Angiotensin Receptor Blocker Associated with a Decreased Risk of Lung Cancer: An Updated Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusive and Exclusive Criteria
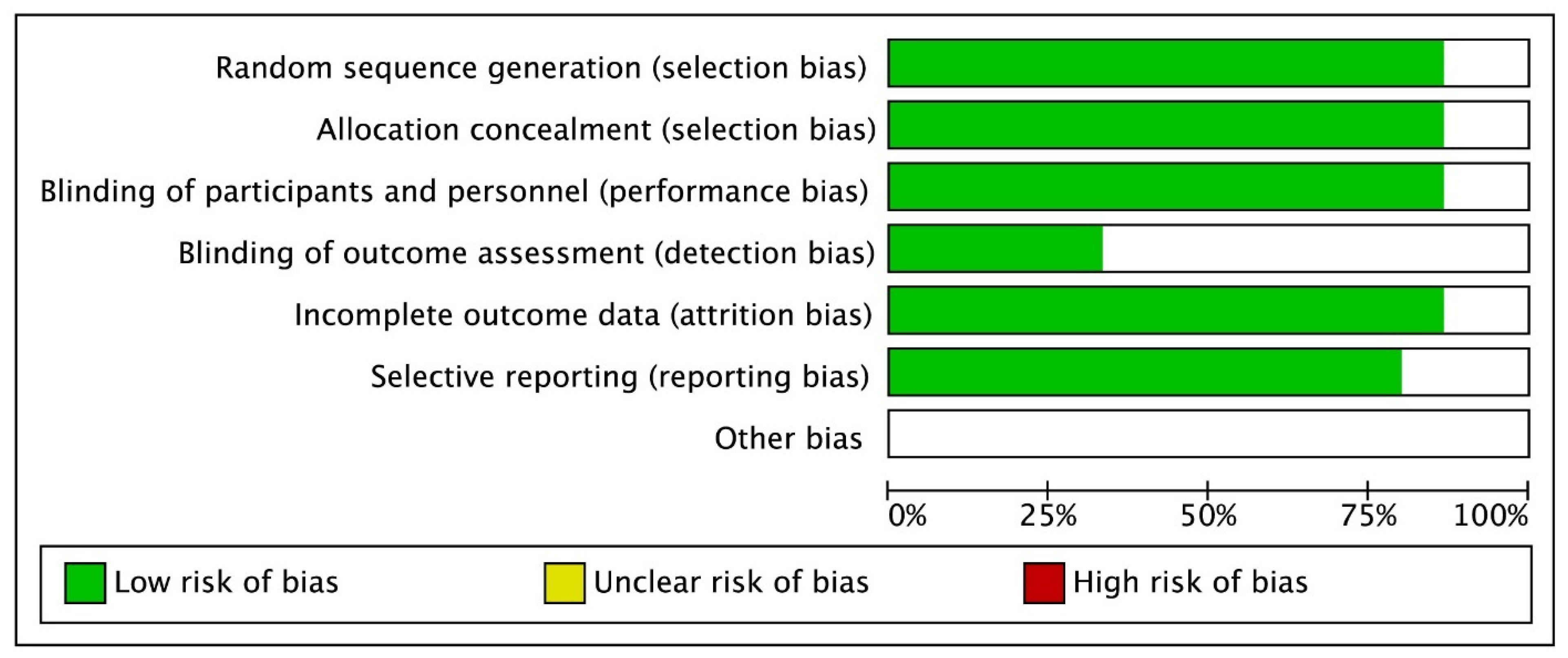
2.3. Quality Evaluation and Statistical Analysis
3. Results
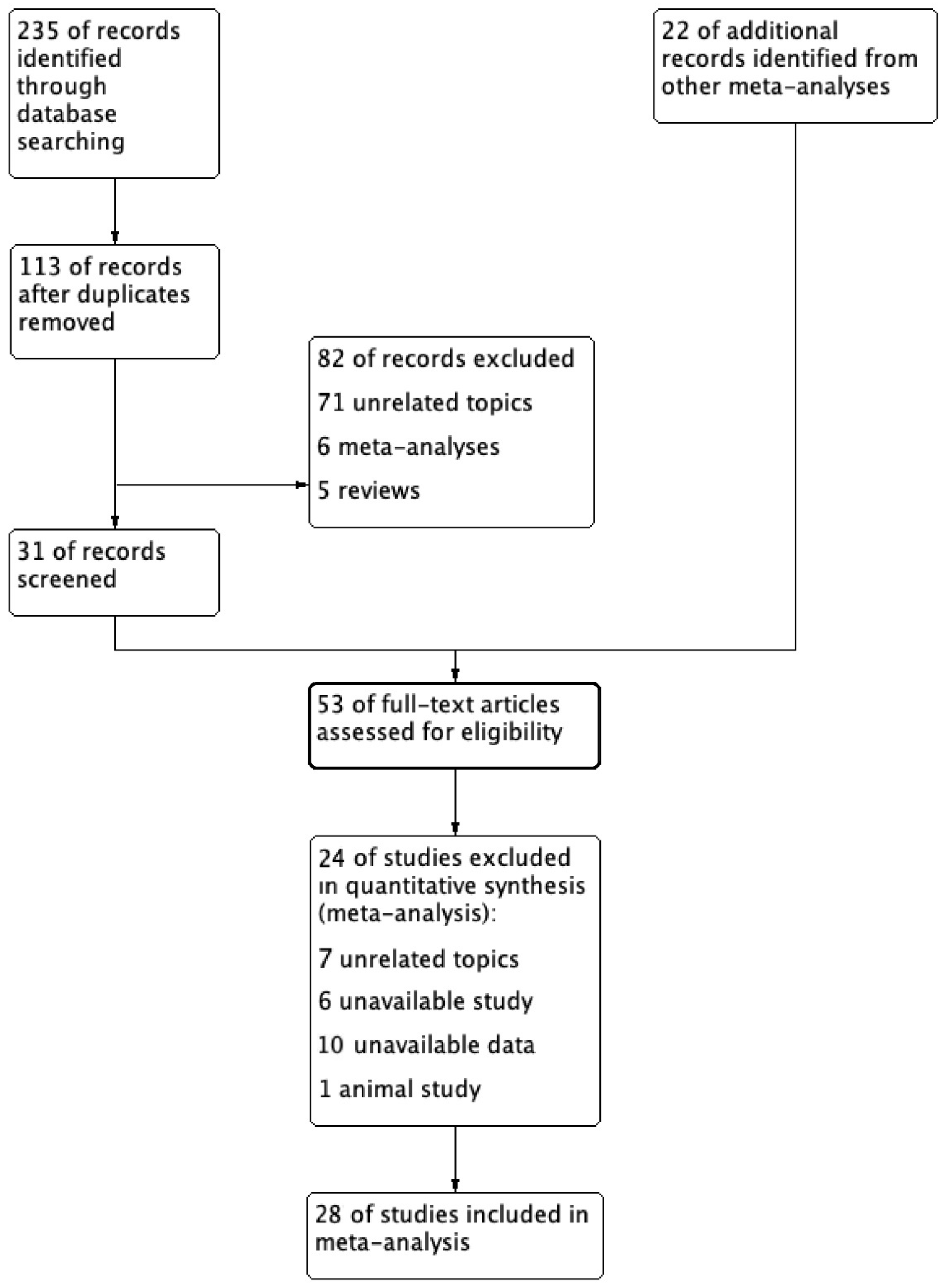
3.1. Literature Selection Results and Characteristics of the Included Studies
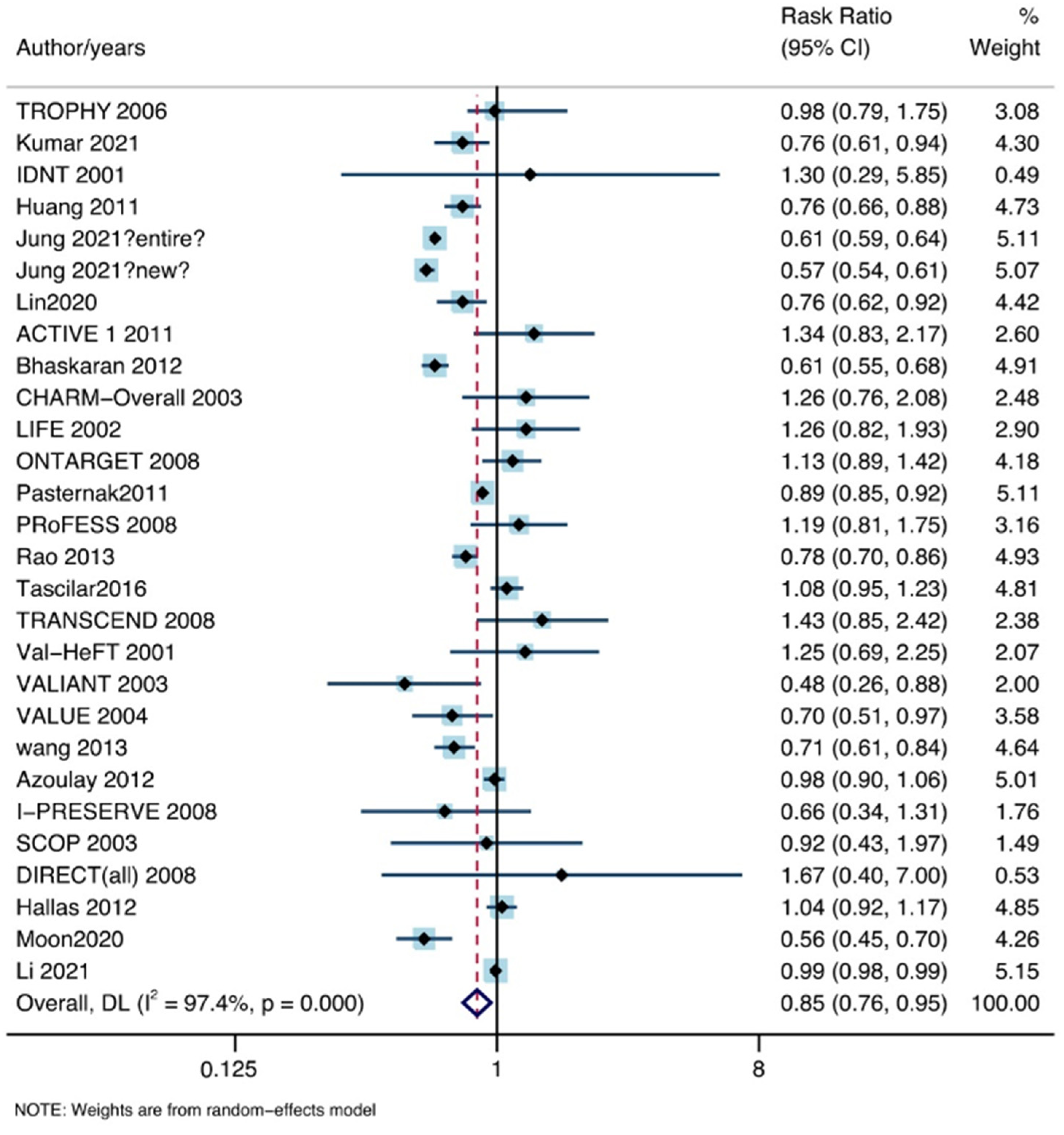
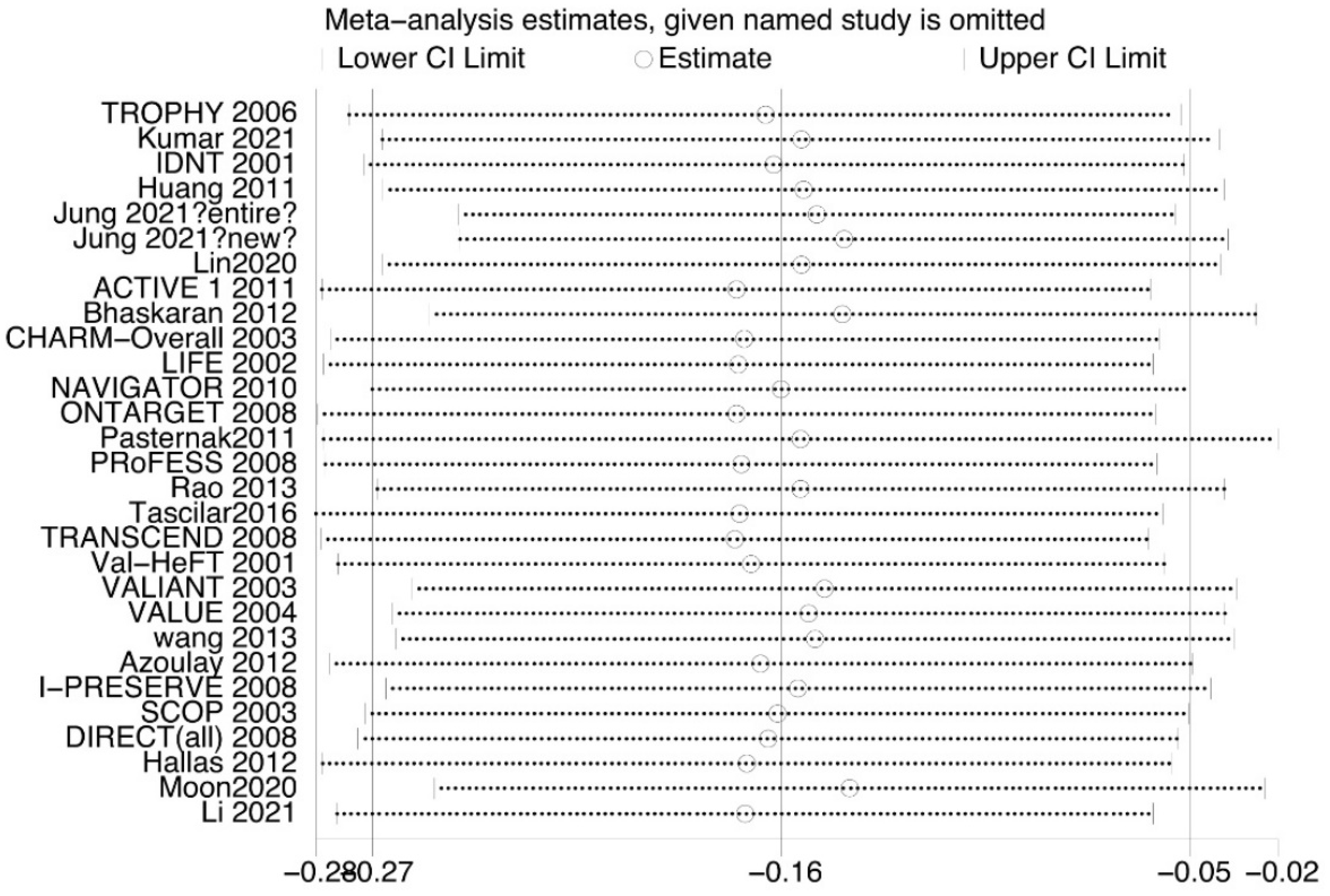
3.2. Overall Analysis
3.3. Subgroup Analysis by Study Type
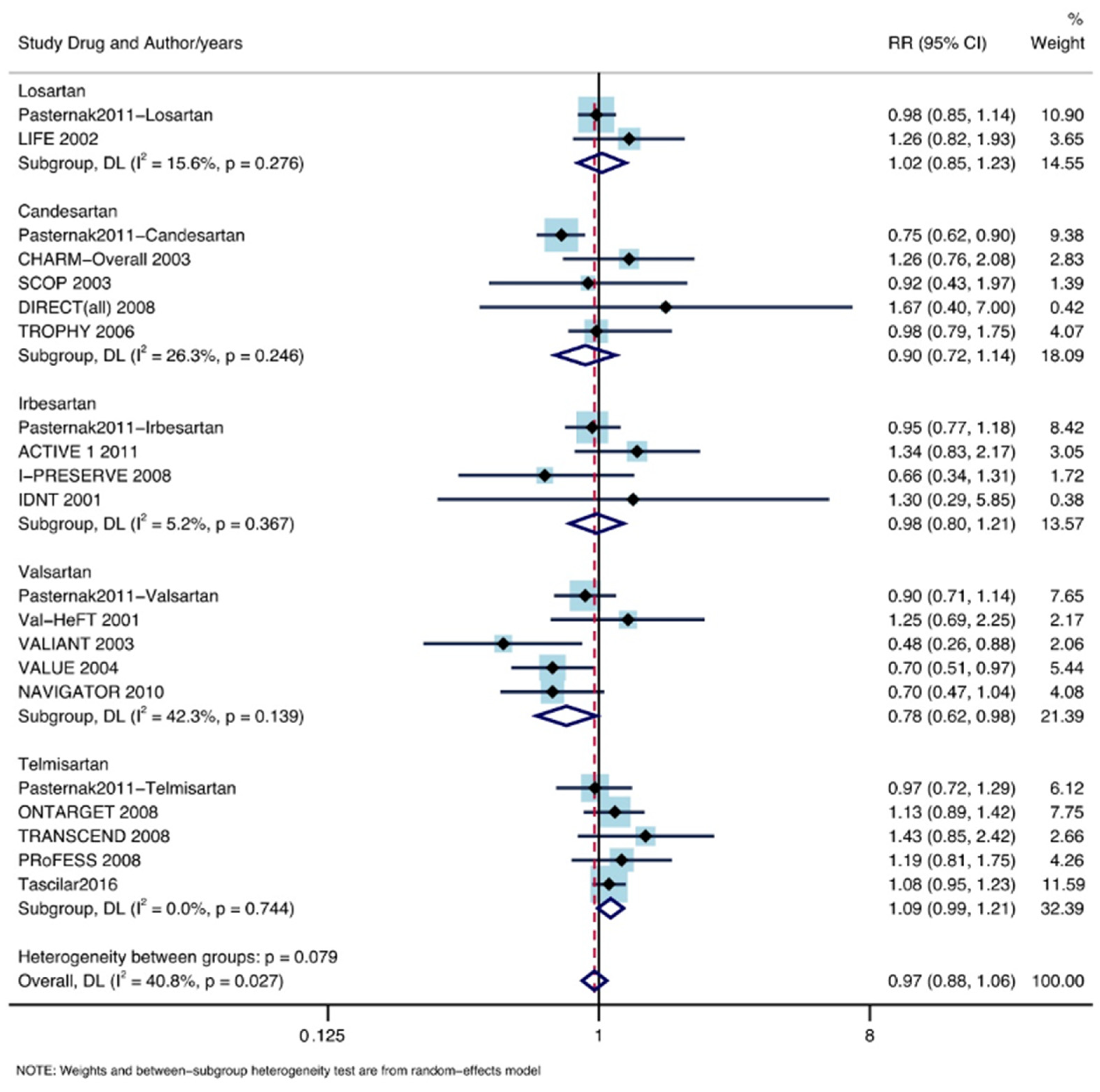
3.4. Subgroup Analysis According to ARB Drugs
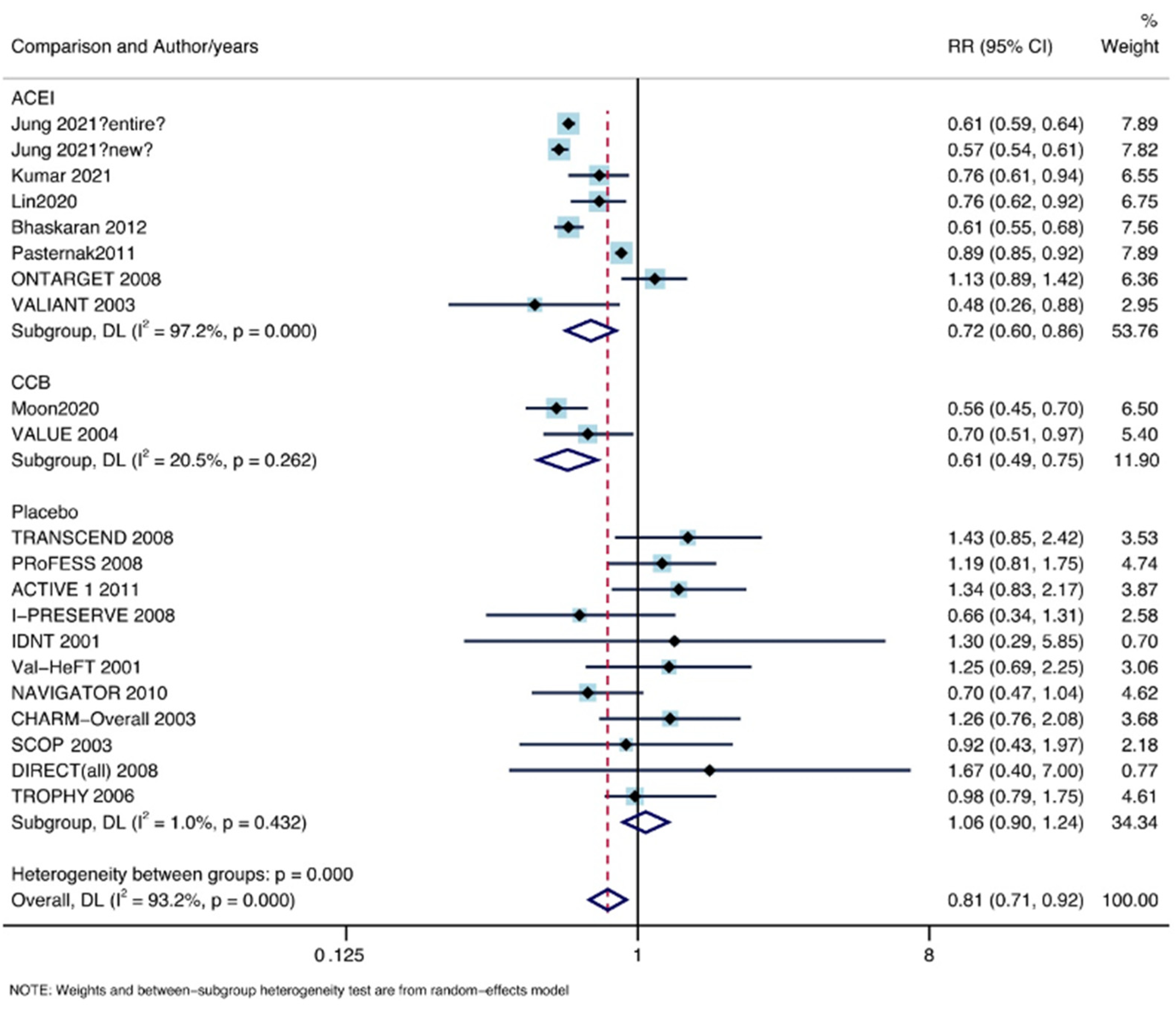
3.5. Subgroup Analysis by Comparison Object
3.6. Subgroup Analysis by Continent
3.7. Subgroup Analysis by Main Race
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escobar, E.; Rodríguez-Reyna, T.S.; Arrieta, O.; Sotelo, J. Angiotensin II, cell proliferation and angiogenesis regulator: Biologic and therapeutic implications in cancer. Curr. Vasc. Pharmacol. 2004, 2, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, S.R.; Zhang, C.H.; He, Y.L.; Zhan, W.H.; Wu, H.; Peng, J.-J. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers on lymphangiogenesis of gastric cancer in a nude mouse model. Chin. Med. J. 2008, 121, 2167–2171. [Google Scholar] [CrossRef]
- Nakamura, K.; Kiniwa, Y.; Okuyama, R. CCL5 production by fibroblasts through a local renin-angiotensin system in malignant melanoma affects tumor immune responses. J. Cancer Res. Clin. Oncol. 2021, 147, 1993–2001. [Google Scholar] [CrossRef]
- Datzmann, T.; Fuchs, S.; Andree, D.; Hohenstein, B.; Schmitt, J.; Schindler, C. Systematic review and meta-analysis of randomised controlled clinical trial evidence refutes relationship between pharmacotherapy with angiotensin-receptor blockers and an increased risk of cancer. Eur. J. Intern. Med. 2019, 64, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mc Menamin Ú, C.; Murray, L.J.; Cantwell, M.M.; Hughes, C.M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: A systematic review. Cancer Causes Control 2012, 23, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, I.; Debanne, S.M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010, 11, 627–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ARB Trialists Collaboration. Effects of telmisartan, irbesartan, Valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J. Hypertens. 