How Wearable Sensors Can Support the Research on Foetal and Pregnancy Outcomes: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
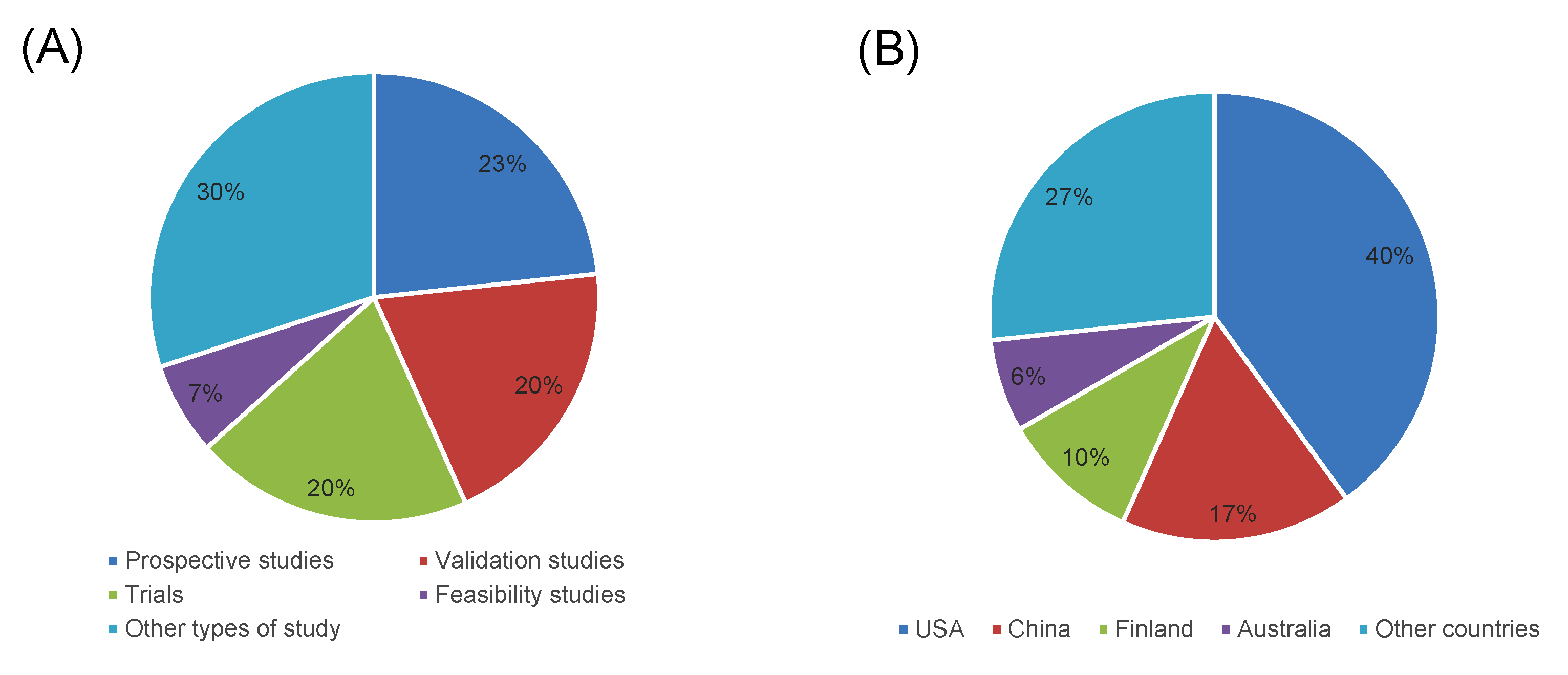
3.1. Study Selection
3.2. Wearable Sensors for Collecting Foetal Parameters
3.2.1. Foetal Movements
3.2.2. Foetal Heart Rate
3.2.3. Other Parameters
3.3. Wearable Sensors for Collecting Maternal Parameters and Activities
3.3.1. Cardiovascular Parameters
3.3.2. Maternal Activities
3.3.3. Simultaneous Collection of Cardiac Parameters and Maternal Activities
3.3.4. Planned Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchant, T.; Boerma, T.; Diaz, T.; Huicho, L.; Kyobutungi, C.; Mershon, C.H.; Schellenberg, J.; Somers, K.; Waiswa, P.; Group, N. Measurement and accountability for maternal, newborn and child health: Fit for 2030? BMJ Glob. Health 2020, 5, e002697. [Google Scholar] [CrossRef] [PubMed]
- Peyroteo, M.; Ferreira, I.A.; Elvas, L.B.; Ferreira, J.C.; Lapão, L.V. Remote Monitoring Systems for Patients with Chronic Diseases in Primary Health Care: Systematic Review. JMIR mHealth uHealth 2021, 9, e28285. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Alexander, C.A. Big data analytics in medical engineering and healthcare: Methods, advances and challenges. J. Med. Eng. Technol. 2020, 44, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswari, V.; Subramaniyaswamy, V.; Logesh, R.; Vijayakumar, V. A study on medical Internet of Things and Big Data in personalized healthcare system. Health Inf. Sci. Syst. 2018, 6, 14. [Google Scholar] [CrossRef]
- DeFrancesco, L.; Jarchum, I. Focus on wearable sensors. Nat. Biotechnol. 2019, 37, 329. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Gulzar Ahmad, S.; Iqbal, T.; Javaid, A.; Ullah Munir, E.; Kirn, N.; Ullah Jan, S.; Ramzan, N. Sensing and Artificial Intelligent Maternal-Infant Health Care Systems: A Review. Sensors 2022, 22, 4362. [Google Scholar] [CrossRef]
- Chan, K.L.; Chen, M. Effects of Social Media and Mobile Health Apps on Pregnancy Care: Meta-Analysis. JMIR mHealth uHealth 2019, 7, e11836. [Google Scholar] [CrossRef] [Green Version]
- Bertini, A.; Gárate, B.; Pardo, F.; Pelicand, J.; Sobrevia, L.; Torres, R.; Chabert, S.; Salas, R. Impact of Remote Monitoring Technologies for Assisting Patients with Gestational Diabetes Mellitus: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 819697. [Google Scholar] [CrossRef]
- Daly, L.M.; Horey, D.; Middleton, P.F.; Boyle, F.M.; Flenady, V. The Effect of Mobile App Interventions on Influencing Healthy Maternal Behavior and Improving Perinatal Health Outcomes: Systematic Review. JMIR mHealth uHealth 2018, 6, e10012. [Google Scholar] [CrossRef] [Green Version]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.; Woodward, R.; Alexandrov, Y.; Ain Munnee, Q.; Lees, C.C.; Vaidyanathan, R.; Nowlan, N.C. Performance of a wearable acoustic system for fetal movement discrimination. PLoS ONE 2018, 13, e0195728. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Peng, J.; Xu, Y. Passive Fetal Movement Signal Detection System Based on Intelligent Sensing Technology. J. Healthc. Eng. 2021, 2021, 1745292. [Google Scholar] [CrossRef]
