Barriers and Facilitators to the Implementation of Personalised Medicine across Europe
Abstract
:1. Introduction
2. The Aim of the Study
3. Materials and Methods
3.1. Ethics Approval
3.2. Study Design
3.3. Study Design
3.4. Questionnaire Development and Data Collection
Participants
3.5. Variables
3.5.1. Quantitative Variables
3.5.2. Qualitative Variables
3.6. Data Sources
3.7. Study Size
4. Results
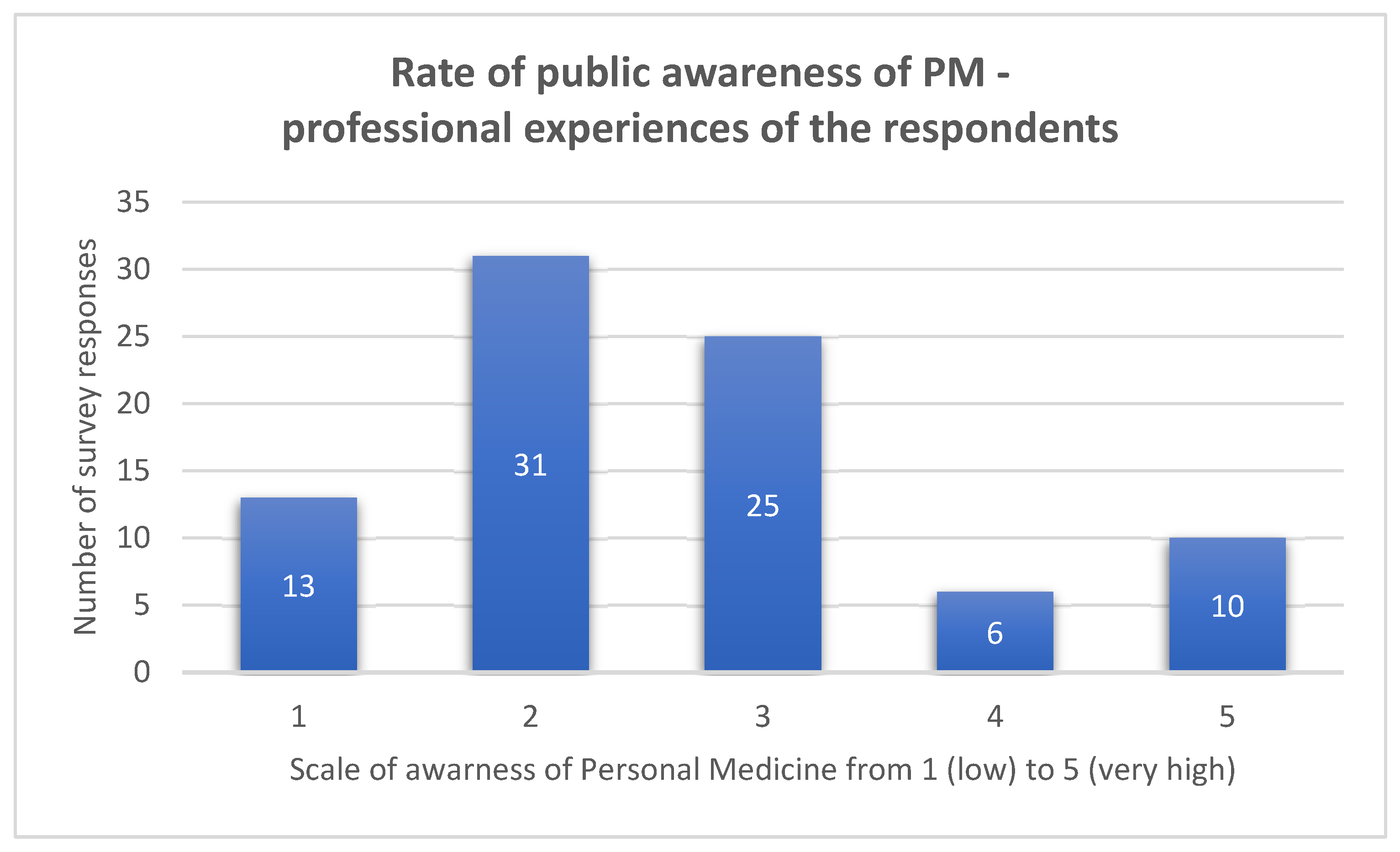
4.1. Participants and Descriptive Data—Survey
4.1.1. Cooperation/Collaboration
4.1.2. Data Protection/IT/Data Sharing
4.1.3. Dissemination
4.1.4. Education
4.1.5. Finances
4.1.6. Public/Citizens
4.1.7. System Changes/Governmental Level
- Medical data-sharing practices and medical data protection;
- Personalised exercise prescription;
- Telemedicine;
- Bioinformatics, artificial intelligence, genomics, machine learning, data analysis;
- Professionals–patients relations, professionals–health managers relations, interdisciplinary and interprofessional approaches to health, emerging specialisations needed in personalised medicine (bioengineers, bionanotechnology specialists, physics applied to health, biodata analysts);
- Health technology assessment in PM, including the patient’s perspective;
- Oncology, internal medicine, public health, healthcare;
- General dissemination;
- Value-based care;
- Paediatrics;
- Omics and advanced diagnostic tests;
- Health and sport;
- Focus group working on how to transform evidence-based medicine into PM, following rational principles;
- Benefits for the individual and the system from the thorough application of PM;
- PM in different disease areas/specialisations, e.g., gastroenterology.