2011, 29, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Jung, M.H.; Lee, J.H.; Lee, C.J.; Shin, J.H.; Kang, S.H.; Kwon, C.H.; Kim, D.; Kim, W.; Kim, H.L.; Kim, H.M.; et al. Effect of angiotensin receptor blockers on the development of cancer: A nationwide cohort study in Korea. J. Clin. Hypertens. 2021, 23, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, V.; Murlidhar, F.; Fatima, A.; Jahangir, M.; Khalid, D.; Memon, M.K.; Memon, S.; Kumar, B. Comparison Between Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers for Incidence of Lung Cancer: A Retrospective Study. Cureus 2021, 13, e14788. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Lin, C.L.; Lin, C.C.; Hsu, W.H.; Lin, C.D.; Wang, I.K.; Hsu, C.Y.; Kao, C.H. Association between Angiotensin-Converting Enzyme Inhibitors and Lung Cancer-A Nationwide, Population-Based, Propensity Score-Matched Cohort Study. Cancers 2020, 12, 747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.; Lee, H.Y.; Jang, J.; Park, S.K. Association Between Angiotensin II Receptor Blockers and the Risk of Lung Cancer Among Patients With Hypertension From the Korean National Health Insurance Service-National Health Screening Cohort. J. Prev. Med. Public Health 2020, 53, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Douglas, I.; Evans, S.; van Staa, T.; Smeeth, L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 2012, 344, e2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Chan, W.-L.; Chen, Y.-C.; Chen, T.-J.; Lin, S.-J.; Chen, J.-W.; Leu, H.-B. Angiotensin II Receptor Blockers and Risk of Cancer in Patients With Systemic Hypertension. Am. J. Cardiol. 2011, 107, 1028–1033. [Google Scholar] [CrossRef]
