Cochlear Implantation in Patients with Bilateral Sudden Sensorineural Hearing Loss after COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cochlear Implant
2.2. Pre-Op Examinations
- Nasopharyngeal swab analysis for SARS-CoV-2;
- Local otoscopy and otomicroscopy exams;
- Pure-tone audiometry (PTA);
- Tympanogram;
- Functional vestibular testing was performed via video head impulse test (vHIT);
- Auditory brain stem response (ABR).
2.3. Intraop Examinations
- Perilymph was taken from the cochlea and sent for virological analysis.
2.4. Postop Examinations
- Pure-tone audiometry (PTA).
3. Results
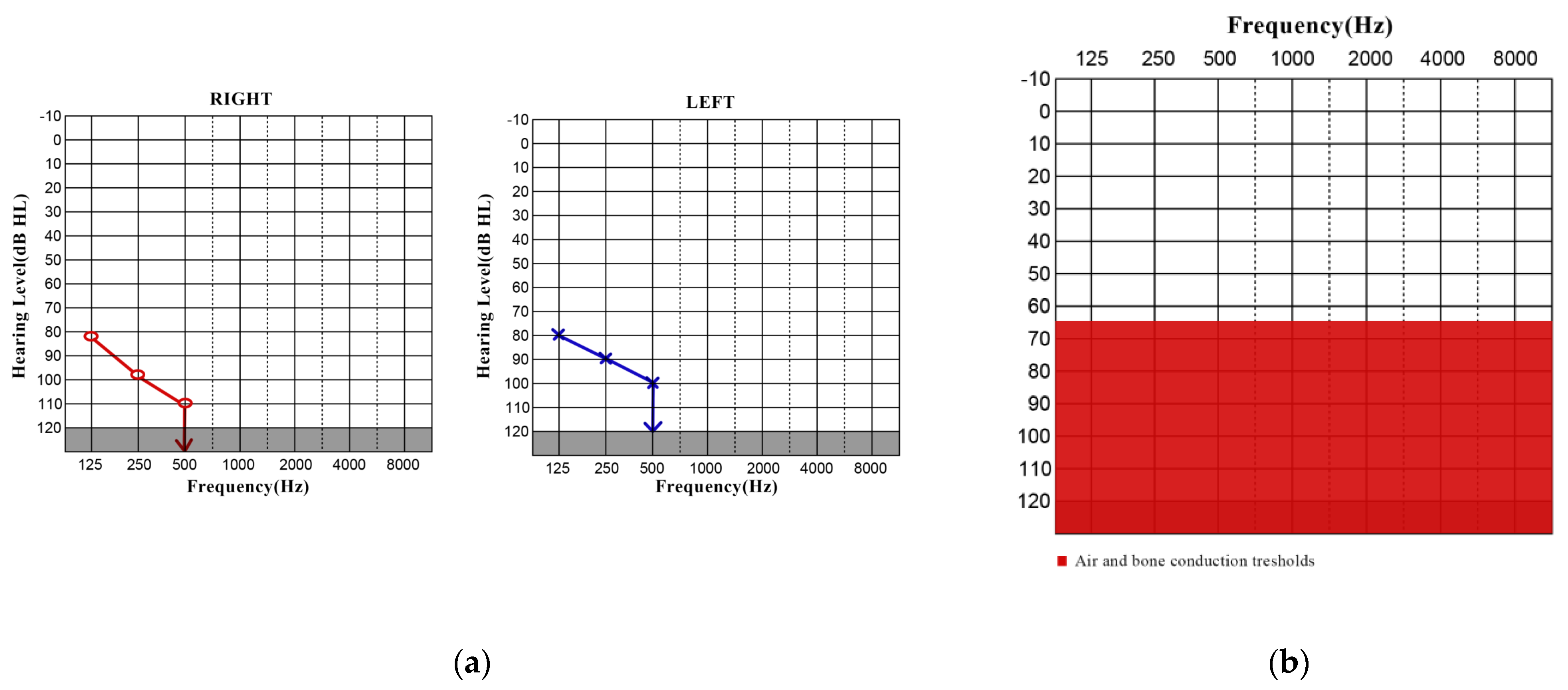
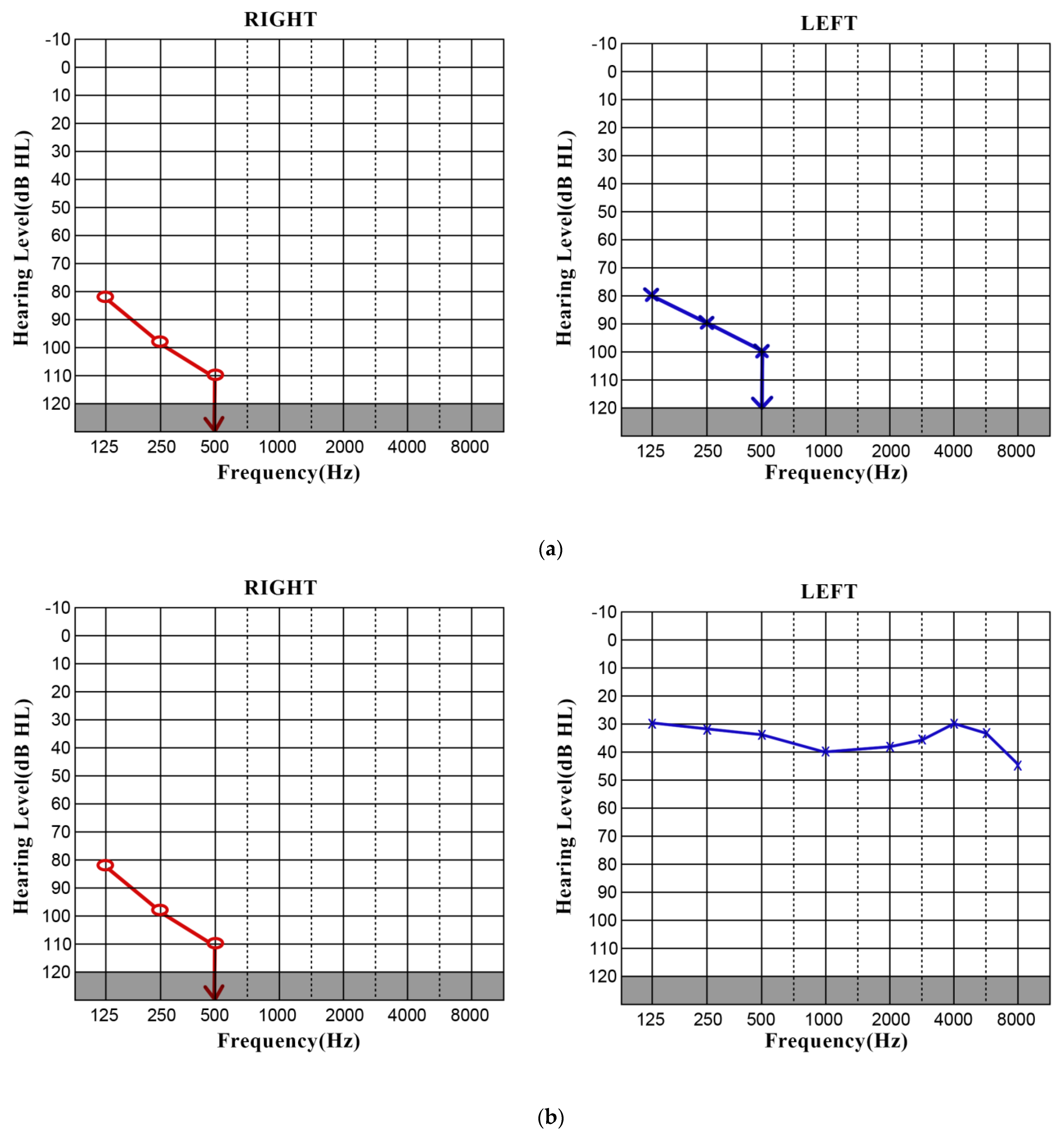
3.1. CASE 1
3.2. CASE 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID Live—Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 15 September 2022).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Harenberg, J.; Jonas, J.B.; Trecca, E.M.C. A Liaison between Sudden Sensorineural Hearing Loss and SARS-CoV-2 Infection. Thromb. Haemost. 2020, 120, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Saniasiaya, J. Hearing Loss in SARS-CoV-2: What Do We Know? Ear Nose Throat J. 2021, 100, 152S–154S. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Kurata, T.; Saito, K. Cochlear changes after herpes simplex virus infection. Acta Otolaryngol. 1985, 99, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; Durand, M.L.; Adams, J.C. Sudden deafness: Is it viral? ORL J. Otorhinolaryngol. Relat. Spec. 2008, 70, 52–60; discussion 60–52. [Google Scholar] [CrossRef]
- Greco, A.; Fusconi, M.; Gallo, A.; Marinelli, C.; Macri, G.F.; De Vincentiis, M. Sudden sensorineural hearing loss: An autoimmune disease? Autoimmun. Rev. 2011, 10, 756–761. [Google Scholar] [CrossRef]
- Chen, X.; Fu, Y.Y.; Zhang, T.Y. Role of viral infection in sudden hearing loss. J. Int. Med. Res. 2019, 47, 2865–2872. [Google Scholar] [CrossRef]
- Fetterman, B.L.; Saunders, J.E.; Luxford, W.M. Prognosis and treatment of sudden sensorineural hearing loss. Am. J. Otol. 1996, 17, 529–536. [Google Scholar]