5. Discussion
5.1. Government and Government Agencies
5.2. Medical Doctors/Practitioners
5.3. Healthcare Systems
5.4. Healthcare Providers
5.5. Patients
5.6. Industry
5.7. Technology Developers
5.8. Financial Institutions
5.9. Media
6. Limitations of the Study
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fournier, V.; Prebet, T.; Dormal, A.; Brunel, M.; Cremer, R.; Schiaratura, L. Definition of Personalized Medicine and Targeted Therapies: Does Medical Familiarity Matter? J. Pers. Med. 2021, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Botham, J.; Shilling, V.; Jones, J. Patient and public understanding of the concept of ‘personalised medicine’ in relation to cancer treatment: A systematic review. Future Health J. 2021, 8, e703–e708. [Google Scholar] [CrossRef] [PubMed]
- Schooneveldt, B.C.; Veldwijk, J.; Weda, M. Application of Personalized Medicine Opportunities and Challenges for Policy. Available online: https://www.rivm.nl/bibliotheek/rapporten/2015-0177.pdf (accessed on 17 December 2022).
- Antoñanzas, F.; Juárez-Castelló, C.A.; Rodríguez-Ibeas, R. Implementing personalized medicine with asymmetric information on prevalence rates. Health Econ. Rev. 2016, 6, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Garrison, L.; Finley, M.J. Linking pharmacogenetics-based diagnostics and drugs for personalized medicine. Health Aff. 2006, 25, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Stefanicka-Wojtas, D.; Kurpas, D. eHealth and mHealth in Chronic Diseases—Identification of Barriers, Existing Solutions, and Promoters Based on a Survey of EU Stakeholders Involved in Regions4PerMed (H2020). J. Pers. Med. 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Holden, C.; Bignell, L.; Mukhopadhyay, S.; Jones, C. The public perception of the facilitators and barriers to implementing personalized medicine: A systematic review. Pers. Med. 2019, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Jansen, M.; Leyens, L.; Lal, J.A.; Sudbrak, R.; Hackenitz, E.; Bußhoff, U.; Ballensiefen, W.; Brand, A. An index of barriers for the implementation of personalised medicine and pharmacogenomics in Europe. Public Health Genom. 2014, 17, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Allotey, P.A.; Chaiyakunapruk, N. Current landscape of personalized medicine adoption and implementation in Southeast Asia. BMC Med Genom. 2018, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Trein, P.; Wagner, J. Governing Personalized Health: A Scoping Review. Front. Genet. 2021, 12, 650504. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.S.; Widmer, D.; Cohidon, C.; Desvergne, B.; Cornuz, J.; Guessous, I.; Cerqui, D. Representations of personalised medicine in family medicine: A qualitative analysis. BMC Prim. Care 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Brand, A.; Holgate, S.T.; Kristiansen, L.V.; Lehrach, H.; Palotie, A.; Prainsack, B. The future of technologies for personalised medicine. New Biotechnol. 2012, 29, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritchard, D.; Hulick, P.J.; Wells, C.J. The integration of personalized medicine into health systems: Progress and a path forward. Pers. Med. 2021, 18, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Aronson, S.J.; Rehm, H.L. Building the foundation for genomics in precision medicine. Nature 2015, 15, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMichael, A.J.; Kane, J.P.M.; Rolison, J.J.; O’Neill, F.A.; Boeri, M.; Kee, F. Implementation of personalised medicine policies in mental healthcare: Results from a stated preference study in the UK. BJPsych Open 2022, 8, e40. [Google Scholar] [CrossRef] [PubMed]
- Koleva-Kolarova, R.; Buchanan, J.; Vellekoop, H.; Huygens, S.; Versteegh, M.; Rutten-van Molken, M.; Szilberhorn, L.; Zelei, T.; Nagy, B.; Wordsworth, S.; et al. Financing and Reimbursement Models for Personalised Medicine: A Systematic Review to Identify Current Models and Future Options. Appl. Health Econ. Health Policy 2022, 20, 501–524. [Google Scholar] [CrossRef] [PubMed]
