Time to Deliver on Promises: The Role of ERBB2 Alterations as Treatment Options for Colorectal Cancer Patients in the Era of Precision Oncology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Tumor Board
2.2. Patient Population
2.3. Sequencing Assays
2.4. Conventional ERBB2 Evaluation
2.5. Statistical Analysis
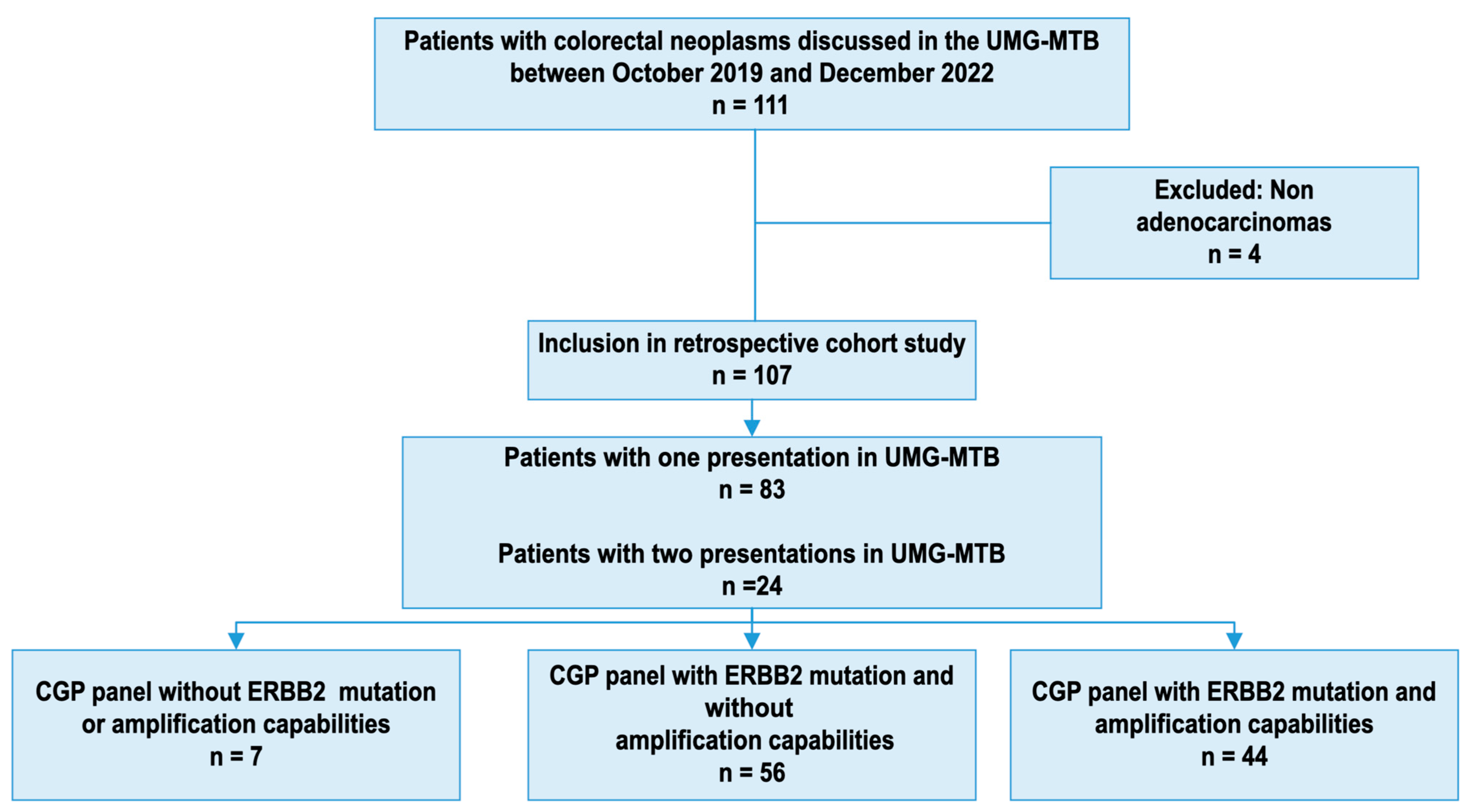
3. Results
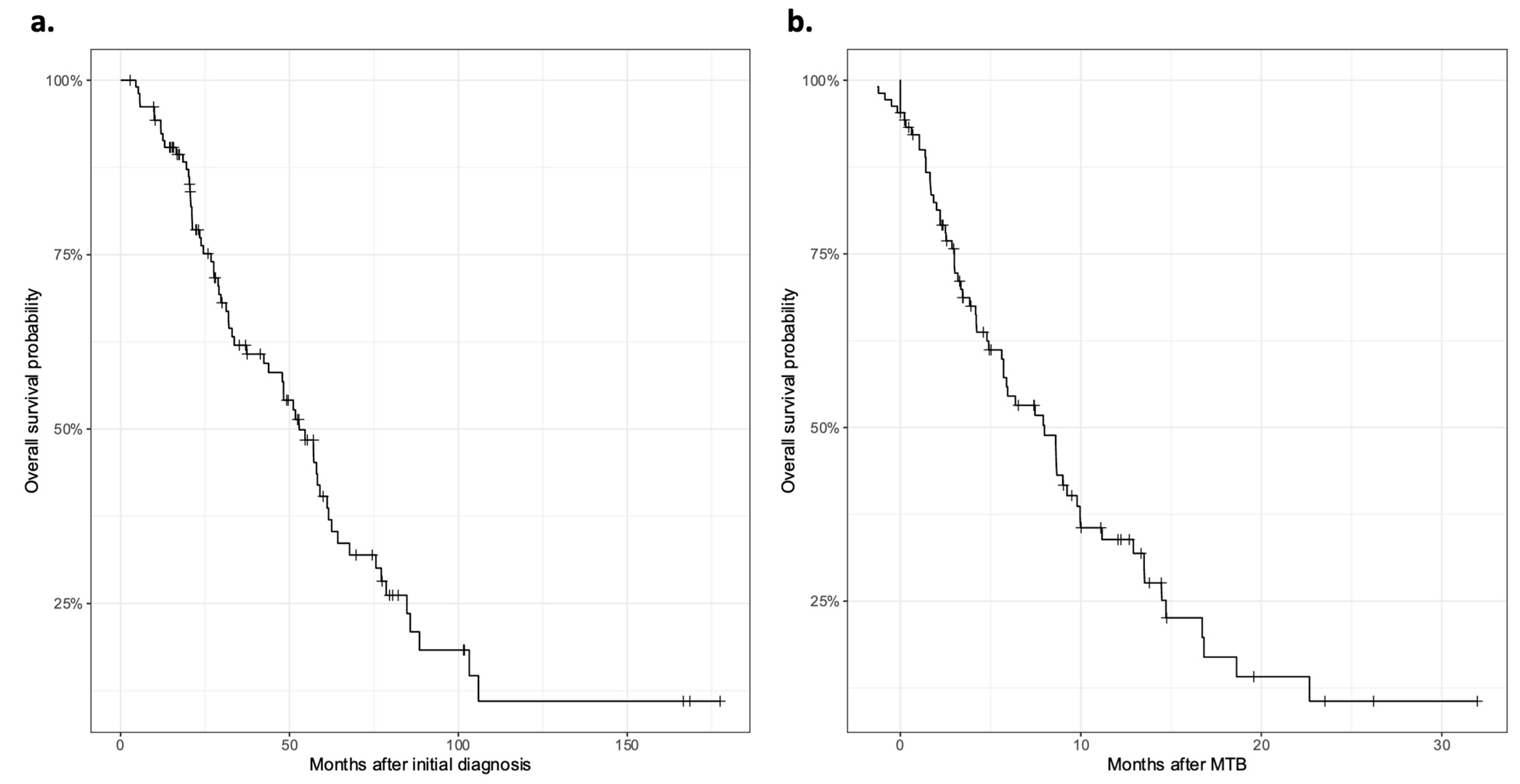
3.1. Patient Characteristics
3.2. Detection and Management of CRC Patients with ERBB2 Amplification or Mutation
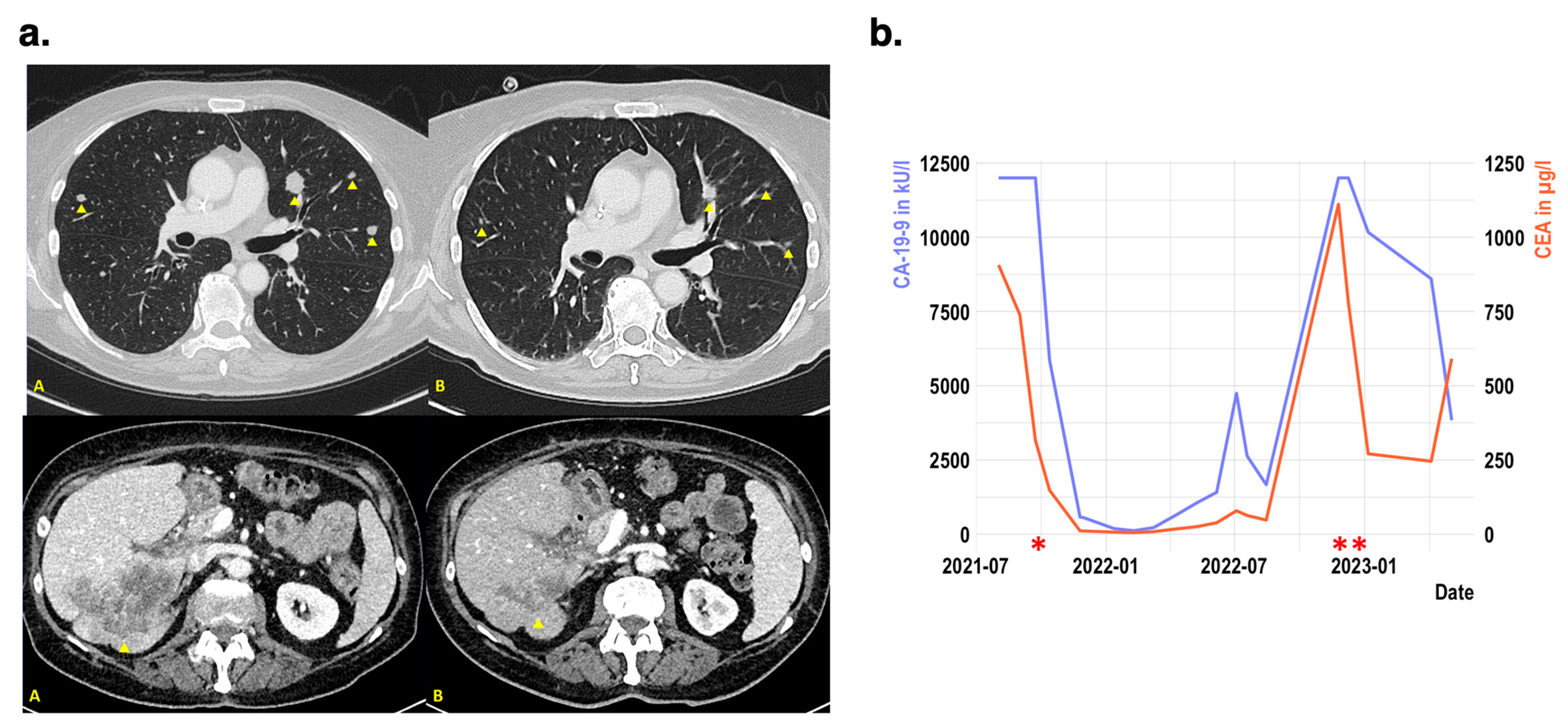
3.3. Exemplatory Case Reviews of Patients with ERBB2 Amplification in mCRC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Treatment of Patients with Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Sartore-Bianchi, A.; Nagy, R.J.; Raghav, K.; Odegaard, J.I.; Lanman, R.B.; Trusolino, L.; Marsoni, S.; Siena, S.; Bardelli, A. Plasma HER2 (ERBB2) Copy Number Predicts Response to HER2-Targeted Therapy in Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 3046–3053. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.D.; Southward, K.; Chambers, P.; Cross, D.; Barrett, J.; Hemmings, G.; Taylor, M.; Wood, H.; Hutchins, G.; Foster, J.M.; et al. HER2 Overexpression and Amplification as a Potential Therapeutic Target in Colorectal Cancer: Analysis of 3256 Patients Enrolled in the QUASAR, FOCUS and PICCOLO Colorectal Cancer Trials. J. Pathol. 2016, 238, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Kavuri, S.M.; Jain, N.; Galimi, F.; Cottino, F.; Leto, S.M.; Migliardi, G.; Searleman, A.C.; Shen, W.; Monsey, J.; Trusolino, L.; et al. HER2 Activating Mutations Are Targets for Colorectal Cancer Treatment. Cancer Discov. 2015, 5, 832–841. [Google Scholar] [CrossRef]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 Scoring System for Colorectal Cancer: Results from a Validation Study. Mod. Pathol. 2015, 28, 1481–1491. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Bang, Y.