- Liang, S.; Peng, J.; Xu, Y.; Ye, H. Passive Fetal Movement Recognition Approaches Using Hyperparameter Tuned LightGBM Model and Bayesian Optimization. Comput. Intell. Neurosci. 2021, 2021, 6252362. [Google Scholar] [CrossRef]
- Mesbah, M.; Khlif, M.S.; Layeghy, S.; East, C.E.; Dong, S.; Brodtmann, A.; Colditz, P.B.; Boashash, B. Automatic fetal movement recognition from multi-channel accelerometry data. Comput. Methods Programs Biomed. 2021, 210, 106377. [Google Scholar] [CrossRef]
- Mhajna, M.; Sadeh, B.; Yagel, S.; Sohn, C.; Schwartz, N.; Warsof, S.; Zahar, Y.; Reches, A. A Novel, Cardiac-Derived Algorithm for Uterine Activity Monitoring in a Wearable Remote Device. Front. Bioeng. Biotechnol. 2022, 10, 933612. [Google Scholar] [CrossRef]
- Mhajna, M.; Schwartz, N.; Levit-Rosen, L.; Warsof, S.; Lipschuetz, M.; Jakobs, M.; Rychik, J.; Sohn, C.; Yagel, S. Wireless, remote solution for home fetal and maternal heart rate monitoring. Am. J. Obstet. Gynecol. MFM 2020, 2, 100101. [Google Scholar] [CrossRef]
- Nguyen, T.; Khaksari, K.; Khare, S.M.; Park, S.; Anderson, A.A.; Bieda, J.; Jung, E.; Hsu, C.D.; Romero, R.; Gandjbakhche, A.H. Non-invasive transabdominal measurement of placental oxygenation: A step toward continuous monitoring. Biomed. Opt. Express 2021, 12, 4119–4130. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Zhou, Z.; Bai, Y.; Wu, S. A Fetal ECG Monitoring System Based on the Android Smartphone. Sensors 2019, 19, 446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gu, A.; Xiao, Z.; Xing, Y.; Yang, C.; Li, J.; Liu, C. Wearable Fetal ECG Monitoring System from Abdominal Electrocardiography Recording. Biosensors 2022, 12, 475. [Google Scholar] [CrossRef]
- Atzmon, Y.; Ben Ishay, E.; Hallak, M.; Littman, R.; Eisenkraft, A.; Gabbay-Benziv, R. Continuous Maternal Hemodynamics Monitoring at Delivery Using a Novel, Noninvasive, Wireless, PPG-Based Sensor. J. Clin. Med. 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Tan, K.H.; Ang, S.B. I-ACT: Integrated study on effect of Activity on ComplicaTions in pregnancy: Study protocol of a multiethnic prospective cohort study. BMJ Open 2019, 9, e025970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.H.; Lee, C.F.; Huang, J.P.; Hsiung, Y.; Chi, L.K. Effectiveness of a nurse-led mHealth app to prevent excessive gestational weight gain among overweight and obese women: A randomized controlled trial. J. Nurs. Sch. 2022, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fang, X.; Wong, T.H.; Chan, S.N.; Akinwunmi, B.; Ming, W.K.; Zhang, C.J.P.; Wang, Z. Physical Activity during Pregnancy: Comparisons between Objective Measures and Self-Reports in Relation to Blood Glucose Levels. Int. J. Environ. Res. Public Health 2022, 19, 8064. [Google Scholar] [CrossRef]
- Cheung, N.W.; Blumenthal, C.; Smith, B.J.; Hogan, R.; Thiagalingam, A.; Redfern, J.; Barry, T.; Cinnadaio, N.; Chow, C.K. A Pilot Randomised Controlled Trial of a Text Messaging Intervention with Customisation Using Linked Data from Wireless Wearable Activity Monitors to Improve Risk Factors Following Gestational Diabetes. Nutrients 2019, 11, 590. [Google Scholar] [CrossRef] [Green Version]