- Rao, G.A.; Mann, J.R.; Shoaibi, A.; Pai, S.G.; Bottai, M.; Sutton, S.S.; Haddock, K.S.; Bennett, C.L.; Hebert, J.R. Angiotensin receptor blockers: Are they related to lung cancer? J. Hypertens. 2013, 31, 1669–1675. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Liu, C.-J.; Chao, T.-F.; Huang, C.-M.; Wu, C.-H.; Chen, T.-J.; Chiang, C.-E. Long-term use of angiotensin II receptor blockers and risk of cancer: A population-based cohort analysis. Int. J. Cardiol. 2013, 167, 2162–2166. [Google Scholar] [CrossRef]
- Tascilar, K.; Azoulay, L.; Dell’Aniello, S.; Bartels, D.B.; Suissa, S. The Use of Telmisartan and the Incidence of Cancer. Am. J. Hypertens. 2016, 29, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, B.; Svanström, H.; Callréus, T.; Melbye, M.; Hviid, A. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011, 123, 1729–1736. [Google Scholar] [CrossRef]
- Heagerty, A.; Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar]
- Yusuf, S.; Teo, K.; Anderson, C.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: A randomised controlled trial. Lancet 2008, 372, 1174–1183. [Google Scholar]
- Yusuf, S.; Diener, H.-C.; Sacco, R.L.; Cotton, D.; Ôunpuu, S.; Lawton, W.A.; Palesch, Y.; Martin, R.H.; Albers, G.W.; Bath, P.; et al. Telmisartan to Prevent Recurrent Stroke and Cardiovascular Events. N. Engl. J. Med. 2008, 359, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- ACTIVE I Investigators; Yusuf, S.; Healey, J.S.; Pogue, J.; Chrolavicius, S.; Flather, M.; Hart, R.G.; Hohnloser, S.H.; Joyner, C.D.; A Pfeffer, M.; et al. Irbesartan in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 364, 928–938. [Google Scholar]
- Massie, B.M.; Carson, P.E.; McMurray, J.J.; Komajda, M.; McKelvie, R.; Zile, M.R.; Anderson, S.; Donovan, M.; Iverson, E.; Staiger, C.; et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N. Engl. J. Med. 2008, 359, 2456–2467. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.J.; Hunsicker, L.G.; Clarke, W.R.; Berl, T.; Pohl, M.A.; Lewis, J.B.; Ritz, E.; Atkins, R.C.; Rohde, R.; Raz, I.; et al. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. N. Engl. J. Med. 2001, 345, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohn, J.N.; Tognoni, G. A randomized trial of the angiotensin-receptor blocker Valsartan in chronic heart failure. N. Engl. J. Med. 2001, 345, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; McMurray, J.J.; Velazquez, E.J.; Rouleau, J.