-J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular Profiling for Precision Cancer Therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Magliocco, A.M.; Kim, J.; Okamoto, W.; Kim, J.E.; Sawada, K.; Nakamura, Y.; Kopetz, S.; Park, W.-Y.; Tsuchihara, K.; et al. International Harmonization of Provisional Diagnostic Criteria for ERBB2-Amplified Metastatic Colorectal Cancer Allowing for Screening by Next-Generation Sequencing Panel. JCO Precis. Oncol. 2020, 4, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Cenaj, O.; Ligon, A.H.; Hornick, J.L.; Sholl, L.M. Detection of ERBB2 Amplification by Next-Generation Sequencing Predicts HER2 Expression in Colorectal Carcinoma. Am. J. Clin. Pathol. 2019, 152, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Bartolomeo, M.D.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab Deruxtecan (DS-8201) in Patients with HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Ng, K.; Cercek, A.; Fountzilas, C.; Sanchez, F.A.; Hubbard, J.M.; Wu, C.; Siena, S.; Tabernero, J.; Van Cutsem, E.; et al. MOUNTAINEER:Open-Label, Phase II Study of Tucatinib Combined with Trastuzumab for HER2-Positive Metastatic Colorectal Cancer (SGNTUC-017, Trial in Progress). J. Clin. Oncol. 2021, 39, TPS153. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): An Updated Report from a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Liu, F.; Ren, C.; Jin, Y.; Xi, S.; He, C.; Wang, F.; Wang, Z.; Xu, R.; Wang, F. Assessment of Two Different HER2 Scoring Systems and Clinical Relevance for Colorectal Cancer. Virchows Arch. 2020, 476, 391–398. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Strickler, J.H.; Yoshino, T.; Graham, R.P.; Siena, S.; Bekaii-Saab, T. Diagnosis and Treatment of ERBB2-Positive Metastatic Colorectal Cancer: A Review. JAMA Oncol. 2022, 8, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef] [PubMed]