- Cummings, P.; Petitclerc, A.; Moskowitz, J.; Tandon, D.; Zhang, Y.; MacNeill, L.A.; Alshurafa, N.; Krogh-Jespersen, S.; Hamil, J.L.; Nili, A.; et al. Feasibility of Passive ECG Bio-sensing and EMA Emotion Reporting Technologies and Acceptability of Just-in-Time Content in a Well-being Intervention, Considerations for Scalability and Improved Uptake. Affect. Sci. 2022, 3, 849–861. [Google Scholar] [CrossRef]
- Ehrlich, S.F.; Maples, J.M.; Barroso, C.S.; Brown, K.C.; Bassett, D.R.; Zite, N.B.; Fortner, K.B. Using a consumer-based wearable activity tracker for physical activity goal setting and measuring steps in pregnant women with gestational diabetes mellitus: Exploring acceptance and validity. BMC Pregnancy Childbirth 2021, 21, 420. [Google Scholar] [CrossRef]
- Galea, J.T.; Ramos, K.; Coit, J.; Friedman, L.E.; Contreras, C.; Dueñas, M.; Hernandez, N.; Muster, C.; Lecca, L.; Gelaye, B. The Use of Wearable Technology to Objectively Measure Sleep Quality and Physical Activity Among Pregnant Women in Urban Lima, Peru: A Pilot Feasibility Study. Matern. Child Health J 2020, 24, 823–828. [Google Scholar] [CrossRef]
- Grym, K.; Niela-Vilén, H.; Ekholm, E.; Hamari, L.; Azimi, I.; Rahmani, A.; Liljeberg, P.; Löyttyniemi, E.; Axelin, A. Feasibility of smart wristbands for continuous monitoring during pregnancy and one month after birth. BMC Pregnancy Childbirth 2019, 19, 34. [Google Scholar] [CrossRef]
- Hasan, S.M.T.; Ahmed, S.I.; Khan, M.A.; Sarker, S.A.; Ahmed, T. Achieving Optimal Gestational Weight Gain, Birth Weight, and Perinatal Outcomes Among Pregnant Women at Risk of Hypertension: Protocol for a Pilot Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e16676. [Google Scholar] [CrossRef]
- Jimah, T.; Borg, H.; Kehoe, P.; Pimentel, P.; Turner, A.; Labbaf, S.; Asgari Mehrabadi, M.; Rahmani, A.M.; Dutt, N.; Guo, Y. A Technology-Based Pregnancy Health and Wellness Intervention (Two Happy Hearts): Case Study. JMIR Form. Res. 2021, 5, e30991. [Google Scholar] [CrossRef]
- Jimah, T.; Kehoe, P.; Borg, H.; Pimentel, P.; Rahmani, A.; Dutt, N.; Guo, Y. A Micro-Level Analysis of Physiological Responses to COVID-19: Continuous Monitoring of Pregnant Women in California. Front. Public Health 2022, 10, 808763. [Google Scholar] [CrossRef]
- Kawajiri, M.; Nakamura, Y.; Yoshida, M.; Takeishi, Y.; Masaki, A.; Iwasaki, Y.; Sato, S.; Kodera, Y.; Chiba, K.; Yoshizawa, T. Acceptability and Feasibility of a Sedentary Behavior Reduction Program during Pregnancy: A Semi-Experimental Study. Healthcare 2020, 8, 439. [Google Scholar] [CrossRef]
- Ng, A.; Wei, B.; Jain, J.; Ward, E.A.; Tandon, S.D.; Moskowitz, J.T.; Krogh-Jespersen, S.; Wakschlag, L.S.; Alshurafa, N. Predicting the Next-Day Perceived and Physiological Stress of Pregnant Women by Using Machine Learning and Explainability: Algorithm Development and Validation. JMIR mHealth uHealth 2022, 10, e33850. [Google Scholar] [CrossRef]
- Nulty, A.K.; Chen, E.; Thompson, A.L. The Ava bracelet for collection of fertility and pregnancy data in free-living conditions: An exploratory validity and acceptability study. Digit. Health 2022, 8, 20552076221084461. [Google Scholar] [CrossRef]
- Ryu, D.; Kim, D.H.; Price, J.T.; Lee, J.Y.; Chung, H.U.; Allen, E.; Walter, J.R.; Jeong, H.; Cao, J.; Kulikova, E.; et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc. Natl. Acad. Sci. USA 2021, 118, e2100466118. [Google Scholar] [CrossRef]