-L.; Køber, L.; Maggioni, A.P.; Solomon, S.D.; Swedberg, K.; Van de Werf, F.; White, H.; et al. Valsartan, Captopril, or Both in Myocardial Infarction Complicated by Heart Failure, Left Ventricular Dysfunction, or Both. N. Engl. J. Med. 2003, 349, 1893–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julius, S.; E Kjeldsen, S.; Weber, M.; Brunner, H.R.; Ekman, S.; Hansson, L.; Hua, T.; Laragh, J.; McInnes, G.T.; Mitchell, L.; et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on Valsartan or amlodipine: The VALUE randomised trial. Lancet 2004, 363, 2022–2031. [Google Scholar] [CrossRef]
- Mcmurray, J.J.; Holman, R.R.; Haffner, S.M.; A Bethel, M.; Holzhauer, B.; A Hua, T.; Belenkov, Y.; Boolell, M.; Buse, J.B.; Buckley, B.M.; et al. Effect of Valsartan on the Incidence of Diabetes and Cardiovascular Events. N. Engl. J. Med. 2010, 362, 1477–1490. [Google Scholar]
- A Pfeffer, M.; Swedberg, K.; Granger, C.B.; Held, P.; Mcmurray, J.; Michelson, E.L.; Olofsson, B.; Östergren, J.; Yusuf, S.; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet 2003, 362, 759–766. [Google Scholar] [CrossRef]
- Julius, S.; Nesbitt, S.D.; Egan, B.M.; Weber, M.A.; Michelson, E.L.; Kaciroti, N.; Black, H.R.; Grimm, R.H.; Messerli, F.H.; Oparil, S.; et al. Feasibility of Treating Prehypertension with an Angiotensin-Receptor Blocker. N. Engl. J. Med. 2006, 354, 1685–1697. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Porta, M.; Klein, R.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Sjølie, A.K. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet 2008, 372, 1394–1402. [Google Scholar] [CrossRef]
- Sjølie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Chaturvedi, N. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008, 372, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Lithell, H.; Hansson, L.; Skoog, I.; Elmfeldt, D.; Hofman, A.; Olofsson, B.; Trenkwalder, P.; Zanchetti, A. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double-blind intervention trial. J. Hypertens. 2003, 21, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlöf, B.; Devereux, R.B.; E Kjeldsen, S.; Julius, S.; Beevers, G.; de Faire, U.; Fyhrquist, F.; Ibsen, H.; Kristiansson, K.; Lederballe-Pedersen, O.; et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet 2002, 359, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Hallas, J.; Christensen, R.; Andersen, M.; Friis, S.; Bjerrum, L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: A population-based case-control study. Br. J. Clin. Pharmacol. 2012, 74, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Azoulay, L.; Assimes, T.L.; Yin, H.; Bartels, D.B.; Schiffrin, E.L.; Suissa, S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS ONE 2012, 7, e50893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaft, J.E.; Rimner, A.; Weder, W.; Azzoli, C.G.; Kris, M.G.; Cascone, T. Evolution of systemic therapy for stages I-III non-metastatic non-small-cell lung cancer. Nat. Rev. Clin. Oncol. 2021, 18, 547–557. [Google Scholar] [CrossRef]
- Docherty, K.F.; Vaduganathan, M.; Solomon, S.D.; McMurray, J.J.V. Sacubitril/Valsartan: Neprilysin Inhibition 5 Years after PARADIGM-HF. JACC Heart Fail. 2020, 8, 800–810. [Google Scholar] [CrossRef]
- Mcmurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, J.P.; Claggett, B.; Seidelmann, S.B.; Seely, E.W.; Packer, M.; Zile, M.; Rouleau, J.L.; Swedberg, K.; Lefkowitz, M.; Shi, V.C.; et al. Effect of sacubitril/Valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: A post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017, 5, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liang, Z.; Li, J.; Cai, S. Angiotensin receptor blockers use and the risk of lung cancer: A meta-analysis. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 768–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uemura, H.; Ishiguro, H.; Nakaigawa, N.; Nagashima, Y.; Miyoshi, Y.; Fujinami, K.; Sakaguchi, A.; Kubota, Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: A possibility of tyrosine kinase inhibitor of growth factor. Mol. Cancer Ther. 2003, 2, 1139–1147. [Google Scholar]
