Predicting a Favorable (mRS 0–2) or Unfavorable (mRS 3–6) Stroke Outcome by Arterial Spin Labeling and Amide Proton Transfer Imaging in Post-Thrombolysis Stroke Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. MRI Study
2.3. Images Analysis
3. Results
3.1. Clinical Characteristics
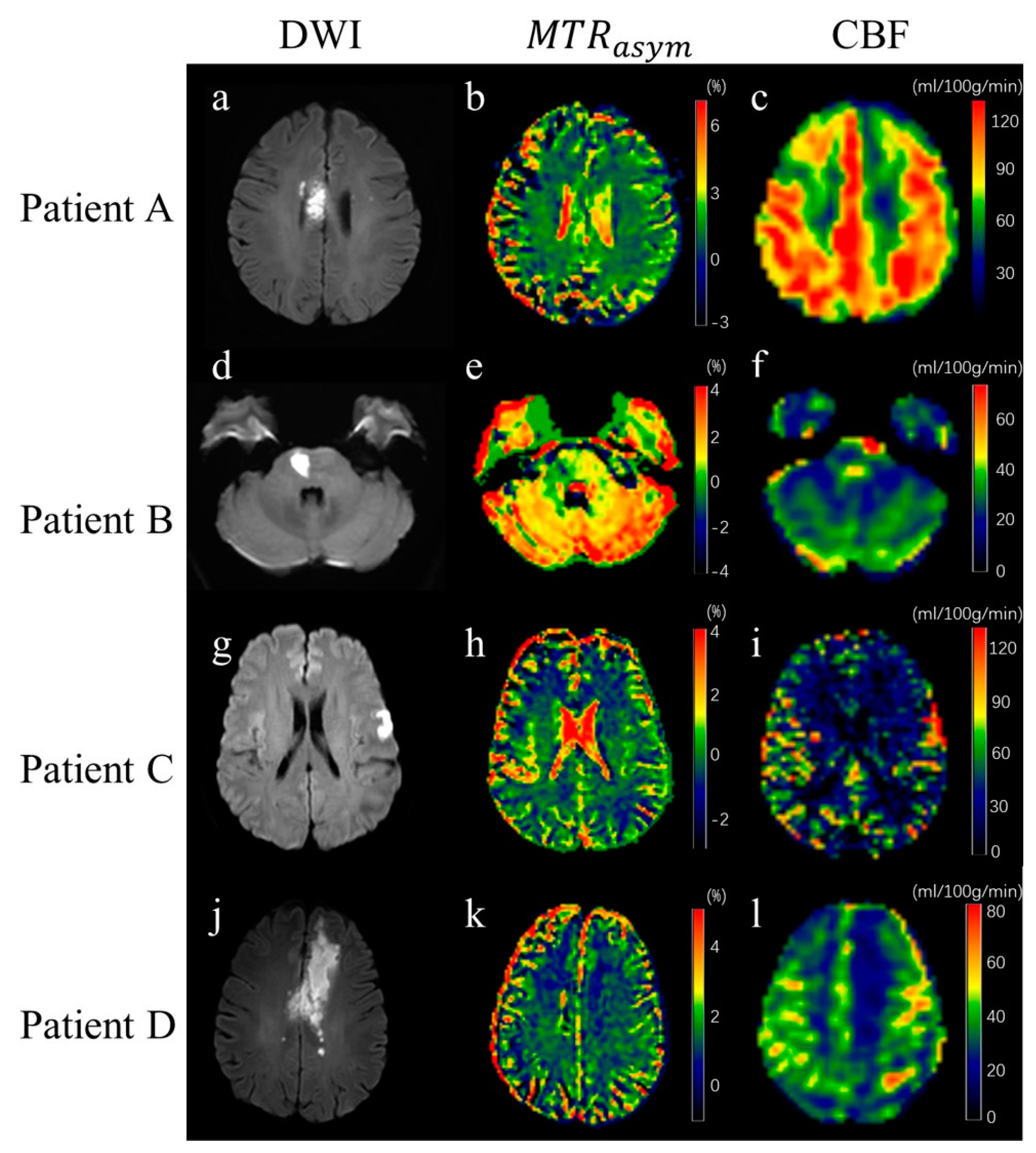
3.2. Image of Stroke Patients
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APT | amide proton transfer |
| ASL | arterial spin labeling |
| CBF | cerebral blood flow |
| CEST | chemical exchange saturation transfer |
| DWI | diffusion-weighted imaging |
| FLAIR | fluid-attenuated inversion recovery |
| LASSO | least absolute shrinkage and selection operator |
| mRS | modified Rankin Score |
| NIHSS | National Institutes of Health Stroke Scale |
| SSFSE | single-shot fast spin echo |
| NOE | Nuclear overhauled enhancement |
| DS | direct saturation |
References
- Boursin, P.; Paternotte, S.; Dercy, B.; Sabben, C.; Maïer, B. Semantics, Epidemiology and Semiology of Stroke. Soins 2018, 63, 24–27. [Google Scholar] [CrossRef]
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, W.; Ma, Y.; Huang, K.; Huang, S.; Chen, Q.; Yun, W.; Xu, G. Early Neurological Deterioration and Hypoperfusion Volume Ratio on Arterial Spin Labeling in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105885. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wu, Y.; Cheung, J.S.; Igarashi, T.; Wu, L.; Zhang, X.; Sun, P.Z. Mapping Tissue Ph in an Experimental Model of Acute Stroke—Determination of Graded Regional Tissue Ph Changes with Non-Invasive Quantitative Amide Proton Transfer Mri. Neuroimage 2019, 191, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Zu, Z.; Afzal, A.; Li, H.; Xie, J.; Gore, J.C. Spin-Lock Imaging of Early Tissue Ph Changes in Ischemic Rat Brain. NMR Biomed. 2018, 31, e3893. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Schmithorst, V.; Panigrahy, A. Arterial Spin Labeling in Pediatric Neuroimaging. Semin. Pediatr. Neurol. 2020, 33, 100799. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and Management of Transient Ischemic Attack and Acute Ischemic Stroke: A Review. Jama 2021, 325, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Heiss, W.D. The Ischemic Penumbra: Correlates in Imaging and Implications for Treatment of Ischemic Stroke. The Johann Jacob Wepfer Award 2011. Cerebrovasc. Dis. 2011, 32, 307–320. [Google Scholar] [CrossRef]
