Etiological Diagnosis and Personalized Therapy for Hypertension: A Hypothesis of the REASOH Classification
Abstract
:1. Introduction
2. Renin-Dependent Hypertension
2.1. Definition and Pathogenesis
2.2. Diagnosis and Hypertension-Mediated Organ Damage
2.3. Treatment
3. Elderly Arteriosclerosis-Based Hypertension
3.1. Definition and Pathogenesis
3.2. Diagnosis and Hypertension-Mediated Organ Damage
3.3. Treatment
4. Sympathetic-Active Hypertension
4.1. Definition and Pathogenesis
4.2. Diagnosis
4.3. Treatment
4.3.1. Drug Therapy
4.3.2. Non-Drug Therapy
5. Secondary Hypertension
5.1. Definition and Pathogenesis
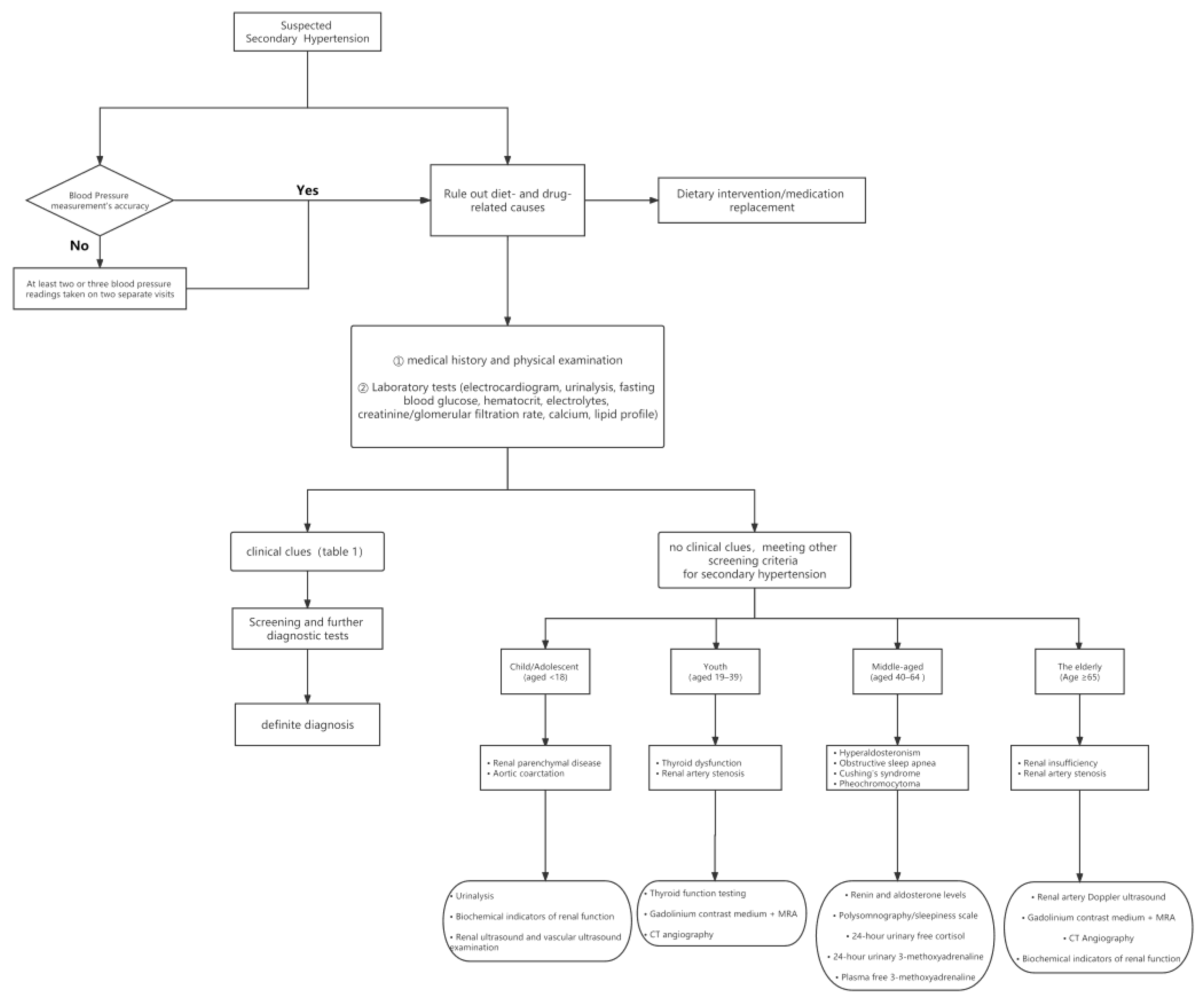
5.2. Screening and Diagnosis
5.3. Treatment
6. Salt-Sensitive Hypertension
6.1. Definition and Pathogenesis
6.2. Diagnosis and Hypertension-Mediated Organ Damage
6.3. Treatment
7. Hyperhomocysteinemia Hypertension
7.1. Definition and Pathogenesis
7.2. Diagnosis and Hypertension-Mediated Organ Damage
7.3. Treatment
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef] [PubMed]
- Tanai, E.; Frantz, S. Pathophysiology of Heart Failure. Compr. Physiol. 2015, 6, 187–214. [Google Scholar] [PubMed]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.J. Chronic kidney disease. Nat. Rev. Dis. Prim. 2017, 3, 17088. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.R.; Laragh, J.H.; Baer, L.; Newton, M.A.; Goodwin, F.T.; Krakoff, L.R.; Bard, R.H.; Bühler, F.R. Essential hypertension: Renin and aldosterone, heart attack and stroke. N. Engl. J. Med. 1972, 286, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Alderman, M.H.; Madhavan, S.; Ooi, W.L.; Cohen, H.; Sealey, J.E.; Laragh, J.H. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N. Engl. J. Med. 1991, 324, 1098–1104. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, M.; Holmes, D.T.; Xu, L.; Teoh, H.; Gupta, S.; Yusuf, S.; Lonn, E.M. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur. Heart J. 2011, 32, 2135–2142. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Morganti, A.; Grammer, T.; Amrein, K.; Boehm, B.O.; März, W. Associations of plasma renin with 10-year cardiovascular mortality, sudden cardiac death, and death due to heart failure. Eur. Heart J. 2011, 32, 2642–2649. [Google Scholar] [CrossRef] [Green Version]
- Gross, F. The renin-angiotensin system and hypertension. Ann. Intern. Med. 1971, 75, 777–787. [Google Scholar] [CrossRef]
- Swales, J.D. Renin-angiotensin system in hypertension. Pharmacol. Ther. 1979, 7, 173–201. [Google Scholar] [CrossRef]
- Te Riet, L.; Van Esch, J.H.; Roks, A.J.; Van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef] [Green Version]
- Laragh, J.H. Vasoconstriction-volume analysis for understanding and treating hypertension: The use of renin and aldosterone profiles. Am. J. Med. 1973, 55, 261–274. [Google Scholar] [CrossRef]
- Laragh, J. Laragh’s lessons in pathophysiology and clinical pearls for treating hypertension. Am. J. Hypertens. 2001, 14, 491–503. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Heizhati, M.; Gan, L.; Hong, J.; Wu, T.; Xiamili, Z.; Tong, L.; Lin, Y.; Li, N. Higher plasma renin activity is associated with increased kidney damage risk in patients with hypertension and glucose metabolic disorders. J. Clin. Hypertens. 2022, 24, 750–759. [Google Scholar] [CrossRef]