- Verbaanderd, C.; Rooman, I.; Meheus, L.; Huys, I. On-Label or Off-Label? Overcoming Regulatory and Financial Barriers to Bring Repurposed Medicines to Cancer Patients. Front. Pharmacol. 2020, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Lawlor, R.T.; Milella, M.; Scarpa, A. Molecular Tumor Boards in Clinical Practice. Trends Cancer 2020, 6, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the Use of Next-Generation Sequencing (NGS) for Patients with Metastatic Cancers: A Report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Ebi, H.; Bando, H. Precision Oncology and the Universal Health Coverage System in Japan. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; Baere, T.D.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B.; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Vanderpoel, J.; Stevens, A.L.; Emond, B.; Lafeuille, M.-H.; Hilts, A.; Lefebvre, P.; Morrison, L. Total Cost of Testing for Genomic Alterations Associated with Next-Generation Sequencing versus Polymerase Chain Reaction Testing Strategies among Patients with Metastatic Non-Small Cell Lung Cancer. J. Med. Econ. 2022, 25, 457–468. [Google Scholar] [CrossRef]
- Berrino, E.; Annaratone, L.; Bellomo, S.E.; Ferrero, G.; Gagliardi, A.; Bragoni, A.; Grassini, D.; Guarrera, S.; Parlato, C.; Casorzo, L.; et al. Integrative Genomic and Transcriptomic Analyses Illuminate the Ontology of HER2-Low Breast Carcinomas. Genome Med. 2022, 14, 98. [Google Scholar] [CrossRef]
- Li, Y.; Tsang, J.Y.; Tam, F.; Loong, T.; Tse, G.M. Comprehensive Characterization of HER2-Low Breast Cancers: Implications in Prognosis and Treatment. eBioMedicine 2023, 91, 104571. [Google Scholar] [CrossRef]
- Daiichi Sankyo, Inc. A Phase 2, Multicenter, Randomized, Study of Trastuzumab Deruxtecan in Participants with HER2-Overexpressing Locally Advanced, Unresectable or Metastatic Colorectal Cancer (DESTINY-CRC02); Daiichi Sankyo, Inc.: Tokyo, Japan, 2023. [Google Scholar]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.J.; Lichinitser, M.; et