- Saarikko, J.; Niela-Vilen, H.; Ekholm, E.; Hamari, L.; Azimi, I.; Liljeberg, P.; Rahmani, A.M.; Löyttyniemi, E.; Axelin, A. Continuous 7-Month Internet of Things-Based Monitoring of Health Parameters of Pregnant and Postpartum Women: Prospective Observational Feasibility Study. JMIR Form. Res. 2020, 4, e12417. [Google Scholar] [CrossRef]
- Sarhaddi, F.; Azimi, I.; Labbaf, S.; Niela-Vilén, H.; Dutt, N.; Axelin, A.; Liljeberg, P.; Rahmani, A.M. Long-Term IoT-Based Maternal Monitoring: System Design and Evaluation. Sensors 2021, 21, 2281. [Google Scholar] [CrossRef]
- Souza, R.T.; Cecatti, J.G.; Mayrink, J.; Galvão, R.B.; Costa, M.L.; Feitosa, F.; Rocha Filho, E.; Leite, D.F.; Vettorazzi, J.; Tedesco, R.P.; et al. Identification of earlier predictors of pregnancy complications through wearable technologies in a Brazilian multicentre cohort: Maternal Actigraphy Exploratory Study I (MAES-I) study protocol. BMJ Open 2019, 9, e023101. [Google Scholar] [CrossRef] [Green Version]
- Maggioni, C.; Cornélissen, G.; Otsuka, K.; Halberg, F.; Consonni, D.; Nicolini, U. Circadian rhythm of maternal blood pressure and fetal growth. Biomed. Pharmacother. 2005, 59 (Suppl. S1), S86–S91. [Google Scholar] [CrossRef] [Green Version]
- Kominiarek, M.A.; Balmert, L.C.; Tolo, H.; Grobman, W.; Simon, M. A feasibility study of activity tracking devices in pregnancy. BMC Pregnancy Childbirth 2019, 19, 401. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019, 11, 1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2022, 10, 67. [Google Scholar]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Riela, P.M.; Guarnera, L.; Battiato, S.; Agodi, A. Development of a Web-App for the Ecological Momentary Assessment of Dietary Habits among College Students: The HEALTHY-UNICT Project. Nutrients 2022, 14, 330. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M. A Systematic Review of Ecological Momentary Assessment of Diet: Implications and Perspectives for Nutritional Epidemiology. Nutrients 2019, 11, 2696. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.; Burnett, W.; Chung, J.W.; Bao, Z. Bring on the bodyNET. Nature 2017, 549, 328–330. [Google Scholar] [CrossRef] [Green Version]
- Maugeri, A.; Barchitta, M.; Agodi, A. Using Google Trends to Predict COVID-19 Vaccinations and Monitor Search Behaviours about Vaccines: A Retrospective Analysis of Italian Data. Vaccines 2022, 10, 119. [Google Scholar] [CrossRef]
- Magnano San Lio, R.; Maugeri, A.; La Rosa, M.C.; Cianci, A.; Panella, M.; Giunta, G.; Agodi, A.; Barchitta, M. The Impact of Socio-Demographic Factors on Breastfeeding: Findings from the “Mamma & Bambino” Cohort. Medicina 2021, 57, 103. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Giunta, G.; Cianci, A.; Agodi, A. Vaccination Status of Mothers and Children from the ‘Mamma & Bambino’ Cohort. Vaccines 2021, 9, 168. [Google Scholar]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef] [Green Version]
- Bjelica, D.; Bjelica, A.; Despotović-Zrakić, M.; Radenković, B.; Barać, D.; Đogatović, M. Designing an IT Ecosystem for Pregnancy Care Management Based on Pervasive Technologies. Healthcare 2020, 9, 12. [Google Scholar] [CrossRef]
