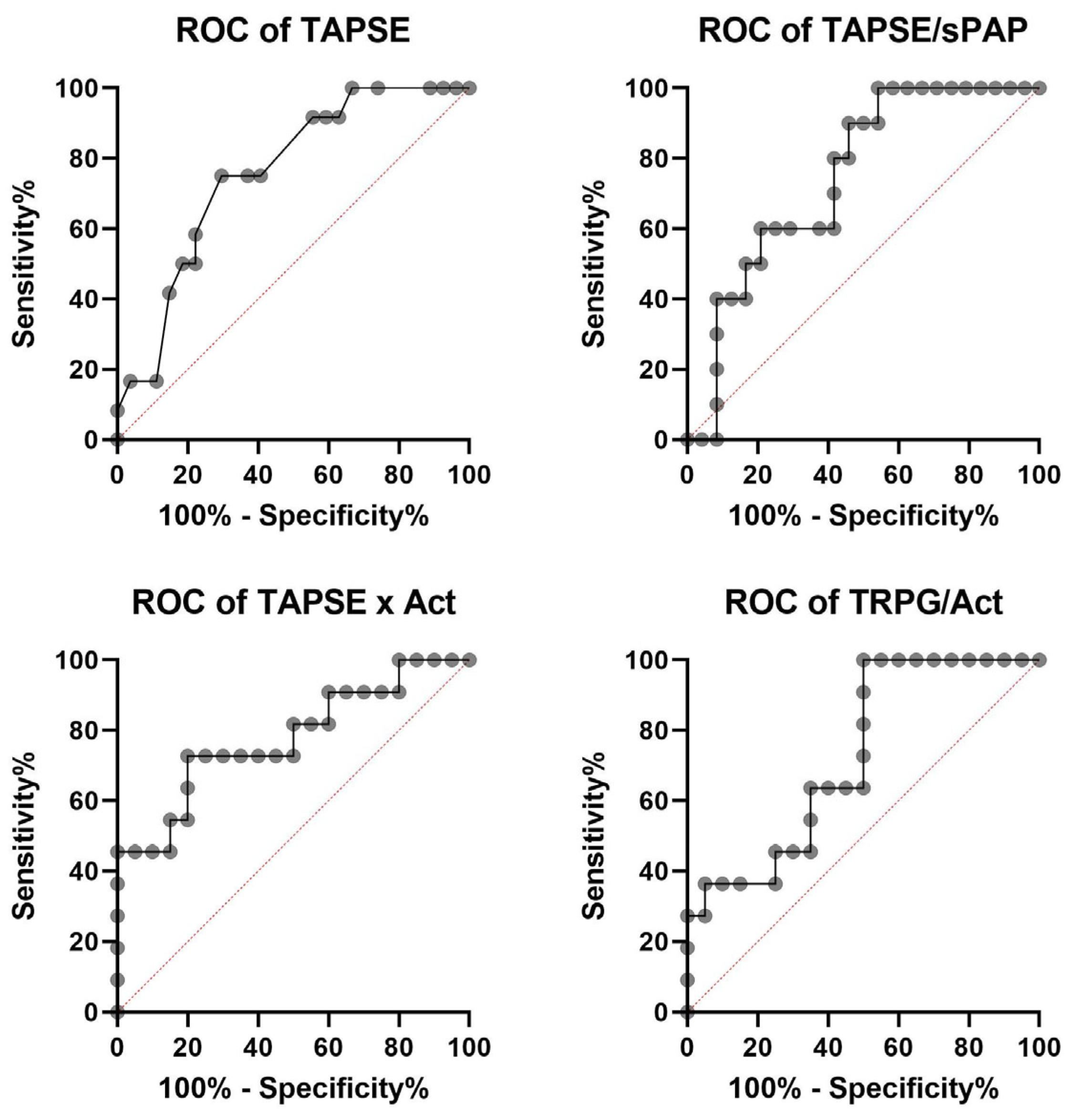

| General Characteristic | |
|---|---|
| Age, years | 63.1 ± 15.9 |
| Female gender, % (n) | 74% (29) |
| BMI, kg/m2 | 23.5 ± 3.1 |
| PH etiology | |
| IPAH, % (n) | 30.8% (12) |
| CTD-PAH, % (n) | 33.3% (13) |
| CHD-PAH, % (n) | 17.9% (7) |
| PoPH, % (n) | 2.6% (1) |
| CTEPH, % (n) | 15.4% (6) |
| Comorbidities | |
| Hypertension, % (n) | 66.7% (26) |
| Diabetes, % (n) | 41.0% (16) |
| Obesity, % (n) | 23.1% (9) |
| Hyperlipidemia, % (n) | 56.4% (22) |
| Chronic kidney disease, % (n) | 17.9% (7) |
| Heart failure, % (n) | 41.0% (16) |
| Atrial fibrillation, % (n) | 43.6% (17) |
| Ischemic heart disease, % (n) | 28.2% (11) |
| Chronic obstructive pulmonary disease, % (n) | 12.8% (5) |
| PH treatment | |
| Endothelin receptor antagonist, % (n) | 61.5% (24) |
| Phosphodiesterase-5 inhibitors, % (n) | 82.1% (32) |
| Prostanoids, % (n) | 43.6% (17) |
| Stimulator of soluble guanylate cyclase, % (n) | 7.7% (3) |
| Agonists of the prostacyclin receptor, % (n) | 15.4% (6) |
| Diuretics, % (n) | 43.6% (17) |
| Survivors | Non-Survivors | p-Value | |
|---|---|---|---|
| Women. % (n) | 72.41% (21) | 27.59% (8) | 0.6927 & |
| Men. % (n) | 60% (6) | 40% (4) | |
| Age. y | 58.48 ± 17.05 | 68.00 ± 12.25 | 0.1360 # |
| Neutrophil-to-lymphocyte ratio | 3.34 ± 2.01 | 7.99 ± 10.23 | 0.0668 # |
| Mean survival time. days * | 593.78 ± 430.69 | 168.08 ± 189.23 | 0.0065 # |
| WHO FC II | 22.22% | 0.00% | 0.0030 & |
| WHO FC III | 48.15% | 16.67% | |
| WHO FC IV | 29.63% | 83.33% | |
| Echocardiographic parameters | |||
| FAC. % | 0.34 ± 0.12 | 0.37 ± 0.16 | 0.6025 ^ |
| RV dp/dt. mmHg/s | 874.66 ± 424.16 | 857.91 ± 327.48 | 0.9266 ^ |