- Sotoudeh, H.; Shafaat, O.; Sotoudeh, E. Misleading Ct Perfusion in Subacute Ischemic Stroke. Emerg. Radiol. 2019, 26, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Shankar, J.; Liebeskind, D.; Terceño, M.; Nael, K.; Demchuk, A.M.; Menon, B.; Dowlatshahi, D.; Leiva-Salinas, C.; Wintermark, M.; et al. From “Time Is Brain” to “Imaging Is Brain”: A Paradigm Shift in the Management of Acute Ischemic Stroke. J. Neuroimaging 2020, 30, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Hurford, R.; Sekhar, A.; Hughes, T.A.T.; Muir, K.W. Diagnosis and Management of Acute Ischaemic Stroke. Pract. Neurol. 2020, 20, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wakhloo, A.K.; Fisher, M. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.J.; Dawson, J.; Walters, M.R.; Lees, K.R. Reliability of the Modified Rankin Scale: A Systematic Review. Stroke 2009, 40, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Kizawa, R.; Sato, T.; Umehara, T.; Komatsu, T.; Omoto, S.; Iguchi, Y. A Case of Epileptic Seizure That Required Differentiation from Hyper-Acute Ischemic Stroke: Usefulness of Comparing Dwi and Flair. Rinsho Shinkeigaku 2021, 61, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Hassen, W.B.; Tisserand, M.; Turc, G.; Charron, S.; Seners, P.; Edjlali, M.; Legrand, L.; Lion, S.; Calvet, D.; Naggara, O.; et al. Comparison between Voxel-Based and Subtraction Methods for Measuring Diffusion-Weighted Imaging Lesion Growth after Thrombolysis. Int. J. Stroke 2016, 11, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Okell, T.W.; Harston, G.W.J.; Chappell, M.A.; Sheerin, F.; Kennedy, J.; Jezzard, P. Measurement of Collateral Perfusion in Acute Stroke: A Vessel-Encoded Arterial Spin Labeling Study. Sci. Rep. 2019, 9, 8181. [Google Scholar] [CrossRef]
- Lin, G.; Zhuang, C.; Shen, Z.; Xiao, G.; Chen, Y.; Shen, Y.; Zong, X.; Wu, R. Apt Weighted Mri as an Effective Imaging Protocol to Predict Clinical Outcome after Acute Ischemic Stroke. Front. Neurol. 2018, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.Y.; Tee, Y.K.; Harston, G.; Leigh, R.; Chappell, M.A. Amide Proton Transfer Imaging in Stroke. NMR Biomed. 2022, e4734. [Google Scholar] [CrossRef]