- Li, J.; Silvera-Tawil, D.; Varnfield, M.; Hussain, M.S.; Math, V. Users’ Perceptions Toward mHealth Technologies for Health and Well-being Monitoring in Pregnancy Care: Qualitative Interview Study. JMIR Form. Res. 2021, 5, e28628. [Google Scholar] [CrossRef]
- Materia, F.T.; Smyth, J.M.; Heron, K.E.; Hillemeier, M.; Feinberg, M.E.; Fonzi, P.; Symons Downs, D. Preconceptional health behavior change in women with overweight and obesity: Prototype for SMART strong healthy women intervention. Mhealth 2018, 4, 24. [Google Scholar] [CrossRef]
- Runkle, J.; Sugg, M.; Boase, D.; Galvin, S.L.; Coulson, C. Use of wearable sensors for pregnancy health and environmental monitoring: Descriptive findings from the perspective of patients and providers. Digit. Health 2019, 5, 2055207619828220. [Google Scholar] [CrossRef] [Green Version]
- Schramm, K.; Grassl, N.; Nees, J.; Hoffmann, J.; Stepan, H.; Bruckner, T.; Haun, M.W.; Maatouk, I.; Haist, M.; Schott, T.C.; et al. Women’s Attitudes Toward Self-Monitoring of Their Pregnancy Using Noninvasive Electronic Devices: Cross-Sectional Multicenter Study. JMIR mHealth uHealth 2019, 7, e11458. [Google Scholar] [CrossRef] [Green Version]

| First Author and Year of Publication | Country | Type of Study | Population | Wearable Design | Feature/s Captured | Main Findings |
|---|---|---|---|---|---|---|
| Lai et al., 2018 [12] | UK | Validation study | 44 pregnant women between 25–36 weeks of gestation | Combination of accelerometers and bespoke acoustic sensors | Foetal movements | The device was able to discriminate startle movements from other forms of activity, and can effectively eliminate artefacts due to maternal movement |
| Liang et al., 2021 [13] | China | Validation study | 4 pregnant women | Accelerometers | Foetal movements | The orthogonal matching pursuit algorithm was more effective than the adaptive filtering algorithm in identifying foetal movement signals |
| Liang et al., 2022 [14] | China | Comparative study | Publicly available dataset of 16 pregnant women | Accelerometers | Foetal movements | Compared with 8 existing methods for foetal movement signal recognition, the proposed method had better accuracy and robustness |
| Mesbah et al., 2021 [15] | Australia | Validation study | 21 pregnant women with gestational age of at least 30 weeks | Tri-axial accelerometers | Foetal movements | The best performance was achieved by Bagging classifier algorithm, with random forest as its basis classifier |
| Mhajna et al., 2020 [16] | USA | Prospective, open-label, multicentre study | 147 pregnant women with a mean gestational age of 37.7 weeks | Self-administered device consisting of 8 electrical sensors and 4 acoustic sensors | Foetal heart rate and maternal heart rate | Foetal heart rate measurements were highly correlated with cardiotocography. Maternal heart rate measured with the device was also highly correlated with that measured using cardiotocography |
| Mhajna et al., 2022 [17] | USA | Two separate prospective, comparative, open-label, multicentre studies | 41 pregnant women with a mean gestational age of 38.8 weeks | Self-administered device consisting of 8 electrical sensors and 4 acoustic sensors | Uterine activity | In both groups (intrapartum and antepartum), the device had better sensitivity than tocodynamometry |