| S′. cm/s | 11.82 ± 2.37 | 10.73 ± 1.49 | 0.2712 ^ |
| AcT. m/s | 86.35 ± 17.16 | 74.27 ± 21.78 | 0.0287 # |
| TRPG. mmHg | 0.81 ± 0.35 | 1.12 ± 0.41 | 0.2915 ^ |
| TRV. m/s | 4.14 ± 0.78 | 4.43 ± 0.53 | 0.2500 ^ |
| TRPG/AcT. mmHg:m/s | 0.81 ± 0.35 | 1.12 ± 0.41 | 0.0300 ^ |
| E/A ratio | 1.08 ± 0.56 | 1.24 ± 0.40 | 0.3500 # |
| Lateral E/e’ratio | 10.39 ± 6.00 | 11.4 ± 8.82 | 0.6958 # |
| Medial E/e’ratio | 5.83 ± 2.70 | 6.45 ± 1.81 | 0.2895 ^ |
| E wave deceleration time. ms | 233.49 ± 116.01 | 180.4 ± 96.57 | 0.3024 ^ |
| TAPSE. mm | 18.48 ± 3.90 | 14.96 ± 3.15 | 0.0094 ^ |
| TAPSE × AcT. mm × s | 166.15 ± 45.33 | 113.99 ± 47.52 | 0.0053 ^ |
| End-systolic eccentricity index | 1.47 ± 1.17 | 1.66 ± 0.55 | 0.1336 # |
| Diastolic eccentricity index | 1.22 ± 0.35 | 1.56 ± 0.57 | 0.0742 # |
| RAA. cm2 | 24.83 ± 6.53 | 29.95 ± 7.93 | 0.0524 ^ |
| RAP. mmHg | 6.61 ± 3.35 | 13.71 ± 3.45 | 0.0016 # |
| RV/LV ratio | 1.16 ± 0.36 | 1.33 ± 0.36 | 0.1733 ^ |
| mPAP. mmHg | 39.50 ± 7.87 | 47.61 ± 7.83 | 0.0182 # |
| sPAP. mmHg | 75.20 ± 25.85 | 88.90 ± 20.49 | 0.1448 ^ |
| TAPSE/sPAP. mm/mmHg | 0.29 ± 0.14 | 0.18 ± 0.06 | 0.0270 # |
| Mortality | ||
|---|---|---|
| HR | p-Value | |
| AcT. m/s | 0.96 (0.92–0.99) | 0.05 |
| TAPSE. mm | 0.82 (0.70–0.95) | 0.007 |
| mPAP. mmHg | 1.13 (1.02–1.24) | 0.02 |
| TAPSE/sPAP. mm/mmHg | 0.0001 (0.00–0.87) | 0.005 |
| RAP mmHg | 1.25 (1.07–1.45) | 0.005 |
| TRPG/AcT ratio. mmHg:m/s | 5.13 (1.19–22.15) | 0.03 |
| TAPSE × Act. mm × s | 0.98 (0.96–0.99) | 0.03 |
| Neutrophil-to-lymphocyte ratio | 1.07 (1.03–1.70) | 0.005 |
| Area under the Curve | p-Value | Best Cut-off Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|