- Sealey, J.E.; Parra, D.; Rosenstein, R.; Laragh, J.H. “Effective” plasma renin activity: A derived measure for assessing residual plasma renin activity in patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Hypertension 2010, 55, e16. [Google Scholar] [CrossRef] [Green Version]
- Pizoń, T.; Rajzer, M.; Wojciechowska, W.; Drożdż, T.; Drożdż, D.; Rojek, M.; Gruszka, K.; Czarnecka, D. Plasma renin activity, serum aldosterone concentration and selected organ damage indices in essential arterial hypertension. Arch. Med. Sci. AMS 2021, 17, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Ruilope, L.M.; Dukat, A.; Böhm, M.; Lacourcière, Y.; Gong, J.; Lefkowitz, M.P. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: A randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010, 375, 1255–1266. [Google Scholar] [CrossRef]
- Olson, N.; DeJongh, B.; Hough, A.; Parra, D. Plasma renin activity-guided strategy for the management of hypertension. Pharmacotherapy 2012, 32, 446–455. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S.; Dimas, B. Current and future status of beta-blockers in the treatment of hypertension. Clin. Cardiol. 2008, 31, 249–252. [Google Scholar] [CrossRef]
- Egan, B.M.; Basile, J.N.; Rehman, S.U.; Davis, P.B.; Grob, C.H., 3rd; Riehle, J.F.; Walters, C.A.; Lackland, D.T.; Merali, C.; Sealey, J.E.; et al. Plasma Renin test-guided drug treatment algorithm for correcting patients with treated but uncontrolled hypertension: A randomized controlled trial. Am. J. Hypertens. 2009, 22, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Leotta, G.; Rabbia, F.; Testa, E.; Totaro, S.; Abram, S.; Milan, A.; Mulatero, P.; Veglio, F. Efficacy of antihypertensive treatment based on plasma renin activity: An open label observational study. Blood Press. 2010, 19, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, J.D.; Laragh, J.H. Renin system analysis: A rational method for the diagnosis and treatment of the individual patient with hypertension. Am. J. Hypertens. 1998, 11, 894–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, K.; Emdin, C.A.; MacMahon, S. The epidemiology of blood pressure and its worldwide management. Circ. Res. 2015, 116, 925–936. [Google Scholar] [CrossRef] [Green Version]
- Epstein, M. Aging and the kidney. J. Am. Soc. Nephrol. 1996, 7, 1106–1122. [Google Scholar] [CrossRef]
- Seals, D.R.; Esler, M.D. Human ageing and the sympathoadrenal system. J. Physiol. 2000, 528, 407–417. [Google Scholar] [CrossRef]
- Avolio, A.P.; Kuznetsova, T.; Heyndrickx, G.R.; Kerkhof, P.L.M.; Li, J.K. Arterial Flow, Pulse Pressure and Pulse Wave Velocity in Men and Women at Various Ages. Adv. Exp. Med. Biol. 2018, 1065, 153–168. [Google Scholar]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef]