- Jezzard, P.; Chappell, M.A.; Okell, T.W. Arterial Spin Labeling for the Measurement of Cerebral Perfusion and Angiography. J. Cereb. Blood Flow Metab. 2018, 38, 603–626. [Google Scholar] [CrossRef]
- Cebeci, H.; Durmaz, M.S.; Arslan, S.; Arslan, A.; Tekin, A.F.; Habibi, H.A.; Koylu, R. Diagnostic Utility of Arterial Spin Labeling in Identifying Changes in Brain Perfusion in Patients with Carbon Monoxide Poisoning. Clin. Imaging 2020, 64, 92–96. [Google Scholar] [CrossRef]
- Wazni, W.; Farooq, S.; Cox, J.A.; Southwood, C.; Rozansky, G.; Kodankandath, T.V.; Johnson, V.; Lynch, J.R. Use of Arterial Spin-Labeling in Patients with Aneurysmal Sub-Arachnoid Hemorrhage. J. Vasc. Interv. Neurol. 2019, 10, 10–14. [Google Scholar] [PubMed]
- Buch, K.; Hakimelahi, R.; Locascio, J.J.; Bolar, D.S.; Gonzalez, R.G.; Schaefer, P.W. Clinical Utility of Arterial Spin Labeling Perfusion Images in the Emergency Department for the Work-up of Stroke-Like Symptoms. Neuroradiology 2022, 64, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Tietze, A.; Blicher, J.; Mikkelsen, I.K.; Østergaard, L.; Strother, M.K.; Smith, S.A.; Donahue, M.J. Assessment of Ischemic Penumbra in Patients with Hyperacute Stroke Using Amide Proton Transfer (Apt) Chemical Exchange Saturation Transfer (Cest) Mri. NMR Biomed. 2014, 27, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Adany, P.; Choi, I.Y. Imaging Based Magnetic Resonance Spectroscopy (Mrs) Localization for Quantitative Neurochemical Analysis and Cerebral Metabolism Studies. Anal. Biochem. 2017, 529, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Suh, D.C.; Cho, S.H.; Sheen, J.J.; Lee, D.H.; Kim, J.S. Subacute Endovascular Recanalization of Symptomatic Cerebral Artery Occlusion: A Propensity Score-Matched Analysis. J. Neurointerv. Surg. 2018, 10, 536–542. [Google Scholar] [CrossRef]