- Huy, P.T.; Sauvaget, E. Idiopathic sudden sensorineural hearing loss is not an otologic emergency. Otol. Neurotol. 2005, 26, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Park, K.; Lee, S.J.; Shin, Y.R.; Choung, Y.H. Bilateral versus unilateral sudden sensorineural hearing loss. Otolaryngol. Head. Neck Surg. 2007, 136, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.K.; Lin, J.R.; Atashband, S.; Irvine, R.A.; Westerberg, B.D. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010, 120, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Mentel, R.; Kaftan, H.; Wegner, U.; Reissmann, A.; Gurtler, L. Are enterovirus infections a co-factor in sudden hearing loss? J. Med. Virol. 2004, 72, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F.; Donovan, E.D. The pathology of idiopathic sudden sensorineural hearing loss. Arch. Otorhinolaryngol. 1986, 243, 243. [Google Scholar] [CrossRef]
- Massa, H.M.; Cripps, A.W.; Lehmann, D. Otitis media: Viruses, bacteria, biofilms and vaccines. Med. J. Aust. 2009, 191, S44–S49. [Google Scholar] [CrossRef]
- Tang, M.; Wang, J.; Zhang, Q. Prevalence of hearing loss in COVID-19 patients: A systematic review and meta-analysis. Acta Otolaryngol. 2023, 143, 416–422. [Google Scholar] [CrossRef]
- Mehraeen, E.; Afzalian, A.; Afsahi, A.M.; Shahidi, R.; Fakhfouri, A.; Karimi, K.; Varshochi, S.; Habibi, M.A.; Molla, A.; Dadjou, A.; et al. Hearing loss and COVID-19: An umbrella review. Eur. Arch. Otorhinolaryngol. 2023, 280, 3515–3528. [Google Scholar] [CrossRef]
- Frosolini, A.; Franz, L.; Daloiso, A.; de Filippis, C.; Marioni, G. Sudden Sensorineural Hearing Loss in the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 3139. [Google Scholar] [CrossRef]
- Edwards, M.; Muzaffar, J.; Naik, P.; Coulson, C. Catastrophic bilateral sudden sensorineural hearing loss following COVID-19. BMJ Case Rep. 2021, 14, e243157. [Google Scholar] [CrossRef]
- Abdel Rhman, S.; Abdel Wahid, A. COVID-19 and sudden sensorineural hearing loss, a case report. Otolaryngol. Case Rep. 2020, 16, 100198. [Google Scholar] [CrossRef] [PubMed]
- Asfour, L.; Kay-Rivest, E.; Roland, J.T., Jr. Cochlear implantation for single-sided deafness after COVID-19 hospitalization. Cochlear Implants Int. 2021, 22, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gerstacker, K.; Speck, I.; Riemann, S.; Aschendorff, A.; Knopf, A.; Arndt, S. Deafness after COVID-19? HNO 2021, 69, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Callan, D.E.; Tsytsarev, V.; Hanakawa, T.; Callan, A.M.; Katsuhara, M.; Fukuyama, H.; Turner, R. Song and speech: Brain regions involved with perception and covert production. Neuroimage 2006, 31, 1327–1342. [Google Scholar] [CrossRef] [PubMed]