- Ren, H.; Du, N.; Feng, J.; Hu, L.-J.; Sun, X.; Sun, H.-B.; Zhao, Y.; Yang, Y.-P. Angiotensin II receptor type 1 blockers suppress the cell proliferation effects of angiotensin II in breast cancer cells by inhibiting AT1R signaling. Oncol. Rep. 2012, 27, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Miyajima, A.; Shirotake, S.; Kikuchi, E.; Hasegawa, M.; Mikami, S.; Oya, M. Ets-1 and hypoxia inducible factor-1α inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate 2009, 70, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Funao, K.; Matsuyama, M.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.-L.; Nakatani, T. Telmisartan as a peroxisome proliferator-activated receptor-γ ligand is a new target in the treatment of human renal cell carcinoma. Mol. Med. Rep. 2009, 2, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, R.; Funao, K.; Matsuyama, M.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.-L.; Nakatani, T. Telmisartan is a potent target for prevention and treatment in human prostate cancer. Oncol. Rep. 1994, 20, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, M.; Funao, K.; Kuratsukuri, K.; Tanaka, T.; Kawahito, Y.; Sano, H.; Chargui, J.; Touraine, J.-L.; Yoshimura, N.; Yoshimura, R. Telmisartan inhibits human urological cancer cell growth through early apoptosis. Exp. Ther. Med. 2010, 1, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujihara, S.; Morishita, A.; Ogawa, K.; Tadokoro, T.; Chiyo, T.; Kato, K.; Kobara, H.; Mori, H.; Iwama, H.; Masaki, T. The angiotensin II type 1 receptor antagonist telmisartan inhibits cell proliferation and tumor growth of esophageal adenocarcinoma via the AMPKα/mTOR pathway in vitro and in vivo. Oncotarget 2016, 8, 8536–8549. [Google Scholar] [CrossRef] [Green Version]
- Samukawa, E.; Fujihara, S.; Oura, K.; Iwama, H.; Yamana, Y.; Tadokoro, T.; Chiyo, T.; Kobayashi, K.; Morishita, A.; Nakahara, M.; et al. Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int. J. Oncol. 2017, 51, 1674–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, Y.; Jung, Y.J. Angiotensin II receptor blockers induce autophagy in prostate cancer cells. Oncol. Lett. 2017, 13, 3579–3585. [Google Scholar] [CrossRef] [Green Version]