- Lou, M.; Ding, J.; Hu, B.; Zhang, Y.; Li, H.; Tan, Z.; Wan, Y.; Xu, A.D. Chinese Stroke Association Guidelines for Clinical Management of Cerebrovascular Disorders: Executive Summary and 2019 Update on Organizational Stroke Management. Stroke Vasc. Neurol. 2020, 5, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, D.Y.; Sohn, M.K.; Lee, J.; Lee, S.G.; Shin, Y.I.; Kim, S.Y.; Oh, G.J.; Lee, Y.H.; Lee, Y.S.; et al. Determining the Cut-Off Score for the Modified Barthel Index and the Modified Rankin Scale for Assessment of Functional Independence and Residual Disability after Stroke. PLoS ONE 2020, 15, e0226324. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (Eso) Guidelines on Intravenous Thrombolysis for Acute Ischaemic Stroke. Eur. Stroke J. 2021, 6, I-lxii. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.; Pomper, M.G.; Golay, X.; Zhou, J. Amide Proton Transfer Imaging of Human Brain Tumors at 3t. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef]
- Gevers, S.; van Osch, M.J.; Bokkers, R.P.; Kies, D.A.; Teeuwisse, W.M.; Majoie, C.B.; Hendrikse, J.; Nederveen, A.J. Intra- and Multicenter Reproducibility of Pulsed, Continuous and Pseudo-Continuous Arterial Spin Labeling Methods for Measuring Cerebral Perfusion. J. Cereb. Blood Flow. Metab. 2011, 31, 1706–1715. [Google Scholar] [CrossRef]
- Zöllner, J.P.; Hattingen, E.; Singer, O.C.; Pilatus, U. Changes of Ph and Energy State in Subacute Human Ischemia Assessed by Multinuclear Magnetic Resonance Spectroscopy. Stroke 2015, 46, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Harston, G.W.; Tee, Y.K.; Blockley, N.; Okell, T.W.; Thandeswaran, S.; Shaya, G.; Sheerin, F.; Cellerini, M.; Payne, S.; Jezzard, P.; et al. Identifying the Ischaemic Penumbra Using Ph-Weighted Magnetic Resonance Imaging. Brain 2015, 138 Pt 1, 36–42. [Google Scholar] [CrossRef]
- Foo, L.S.; Harston, G.; Mehndiratta, A.; Yap, W.S.; Hum, Y.C.; Lai, K.W.; Mukari, S.A.M.; Zaki, F.M.; Tee, Y.K. Clinical Translation of Amide Proton Transfer (Apt) Mri for Ischemic Stroke: A Systematic Review (2003–2020). Quant. Imaging Med. Surg. 2021, 11, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.J.; Yan, C.G.; Zhao, Z.G.; Bai, Q.K. Prognostic Value of Diffusion-Weighted Imaging (Dwi) Apparent Diffusion Coefficient (Adc) in Patients with Hyperacute Cerebral Infarction Receiving Rt-Pa Intravenous Thrombolytic Therapy. Med. Sci. Monit. 2016, 22, 4438–4445. [Google Scholar] [CrossRef]