| Nguyen et al., 2021 [18] | USA | Case-control study | 12 pregnant women with gestational age greater than 28 weeks (5 with pregnancy complications) | Near-Infrared Spectroscopy device | Placental oxygenation | Women with maternal pregnancy complications reported lower placental oxygenation level than those with uncomplicated pregnancy |
| Yuan et al., 2019 [19] | China | Validation study | Maternal abdominal signal generator developed to simulate the abdominal surface signal of a pregnant woman | Foetal electrocardiogram collector with five electrodes | Foetal heart rate | The proposed system may be feasible for non-invasive, real-time monitoring of foetal electrocardiogram |
| Zhang et al., 2022 [20] | China | Validation study | 3 pregnant women | Foetal electrocardiogram monitoring system with three electrodes | Foetal heart rate | The proposed system had a promising application in foetal health monitoring |
| First Author and Year of Publication | Country | Type of Study | Population | Wearable Design | Feature/s Captured |
|---|---|---|---|---|---|
| Atzmon et al., 2020 [21] | Israel | Prospective study | 81 pregnant women at 37–42 gestational weeks | Wristband photoplethysmography monitoring device | Cardiac output, blood pressure, stroke volume, systemic vascular resistance, heart rate |
| Cai et al., 2019 [22] | Singapore | Protocol for a prospective study | 408 women at <12 weeks of gestation | Wristband activity tracker | Step count |
| Chen et al., 2022 [23] | Taiwan | Randomized Controlled Trial | 92 pregnant women assigned to the intervention and control groups | Wristband activity tracker | Step count |
| Chen et al., 2022b [24] | China | Prospective study | 197 pregnant women at 10–14 gestational weeks | Wristband activity tracker | Objective physical activity |
| Cheung et al., 2019 [25] | Australia | Randomised Controlled Trial | 60 pregnant women with gestational diabetes mellitus assigned to the intervention and control groups | Wristband activity tracker integrated with text-messaging | Objective physical activity |
| Cummings et al., 2022 [26] | USA | Randomized Controlled Trial | 99 pregnant women at 18–22 gestational weeks assigned to the intervention and control group | Body-conforming flexible electrocardiograph sensors | Heart rate |
| Ehrlich et al., 2021 [27] | USA | Validation study | 15 pregnant women with gestational diabetes mellitus and a mean gestational age of 32.8 weeks | Wristband activity tracker | Step count |
| Galea et al., 2020 [28] | Perú | Feasibility Study | 13 pregnant women with a mean gestational age of 22 weeks | Wristband activity tracker | Step count and sleep characteristics |
| Grym et al., 2019 [29] | Finland | Prospective study | 20 pregnant women at a median of 12.9 weeks of gestation | Wristband activity tracker | Step count, used calories, heart rate, stairs climbed, intensity of physical activity, total hours of sleep, sleep levels, sleep movements |
| Hasan et al., 2020 [30] | Bangladesh | Protocol for a Pilot Randomized Controlled Trial | 70 pregnant women assigned to the intervention and control group | Wristband blood pressure monitoring device | Blood pressure |