- Strelnikov, K.; Marx, M.; Lagleyre, S.; Fraysse, B.; Deguine, O.; Barone, P. PET-imaging of brain plasticity after cochlear implantation. Hear. Res. 2015, 322, 180–187. [Google Scholar] [CrossRef]
- Iba, T.; Connors, J.M.; Levy, J.H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Connors, J.M.; Thachil, J. The authors reply. Crit. Care Med. 2020, 48, e1160–e1161. [Google Scholar] [CrossRef]
- Fancello, V.; Hatzopoulos, S.; Corazzi, V.; Bianchini, C.; Skarzynska, M.B.; Pelucchi, S.; Skarzynski, P.H.; Ciorba, A. SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211027373. [Google Scholar] [CrossRef]
- Byl, F.M., Jr. Sudden hearing loss: Eight years’ experience and suggested prognostic table. Laryngoscope 1984, 94, 647–661. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsović, N.; Jovanović, M.; Babac, S.; Čvorović, L.; Radivojević, N.; Arsović, K. Cochlear Implantation in Patients with Bilateral Sudden Sensorineural Hearing Loss after COVID-19 Infection. J. Pers. Med. 2023, 13, 1708. https://doi.org/10.3390/jpm13121708
Arsović N, Jovanović M, Babac S, Čvorović L, Radivojević N, Arsović K. Cochlear Implantation in Patients with Bilateral Sudden Sensorineural Hearing Loss after COVID-19 Infection. Journal of Personalized Medicine. 2023; 13(12):1708. https://doi.org/10.3390/jpm13121708
Chicago/Turabian StyleArsović, Nenad, Marija Jovanović, Snežana Babac, Ljiljana Čvorović, Nemanja Radivojević, and Konstantin Arsović. 2023. "Cochlear Implantation in Patients with Bilateral Sudden Sensorineural Hearing Loss after COVID-19 Infection" Journal of Personalized Medicine 13, no. 12: 1708. https://doi.org/10.3390/jpm13121708
APA StyleArsović, N., Jovanović, M., Babac, S., Čvorović, L., Radivojević, N., & Arsović, K. (2023). Cochlear Implantation in Patients with Bilateral Sudden Sensorineural Hearing Loss after COVID-19 Infection. Journal of Personalized Medicine, 13(12), 1708. https://doi.org/10.3390/jpm13121708