al. Neoadjuvant and Adjuvant Trastuzumab in Patients with HER2-Positive Locally Advanced Breast Cancer (NOAH): Follow-up of a Randomised Controlled Superiority Trial with a Parallel HER2-Negative Cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Jonsson Comprehensive Cancer Center. A Phase II, Multicenter, Open-Label Trial to Evaluate the Safety and Efficacy of Trastuzumab Deruxtecan (DS-8201a) with or without Anastrozole for HER2 Low Hormone Receptor Positive (HR+) Breast Cancer in the Neoadjuvant Setting; Jonsson Comprehensive Cancer Center: Los Angeles, CA, USA, 2022. [Google Scholar]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
| Characteristic | Number of Patients | |
|---|---|---|
| Sex | ||
| Male | 65 (60.7%) | |
| Female | 42 (39.3%) | |
| Primary tumor localization | ||
| Caecum/appendix | 10 (9.3%) | |
| Ascending colon | 8 (7.5%) | |
| Transverse colon | 5 (4.7%) | |
| Descending colon | 3 (2.8%) | |
| Sigmoid colon | 21 (19.6%) | |
| Rectum | 59 (55.1%) | |
| More than one primary tumor location | 1 (0.9%) | |
| UICC stage at diagnosis | ||
| I | 1 (0.9%) | |
| II | 9 (8.4%) | |
| III | 28 (26.2%) | |
| IV | 65 (60.7%) | |
| Unknown | 4 (3.7%) | |
| Age at diagnosis in years | ||
| Median | 54.3 | |
| Range | 23.4 to 83.1 | |
| Age at presentation in MTB in years | ||
| Median | 57.3 | |
| Range | 23.8 to 83.3 | |
| Number of MTB presentations with individual GCP | ||
| One | 83 (77.6%) | |
| Two | 24 (22.4%) | |
| ERBB2 scope of most comprehensive panel used for GCP | ||
| No analysis of ERBB2 | 7 (7.5%) | |
| Detection of ERBB2 mutations | 56 (52.3%) | |
| Detection of ERBB2 mutations & alterations | 44 (41.1%) | |
| Status at last follow up | ||
| Deceased | 64 (59.8%) | |
| Alive | 43 (40.2%) | |
| Characteristic | Number of Patients | |
|---|---|---|
| Sex | ||
| Male | 2 (50%) | |
| Female | 2 (50%) | |
| Primary tumor localisation | ||
| Sigmoid colon | 1 (25%) | |
| Rectum | 3(75%) | |