| Jimah et al., 2021 [31] | USA | Case study | A pregnant woman at 33 weeks of gestation | Finger-based health tracker | Resting heart rate, resting heart rate variability, sleep, and physical activity |
| Jimah et al., 2022 [32] | USA | Case study | 2 pregnant women with COVID-19 | Finger-based health tracker | Resting heart rate, resting heart rate variability, sleep, and physical activity |
| Kawajiri et al., 2020 [33] | Japan | Semi-Experimental Study | 56 pregnant women in the intervention group compared with an historical control group | Wristband activity tracker | Objective physical activity and sedentary behaviour |
| Kominiarek et al., 2019 [41] | USA | Feasibility Study | 25 pregnant women at <16 weeks of gestation | Wristband activity tracker | Objective physical activity |
| Nulty et al., 2022 [35] | USA | Validation study | 5 pregnant women | Wristband activity tracker | Step count, used calories, heart rate, stairs climbed, intensity of physical activity, total hours of sleep, sleep levels, sleep movements |
| Ryu et al., 2021 [36] | USA and Zambia | Field trial | 576 pregnant women at 25–41 weeks of gestation | Maternal–foetal sensor system based on three chest, limb, and abdominal sensors | Maternal heart rate, respiratory rate, central temperature, SpO2, peripheral temperature, foetal heart rate, uterine contraction |
| Saarikko et al., 2020 [37] | Finland | Prospective study | 20 pregnant women at ≤15 of weeks of gestation | Wristband activity tracker | Resting heart rate, resting heart rate variability, sleep, and physical activity |
| Sarhaddi et al., 2021 [38] | Finland | Prospective study | 28 pregnant women at 12–15 gestational weeks | Wristband activity tracker | Resting heart rate, resting heart rate variability, sleep, and physical activity |
| Souza et al., 2019 [39] | Brazil | Protocol for a prospective study | 400 pregnant women at 19–21 weeks of gestation | Wristband activity tracker | Objective physical activity and sleep pattern |
| Maggioni et al., 2005 [40] | USA | Prospective study | 52 pregnant women during the third trimester | Automated wearable device | Blood pressure |
| Ng et al., 2022 [34] | USA | Prospective study | 16 pregnant women at 10–18 weeks of gestation | Body-conforming flexible wearable sensor | Heart rate, heart rate variability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, A.; Barchitta, M.; Agodi, A. How Wearable Sensors Can Support the Research on Foetal and Pregnancy Outcomes: A Scoping Review. J. Pers. Med. 2023, 13, 218. https://doi.org/10.3390/jpm13020218
Maugeri A, Barchitta M, Agodi A. How Wearable Sensors Can Support the Research on Foetal and Pregnancy Outcomes: A Scoping Review. Journal of Personalized Medicine. 2023; 13(2):218. https://doi.org/10.3390/jpm13020218
Chicago/Turabian StyleMaugeri, Andrea, Martina Barchitta, and Antonella Agodi. 2023. "How Wearable Sensors Can Support the Research on Foetal and Pregnancy Outcomes: A Scoping Review" Journal of Personalized Medicine 13, no. 2: 218. https://doi.org/10.3390/jpm13020218
APA StyleMaugeri, A., Barchitta, M., & Agodi, A. (2023). How Wearable Sensors Can Support the Research on Foetal and Pregnancy Outcomes: A Scoping Review. Journal of Personalized Medicine, 13(2), 218. https://doi.org/10.3390/jpm13020218