- Perrone-Filardi, P.; Coca, A.; Galderisi, M.; Paolillo, S.; Alpendurada, F.; De Simone, G.; Donal, E.; Kahan, T.; Mancia, G.; Redon, J.; et al. Noninvasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients: A consensus article from the European Association of Cardiovascular Imaging, the European Society of Cardiology Council on Hypertension and the European Society of Hypertension. J. Hypertens. 2017, 35, 1727–1741. [Google Scholar]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Najjar, S.S.; Scuteri, A.; Shetty, V.; Wright, J.G.; Muller, D.C.; Fleg, J.L.; Spurgeon, H.P.; Ferrucci, L.; Lakatta, E.G. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 2008, 51, 1377–1383. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Ihle-Hansen, H.; Thommessen, B.; Fagerland, M.W.; Øksengård, A.R.; Wyller, T.B.; Engedal, K.; Fure, B. Blood pressure control to prevent decline in cognition after stroke. Vasc. Health Risk Manag. 2015, 11, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Segev-Jacubovski, O.; Herman, T.; Yogev-Seligmann, G.; Mirelman, A.; Giladi, N.; Hausdorff, J.M. The interplay between gait, falls and cognition: Can cognitive therapy reduce fall risk? Expert Rev. Neurother. 2011, 11, 1057–1075. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef]
- Thomopoulos, C.; Parati, G.; Zanchetti, A. Effects of blood pressure-lowering on outcome incidence in hypertension: 5. Head-to-head comparisons of various classes of antihypertensive drugs—Overview and meta-analyses. J. Hypertens. 2015, 33, 1321–1341. [Google Scholar] [CrossRef]
- Mann, S.J. Neurogenic hypertension: Pathophysiology, diagnosis and management. Clin. Auton. Res. Off. J. Clin. Auton. Res. Soc. 2018, 28, 363–374. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Sympathetic Nervous System, Hypertension, Obesity and Metabolic Syndrome. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2016, 23, 175–179. [Google Scholar] [CrossRef]
- Grassi, G. The Sympathetic Nervous System in Hypertension: Roadmap Update of a Long Journey. Am. J. Hypertens. 2021, 34, 1247–1254. [Google Scholar] [CrossRef]
- Grassi, G.; Ram, V.S. Evidence for a critical role of the sympathetic nervous system in hypertension. J. Am. Soc. Hypertens. 2016, 10, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L. The Mechanism of Cardiac Sympathetic Activity Assessment Methods: Current Knowledge. Front. Cardiovasc. Med. 2022, 9, 931219. [Google Scholar] [CrossRef] [PubMed]
- Doytchinova, A.; Hassel, J.L.; Yuan, Y.; Lin, H.; Yin, D.; Adams, D.; Straka, S.; Wright, K.; Smith, K.; Wagner, D.; et al. Simultaneous noninvasive recording of skin sympathetic nerve activity and electrocardiogram. Heart Rhythm 2017, 14, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolis, A.J.; Poulimenos, L.E.; Kallistratos, M.S.; Gavras, I.; Gavras, H. Sympathetic overactivity in hypertension and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 4–15. [Google Scholar]
- Verloop, W.L.; Beeftink, M.M.; Santema, B.T.; Bots, M.L.; Blankestijn, P.J.; Cramer, M.J.; Doevendans, P.A.; Voskuil, M. A systematic review concerning the relation between the sympathetic nervous system and heart failure with preserved left ventricular ejection fraction. PloS ONE 2015, 10, e0117332. [Google Scholar] [CrossRef]
- Grassi, G.; Quarti-Trevano, F.; Seravalle, G.; Dell’Oro, R.; Facchetti, R.; Mancia, G. Association Between the European Society of Cardiology/European Society of Hypertension Heart Rate Thresholds for Cardiovascular Risk and Neuroadrenergic Markers. Hypertension 2020, 76, 577–582. [Google Scholar] [CrossRef]