- Hicks-Courant, K.; Shen, J.; Stroupe, A.; Cronin, A.; Bair, E.F.; Wing, S.E.; Sosa, E.; Nagler, R.H.; Gray, S.W. Personalized Cancer Medicine in the Media: Sensationalism or Realistic Reporting? J Pers Med. 2021, 11, 741. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.E.; Moeckel, F.; Villa, M.S.; Housman, L.T.; McCarty, C.A.; McLeod, H.L. Strategies for integrating personalized medicine into healthcare practice. Pers. Med. 2017, 14, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayers, A. Personalized Medicine—Future Impact, Pharma Industry Perspective. J. Biomol. Tech. 2010, 21 (Suppl. 3), S5. [Google Scholar]
- Schee Genannt Halfmann, S.; Evangelatos, N.; Schröder-Bäck, P.; Brand, A. European healthcare systems readiness to shift from ‘one-size fits all’ to personalized medicine. Pers. Med. 2017, 14, 63–74. [Google Scholar] [CrossRef] [PubMed]


| Country | Rate of Public Awareness of PM | Number of Survey Responses | Country’s GDP (in USD) per capita (2022 Year—Estimated Data)—Source of Data—The International Monetary Fund | Division of the Countries—High-Income, Middle—Income, Low—Income Country Source of Data—World Bank List of Economies 2022 |
|---|---|---|---|---|
| Ukraine | 2 | 1 | 4862 (2021) | Lower middle income |
| 3 | 3 | |||
| 4 | 1 | |||
| Turkey | 2 | 1 | 9961 | Upper middle income |
| Kazakhstan | 4 | 1 | 11,591 | Upper middle income |
| Romania | 2 | 1 | 15,619 | Upper middle income |
| Poland | 1 | 1 | 19,023 | High income |
| 2 | 2 | |||
| 3 | 1 | |||
| Greece | 1 | 2 | 20,876 | High income |
| Latvia | 2 | 1 | 21,482 | High income |
| Lithuania | 5 | 1 | 24,032 | High income |
| 4 | 1 | |||
| Portugal | 2 | 1 | 24,910 | High income |
| Spain | 2 | 4 | 29,198 | High income |
| 3 | 3 | |||
| Estonia | 3 | 1 | 29,344 | High income |
| Italy | 1 | 5 | 33,740 | High income |
| 2 | 9 | |||
| 3 | 7 | |||
| 4 | 1 | |||
| 5 | 5 | |||
| European Union | 1 | 1 | 37,280 | |
| France | 1 | 1 | 42,330 | High income |
| 5 | 1 | |||
| United Kingdom | 2 | 1 | 47,318 | High income |
| 5 | 1 | |||
| Germany | 1 | 3 | 48,398 | High income |
| 2 | 9 | |||
| 3 | 6 | |||
| 4 | 2 | |||
| 3 | 2 | 50,598 | High income | |
| Netherlands | 2 | 1 | 56,298 | High income |
| 3 | 1 | |||
| 5 | 1 | |||
| Sweden | 3 | 1 | 56,361 | High income |
| Canada | 1 | 1 | 56,794 | High income |
| Key Stakeholders of the Implementation Barriers | Barriers to the Implementation of PM Interventions | Facilitators of the Implementation of PM Interventions (Ułatwienie) |
|---|---|---|
| Government and government agencies |
|
|
| Medical doctors/practitioners |
|
|
| Healthcare systems |
|
|
| Healthcare providers |
|
|
| Patients and patient organisations |
|
|
| Medical sector, scientific community, researchers, stakeholders |
|
|
| Industry |
|
|
| Technology developers |
|
|
| Financial institutions |
|
|
| Media |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanicka-Wojtas, D.; Kurpas, D. Barriers and Facilitators to the Implementation of Personalised Medicine across Europe. J. Pers. Med. 2023, 13, 203. https://doi.org/10.3390/jpm13020203
Stefanicka-Wojtas D, Kurpas D. Barriers and Facilitators to the Implementation of Personalised Medicine across Europe. Journal of Personalized Medicine. 2023; 13(2):203. https://doi.org/10.3390/jpm13020203
Chicago/Turabian StyleStefanicka-Wojtas, Dorota, and Donata Kurpas. 2023. "Barriers and Facilitators to the Implementation of Personalised Medicine across Europe" Journal of Personalized Medicine 13, no. 2: 203. https://doi.org/10.3390/jpm13020203
APA StyleStefanicka-Wojtas, D., & Kurpas, D. (2023). Barriers and Facilitators to the Implementation of Personalised Medicine across Europe. Journal of Personalized Medicine, 13(2), 203. https://doi.org/10.3390/jpm13020203