- De Araújo Júnior, R.F.; Leitão Oliveira, A.L.; de Melo Silveira, R.F.; de Oliveira Rocha, H.A.; de França Cavalcanti, P.; de Araújo, A.A. Telmisartan induces apoptosis and regulates Bcl-2 in human renal cancer cells. Exp. Biol. Med. 2015, 240, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheduzzaman, M.; Park, S.Y. Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp. Cell Res. 2018, 368, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, B.J.; Cederberg, R.A.; Lee, C.-M.; Firmino, N.S.; Franks, S.E.; Pan, J.; Colpo, N.; Lin, K.-S.; Benard, F.; Bennewith, K.L. Angiotensin II type 1 receptor blocker telmisartan inhibits the development of transient hypoxia and improves tumour response to radiation. Cancer Lett. 2020, 493, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, D.N.; Hofschröer, V.; Nielsen, N.; Seidler, D.G.; Schwab, A.; Stock, C. The Angiotensin II Type 1 Receptor Antagonist Losartan Affects NHE1-Dependent Melanoma Cell Behavior. Cell. Physiol. Biochem. 2018, 45, 2560–2576. [Google Scholar] [CrossRef] [PubMed]




| Author/Years | Study Type | Main Race | Continent | Mean Age | Study Drug | Comparison | QE |
|---|---|---|---|---|---|---|---|
| Jung 2021 (entire) [11] | RS | Yellow | Asia | 56.9 | NA | ACEI | 6 |
| Jung 2021 (new) [11] | RS | Yellow | Asia | 56.5 | NA | ACEI | 6 |
| Kumar 2021 [12] | RS | Yellow | Asia | 49.6 | NA | ACEI | 5 |
| Lin2020 [13] | RS | Yellow | Asia | 58.9 | NA | ACEI | 8 |
| Moon2020 [14] | RS | Yellow | Asia | NA | NA | CCB | 8 |
| Bhaskaran 2012 [15] | RS | White | Europe | 64 | NA | ACEI | 8 |
| Huang 2011 [16] | RS | Yellow | Asia | 58.5 | NA | non-ARB | 5 |
| Rao 2013 [17] | RS | White | America | 63 | NA | non-ARB | 8 |
| wang 2013 [18] | RS | Yellow | Asia | 62 | NA | non-ARB | 7 |
| Tascilar2016 [19] | RS | White | America | 62.5 | Telmisartan | Other ARBs | 7 |
| Pasternak2011 [20] | RS | White | Europe | 64.3 | NA | ACEI | 8 |
| ONTARGET 2008 [21] | RCT | White | America | 66.4 | Telmisartan | Ramipril | - |