- Lindholm, L.H.; Carlberg, B.; Samuelsson, O. Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 2005, 366, 1545–1553. [Google Scholar] [CrossRef]
- Böhm, M.; Tsioufis, K.; Kandzari, D.E.; Kario, K.; Weber, M.A.; Schmieder, R.E.; Townsend, R.R.; Kulenthiran, S.; Ukena, C.; Pocock, S.; et al. Effect of Heart Rate on the Outcome of Renal Denervation in Patients With Uncontrolled Hypertension. J. Am. Coll. Cardiol. 2021, 78, 1028–1038. [Google Scholar] [CrossRef]
- Ahmad, Y.; Francis, D.P.; Bhatt, D.L.; Howard, J.P. Renal Denervation for Hypertension: A Systematic Review and Meta-Analysis of Randomized, Blinded, Placebo-Controlled Trials. JACC Cardiovasc. Interv. 2021, 14, 2614–2624. [Google Scholar] [CrossRef]
- Scheffers, I.J.; Kroon, A.A.; Schmidli, J.; Jordan, J.; Tordoir, J.J.; Mohaupt, M.G.; Luft, F.C.; Haller, H.; Menne, J.; Engeli, S.; et al. Novel baroreflex activation therapy in resistant hypertension: Results of a European multi-center feasibility study. J. Am. Coll. Cardiol. 2010, 56, 1254–1258. [Google Scholar] [CrossRef]
- Bisognano, J.D.; Bakris, G.; Nadim, M.K.; Sanchez, L.; Kroon, A.A.; Schafer, J.; De Leeuw, P.W.; Sica, D.A. Baroreflex activation therapy lowers blood pressure in patients with resistant hypertension: Results from the double-blind, randomized, placebo-controlled rheos pivotal trial. J. Am. Coll. Cardiol. 2011, 58, 765–773. [Google Scholar] [CrossRef] [Green Version]
- Sarathy, H.; Salman, L.A.; Lee, C.; Cohen, J.B. Evaluation and Management of Secondary Hypertension. Med. Clin. North Am. 2022, 106, 269–283. [Google Scholar] [CrossRef]
- Viera, A.J.; Neutze, D.M. Diagnosis of secondary hypertension: An age-based approach. Am. Fam. Physician 2010, 82, 1471–1478. [Google Scholar]
- Rossi, G.P.; Bisogni, V.; Rossitto, G.; Maiolino, G.; Cesari, M.; Zhu, R.; Seccia, T.M. Practice Recommendations for Diagnosis and Treatment of the Most Common Forms of Secondary Hypertension. High Blood Press. Cardiovasc. Prev. Off. J. Ital. Soc. Hypertens. 2020, 27, 547–560. [Google Scholar] [CrossRef]
- Charles, L.; Triscott, J.; Dobbs, B. Secondary Hypertension: Discovering the Underlying Cause. Am. Fam. Physician 2017, 96, 453–461. [Google Scholar]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Van Ryswyk, E.; Mukherjee, S.; Chai-Coetzer, C.L.; Vakulin, A.; McEvoy, R.D. Sleep Disorders, Including Sleep Apnea and Hypertension. Am. J. Hypertens. 2018, 31, 857–864. [Google Scholar] [CrossRef]
- Elijovich, F.; Weinberger, M.H.; Anderson, C.A.; Appel, L.J.; Bursztyn, M.; Cook, N.R.; Dart, R.A.; Newton-Cheh, C.H.; Sacks, F.M.; Laffer, C.L. Salt Sensitivity of Blood Pressure: A Scientific Statement from the American Heart Association. Hypertension 2016, 68, e7–e46. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.H.; Miller, J.Z.; Luft, F.C.; Grim, C.E.; Fineberg, N.S. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986, 8, Ii127–Ii134. [Google Scholar] [CrossRef] [Green Version]
- Balafa, O.; Kalaitzidis, R.G. Salt sensitivity and hypertension. J. Hum. Hypertens. 2021, 35, 184–192. [Google Scholar] [CrossRef]