- Sun, P.Z.; Cheung, J.S.; Wang, E.; Lo, E.H. Association between Ph-Weighted Endogenous Amide Proton Chemical Exchange Saturation Transfer Mri and Tissue Lactic Acidosis During Acute Ischemic Stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, W.; Jiang, S.; Zhang, Y.; Heo, H.Y.; Wang, X.; Peng, Y.; Wang, J.; Zhou, J. Amide Proton Transfer-Weighted Mri Detection of Traumatic Brain Injury in Rats. J. Cereb. Blood Flow Metab. 2017, 37, 3422–3432. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, I.Y.; Lu, D.; Manderville, E.; Lo, E.H.; Zheng, H.; Sun, P.Z. Ph-Sensitive Amide Proton Transfer Effect Dominates the Magnetization Transfer Asymmetry Contrast During Acute Ischemia-Quantification of Multipool Contribution to in Vivo Cest Mri. Magn. Reson. Med. 2018, 79, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.S.; Larkin, J.R.; Sutherland, B.A.; Ray, K.J.; Yap, W.S.; Hum, Y.C.; Lai, K.W.; Manan, H.A.; Sibson, N.R.; Tee, Y.K. Study of Common Quantification Methods of Amide Proton Transfer Magnetic Resonance Imaging for Ischemic Stroke Detection. Magn. Reson. Med. 2021, 85, 2188–2200. [Google Scholar] [CrossRef]
- Tee, Y.K.; Harston, G.W.; Blockley, N.; Okell, T.W.; Levman, J.; Sheerin, F.; Cellerini, M.; Jezzard, P.; Kennedy, J.; Payne, S.J.; et al. Comparing Different Analysis Methods for Quantifying the Mri Amide Proton Transfer (Apt) Effect in Hyperacute Stroke Patients. NMR Biomed. 2014, 27, 1019–1029. [Google Scholar] [CrossRef]
- Aracki-Trenkic, A.; Law-Ye, B.; Radovanovic, Z.; Stojanov, D.; Dormont, D.; Pyatigorskaya, N. Asl Perfusion in Acute Ischemic Stroke: The Value of Cbf in Outcome Prediction. Clin. Neurol. Neurosurg. 2020, 194, 105908. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular Ph. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, G.; Avitsian, R. Revisiting Ischemia after Brain Injury: Oxygen May Not Be the Only Problem. J. Neurosurg. Anesthesiol. 2020, 32, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Stanwell, P.; Levi, C.; Parsons, M. Arterial Spin Labeling Identifies Tissue Salvage and Good Clinical Recovery after Acute Ischemic Stroke. J. Neuroimaging 2013, 23, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood-Brain Barrier Tight Junction Permeability and Ischemic Stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Pantsios, C.; Kapelios, C.; Vakrou, S.; Diakos, N.; Pozios, I.; Kontogiannis, C.; Nanas, J.; Malliaras, K. Effect of Elevated Reperfusion Pressure on “No Reflow” Area and Infarct Size in a Porcine Model of Ischemia-Reperfusion. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 405–411. [Google Scholar] [CrossRef]
- Xu, X.; Tan, Z.; Fan, M.; Ma, M.; Fang, W.; Liang, J.; Xiao, Z.; Shi, C.; Luo, L. Comparative Study of Multi-Delay Pseudo-Continuous Arterial Spin Labeling Perfusion Mri and Ct Perfusion in Ischemic Stroke Disease. Front. Neuroinform. 2021, 15, 719719. [Google Scholar] [CrossRef]

| Clinical Information | mRS (0–2) | mRS (3–6) | p Value |
|---|---|---|---|
| Sex (male: female) | 13:5 | 30:10 | 0.823 |
| Age (years) | 62.2 ± 11.4 | 59.7 ± 11.1 | 0.196 |
| NIHSS | 10.8 ± 2.1 | 15.1 ± 3.1 | <0.001 * |
| Hypertension | 16(88.89%) | 37(92.5%) | 0.65 |
| Diabetes mellitus | 4(22.22%) | 4(10.0%) | 0.21 |
| Hyperlipemia | 11(61.11%) | 19(47.5%) | 0.34 |
| Gouty arthropathy | 4(22.22%) | 11(27.5%) | 0.67 |
| mRS (admission) | 1.8 ± 0.4 | 3.9 ± 0.8 | <0.001 * |
| Parameter | Power |
|---|---|
| rAPT mean | 0.6108 |
| rAPT max | 0.6465 |
| rASL max | 0.7779 |
| rASL Min | 0.7992 |
| rASL 90th | 0.7793 |
| rAPT(%) | rASL(mL/100 g/min) | |||||
|---|---|---|---|---|---|---|
| mRS < 2 | mRS ≥ 2 | p Value | mRS < 2 | mRS ≥ 2 | p Value | |
| Mean | 0.14 ± 0.68 | 0.21 ± 0.73 | 0.005 * | 27.45 ± 36.55 | −7.86 ± 39.8 | <0.001 * |
| Max | −0.39 ± 1.7 | −1.34 ± 7.91 | 0.011 * | 30.61 ± 36.44 | −11.55 ± 79.45 | <0.001 * |
| Min | 0.46 ± 1.26 | 0.89 ± 2.37 | 0.881 | 18.94 ± 30.58 | −6.6 ± 12.58 | <0.001 * |
| Kurtosis | −0.59 ± 1.62 | 0.23 ± 2.84 | 0.076 | −0.11 ± 0.49 | −0.11 ± 0.78 | 0.635 |
| Skewness | −0.17 ± 0.95 | −0.03 ± 1.12 | 0.565 | −0.25 ± 0.62 | 0.06 ± 0.77 | 0.532 |
| 10th | 0.26 ± 0.86 | 0.48 ± 1.05 | 0.453 | 21.72 ± 31.83 | −9.48 ± 14.95 | <0.001 * |
| 25th | 0.17 ± 0.63 | 0.38 ± 0.73 | 0.094 | 25.56 ± 37.41 | −10.73 ± 17.93 | <0.001 * |
| 50th | 0.18 ± 0.72 | 0.32 ± 0.76 | 0.019 * | 28.25 ± 39.63 | −7.91 ± 37.39 | <0.001 * |
| 75th | 0.08 ± 0.93 | 0.11 ± 0.8 | 0.054 | 29.63 ± 38.3 | −5.57 ± 65.93 | <0.001 * |