| Age at diagnosis in years | ||
| Median | 62 | |
| Range | 25–66 | |
| Received ERBB2 specific treatment | ||
| Yes | 2 (50%) | |
| No | 2 (50%) | |
| Status at last follow up | ||
| Deceased | 2 (50%) | |
| Alive | 2 (50%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchholz, S.M.; Nause, N.; König, U.; Reinecke, J.; Steuber, B.; Ammer-Herrmenau, C.; Reuter-Jessen, K.; Bohnenberger, H.; Biggemann, L.; Braulke, F.; et al. Time to Deliver on Promises: The Role of ERBB2 Alterations as Treatment Options for Colorectal Cancer Patients in the Era of Precision Oncology. J. Pers. Med. 2023, 13, 1701. https://doi.org/10.3390/jpm13121701
Buchholz SM, Nause N, König U, Reinecke J, Steuber B, Ammer-Herrmenau C, Reuter-Jessen K, Bohnenberger H, Biggemann L, Braulke F, et al. Time to Deliver on Promises: The Role of ERBB2 Alterations as Treatment Options for Colorectal Cancer Patients in the Era of Precision Oncology. Journal of Personalized Medicine. 2023; 13(12):1701. https://doi.org/10.3390/jpm13121701
Chicago/Turabian StyleBuchholz, Soeren M., Nelia Nause, Ute König, Johanna Reinecke, Benjamin Steuber, Christoph Ammer-Herrmenau, Kirsten Reuter-Jessen, Hanibal Bohnenberger, Lorenz Biggemann, Friederike Braulke, and et al. 2023. "Time to Deliver on Promises: The Role of ERBB2 Alterations as Treatment Options for Colorectal Cancer Patients in the Era of Precision Oncology" Journal of Personalized Medicine 13, no. 12: 1701. https://doi.org/10.3390/jpm13121701
APA StyleBuchholz, S. M., Nause, N., König, U., Reinecke, J., Steuber, B., Ammer-Herrmenau, C., Reuter-Jessen, K., Bohnenberger, H., Biggemann, L., Braulke, F., Neesse, A., Ellenrieder, V., Ströbel, P., Adler, M., & König, A. (2023). Time to Deliver on Promises: The Role of ERBB2 Alterations as Treatment Options for Colorectal Cancer Patients in the Era of Precision Oncology. Journal of Personalized Medicine, 13(12), 1701. https://doi.org/10.3390/jpm13121701