| TAPSE | 0.756 (0.602–0.911) | 0.0011 | 16.50 mm | 0.750 | 0.704 |
| TAPSE/sPAP | 0.746 (0.579–0.913) | 0.0039 | 0.242 mm/mmHg | 0.900 | 0.542 |
| TAPSE × AcT | 0.777 (0.595–0.959) | 0.0028 | 126.36 mm × s | 0.727 | 0.800 |
| TRPG/AcT | 0.727 (0.546–0.908) | 0.0138 | 0.91 mmHg:m/s | 0.636 | 0.650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topyła-Putowska, W.; Tomaszewski, M.; Wojtkowska, A.; Wysokiński, A. Novel Echocardiographic Measurements of Right Ventricular–Pulmonary Artery Coupling in Predicting the Prognosis of Precapillary Pulmonary Hypertension. J. Pers. Med. 2023, 13, 1627. https://doi.org/10.3390/jpm13121627
Topyła-Putowska W, Tomaszewski M, Wojtkowska A, Wysokiński A. Novel Echocardiographic Measurements of Right Ventricular–Pulmonary Artery Coupling in Predicting the Prognosis of Precapillary Pulmonary Hypertension. Journal of Personalized Medicine. 2023; 13(12):1627. https://doi.org/10.3390/jpm13121627
Chicago/Turabian StyleTopyła-Putowska, Weronika, Michał Tomaszewski, Agnieszka Wojtkowska, and Andrzej Wysokiński. 2023. "Novel Echocardiographic Measurements of Right Ventricular–Pulmonary Artery Coupling in Predicting the Prognosis of Precapillary Pulmonary Hypertension" Journal of Personalized Medicine 13, no. 12: 1627. https://doi.org/10.3390/jpm13121627
APA StyleTopyła-Putowska, W., Tomaszewski, M., Wojtkowska, A., & Wysokiński, A. (2023). Novel Echocardiographic Measurements of Right Ventricular–Pulmonary Artery Coupling in Predicting the Prognosis of Precapillary Pulmonary Hypertension. Journal of Personalized Medicine, 13(12), 1627. https://doi.org/10.3390/jpm13121627