| TRANSCEND 2008 [22] | RCT | White | America | 66.9 | Telmisartan | Placebo | - |
| PRoFESS 2008 [23] | RCT | White | America | 66.1 | Telmisartan | Placebo | - |
| ACTIVE 1 2011 [24] | RCT | White | America | 69.5 | Irbesartan | Placebo | - |
| I-PRESERVE 2008 [25] | RCT | White | America | 72 | Irbesartan | Placebo | - |
| IDNT 2001 [26] | RCT | White | America | 59.3 | Irbesartan | Placebo | - |
| Val-HeFT 2001 [27] | RCT | White | America | 62.7 | Valsartan | Placebo | - |
| VALIANT 2003 [28] | RCT | White | America | 64.8 | Valsartan | captopril | - |
| VALUE 2004 [29] | RCT | White | America | 67.3 | Valsartan | Amlodipine | - |
| NAVIGATOR 2010 [30] | RCT | White | America | 63.7 | Valsartan | Placebo | - |
| CHARM-Overall 2003 [31] | RCT | White | America | 65.9 | Candesartan | Placebo | - |
| TROPHY 2006 [32] | RCT | NA | America | 48.5 | Candesartan | Placebo | - |
| DIRECT (all) 2008 [33,34] | RCT | NA | America | NA | Candesartan | Placebo | - |
| SCOP 2003 [35] | RCT | White | America | 76.4 | Candesartan | Placebo | - |
| LIFE 2002 [36] | RCT | White | America | 66.9 | Losartan | Atenolol | - |
| Hallas 2012 [37] | CS | White | Europe | NA | NA | non-ARB | 6 |
| Azoulay 2012 [38] | CS | White | America | 72.4 | NA | non-ARB | 8 |
| Li 2021 [39] | CS | Yellow | Asia | NA | NA | Non-ARB | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wei, L.; Yin, C.; Li, W.; Wan, B. Angiotensin Receptor Blocker Associated with a Decreased Risk of Lung Cancer: An Updated Meta-Analysis. J. Pers. Med. 2023, 13, 243. https://doi.org/10.3390/jpm13020243
Wang Z, Wei L, Yin C, Li W, Wan B. Angiotensin Receptor Blocker Associated with a Decreased Risk of Lung Cancer: An Updated Meta-Analysis. Journal of Personalized Medicine. 2023; 13(2):243. https://doi.org/10.3390/jpm13020243
Chicago/Turabian StyleWang, Zexu, Lingyun Wei, Cheng Yin, Wang Li, and Bing Wan. 2023. "Angiotensin Receptor Blocker Associated with a Decreased Risk of Lung Cancer: An Updated Meta-Analysis" Journal of Personalized Medicine 13, no. 2: 243. https://doi.org/10.3390/jpm13020243