| 90th | 0.07 ± 1.17 | −0.22 ± 2.21 | 0.045 * | 31.04 ± 35.69 | −8.42 ± 73.87 | <0.001 * |
| Parameter | NIHSS on Admission | mRS on Admission | The Dimension |
|---|---|---|---|
| rAPT mean | 0. 0096 * | 0. 0024 * | 0. 0071 * |
| rAPT max | 0. 0068 * | 0. 0055 * | 0. 0038 * |
| rASL max | 0. 0001 * | 0. 0038 * | 0. 0061 * |
| rASL Min | 0. 0019 * | 0. 0026 * | 0. 0041 * |
| rASL 90th | 0. 0004 * | 0. 0084 * | 0. 0071 * |
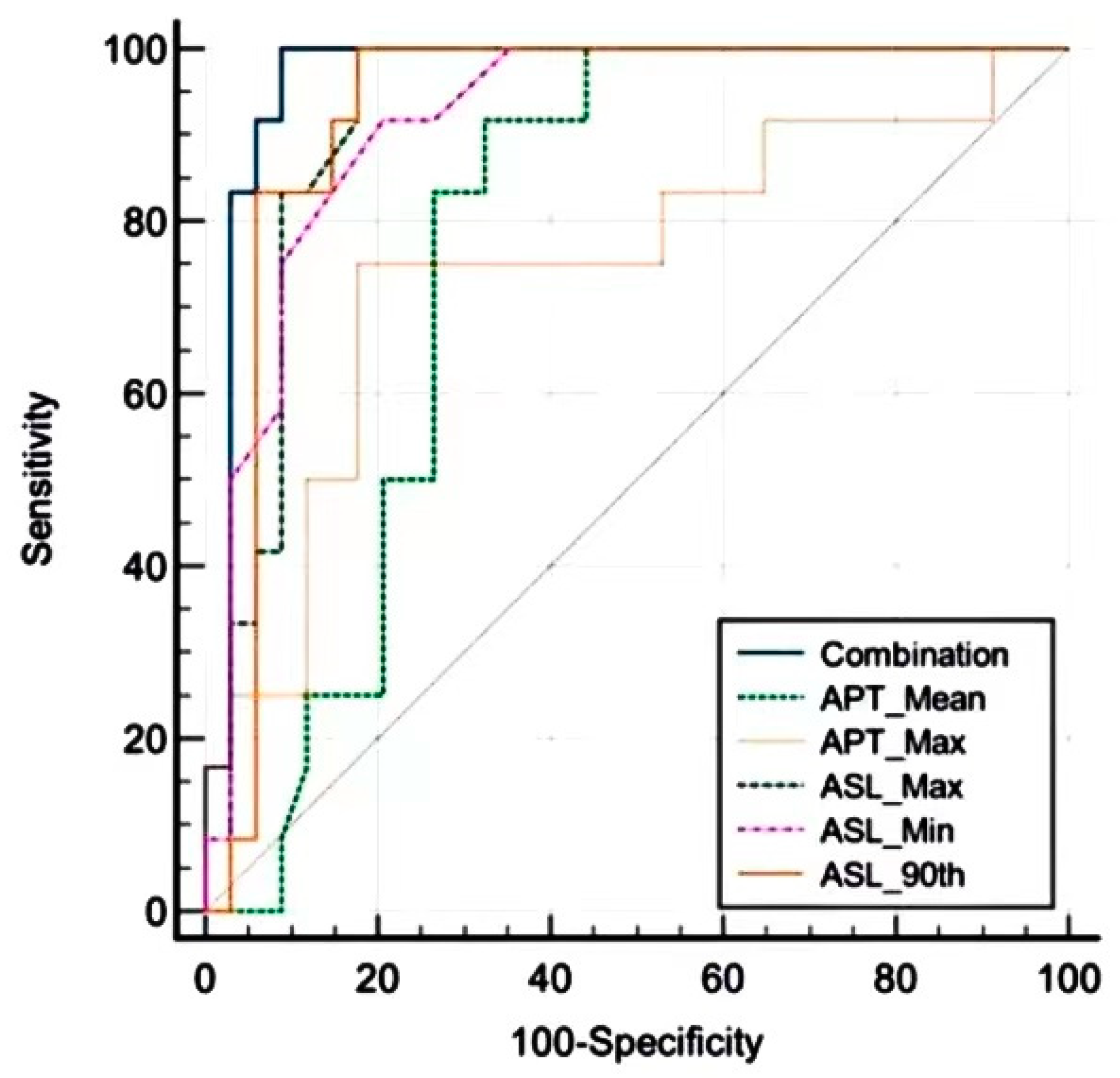
| Variable. | AUC | Sensitivity (%) | Specificity (%) | SE | 95% CI |
|---|---|---|---|---|---|
| Combination | 0.968 | 100 | 91.2 | 0.0258 | 0.869 to 0.998 |
| rAPT mean | 0.771 | 91.7 | 67.6 | 0.0679 | 0.623 to 0.882 |
| rAPT max | 0.75 | 75 | 82.4 | 0.0919 | 0.600 to 0.866 |
| rASL max | 0.922 | 100 | 82.4 | 0.0406 | 0.803 to 0.980 |
| rASL min | 0.918 | 100 | 88.2 | 0.0408 | 0.798 to 0.978 |
| rASL 90th | 0.926 | 100 | 82.4 | 0.0413 | 0.810 to 0.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Q.; Li, G.; Jiang, M.; Zhou, Q.; Gao, Y.; Yan, J. Predicting a Favorable (mRS 0–2) or Unfavorable (mRS 3–6) Stroke Outcome by Arterial Spin Labeling and Amide Proton Transfer Imaging in Post-Thrombolysis Stroke Patients. J. Pers. Med. 2023, 13, 248. https://doi.org/10.3390/jpm13020248
He Q, Li G, Jiang M, Zhou Q, Gao Y, Yan J. Predicting a Favorable (mRS 0–2) or Unfavorable (mRS 3–6) Stroke Outcome by Arterial Spin Labeling and Amide Proton Transfer Imaging in Post-Thrombolysis Stroke Patients. Journal of Personalized Medicine. 2023; 13(2):248. https://doi.org/10.3390/jpm13020248
Chicago/Turabian StyleHe, Qinmeng, Guomin Li, Meien Jiang, Qianling Zhou, Yunyu Gao, and Jianhao Yan. 2023. "Predicting a Favorable (mRS 0–2) or Unfavorable (mRS 3–6) Stroke Outcome by Arterial Spin Labeling and Amide Proton Transfer Imaging in Post-Thrombolysis Stroke Patients" Journal of Personalized Medicine 13, no. 2: 248. https://doi.org/10.3390/jpm13020248
APA StyleHe, Q., Li, G., Jiang, M., Zhou, Q., Gao, Y., & Yan, J. (2023). Predicting a Favorable (mRS 0–2) or Unfavorable (mRS 3–6) Stroke Outcome by Arterial Spin Labeling and Amide Proton Transfer Imaging in Post-Thrombolysis Stroke Patients. Journal of Personalized Medicine, 13(2), 248. https://doi.org/10.3390/jpm13020248