- Elliott, P.; Stamler, J.; Nichols, R.; Dyer, A.R.; Stamler, R.; Kesteloot, H.; Marmot, M. Intersalt revisited: Further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ Clin. Res. Ed. 1996, 312, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Ingole, S.; Jain, R. Salt sensitivity and its implication in clinical practice. Indian Heart J. 2018, 70, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Elijovich, F.; Laffer, C.L.; Schiffrin, E.L.; Gavras, H.; Amador, E. Endothelin-aldosterone interaction and proteinuria in low-renin hypertension. J. Hypertens. 2004, 22, 573–582. [Google Scholar] [CrossRef]
- Yatabe, M.S.; Yatabe, J.; Yoneda, M.; Watanabe, T.; Otsuki, M.; Felder, R.A.; Jose, P.A.; Sanada, H. Salt sensitivity is associated with insulin resistance, sympathetic overactivity, and decreased suppression of circulating renin activity in lean patients with essential hypertension. Am. J. Clin. Nutr. 2010, 92, 77–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund-Johansen, P. Hemodynamic long-term effects of timolol at rest and during exercise in essential hypertension. Acta Med. Scand. 1976, 199, 263–267. [Google Scholar] [CrossRef]
- Thrasher, T.N. Unloading arterial baroreceptors causes neurogenic hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1044–R1053. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.H. Salt sensitivity of blood pressure in humans. Hypertension 1996, 27, 481–490. [Google Scholar] [CrossRef]
- Castiglioni, P.; Parati, G.; Brambilla, L.; Brambilla, V.; Gualerzi, M.; Di Rienzo, M.; Coruzzi, P. Detecting sodium-sensitivity in hypertensive patients: Information from 24-hour ambulatory blood pressure monitoring. Hypertension 2011, 57, 180–185. [Google Scholar] [CrossRef] [Green Version]
- Rakova, N.; Jüttner, K.; Dahlmann, A.; Schröder, A.; Linz, P.; Kopp, C.; Rauh, M.; Goller, U.; Beck, L.; Agureev, A.; et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013, 17, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Du, X.; Bai, Y.; Fang, L.; Liu, M.; Ji, N.; Zhong, J.; Yu, M.; Wu, J. Assessment and validation of spot urine in estimating the 24-h urinary sodium, potassium, and sodium/potassium ratio in Chinese adults. J. Hum. Hypertens. 2020, 34, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.H.; Fineberg, N.S. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 1991, 18, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.T.; Edwards, D.G.; Farquhar, W.B. The Influence of Dietary Salt Beyond Blood Pressure. Curr. Hypertens. Rep. 2019, 21, 42. [Google Scholar] [CrossRef]
- Rhee, M.Y.; Kim, J.H.; Na, S.H.; Chung, J.W.; Bae, J.H.; Nah, D.Y.; Gu, N.; Kim, H.Y. Elevation of heart-femoral pulse wave velocity by short-term low sodium diet followed by high sodium diet in hypertensive patients with sodium sensitivity. Nutr. Res. Pract. 2016, 10, 288–293. [Google Scholar] [CrossRef] [Green Version]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef]
- Bihorac, A.; Tezcan, H.; Ozener, C.; Oktay, A.; Akoglu, E. Association between salt sensitivity and target organ damage in essential hypertension. Am. J. Hypertens. 2000, 13, 864–872. [Google Scholar] [CrossRef] [Green Version]
- He, F.J.; Marciniak, M.; Visagie, E.; Markandu, N.D.; Anand, V.; Dalton, R.N.; MacGregor, G.A. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension 2009, 54, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Powles, J.; Fahimi, S.; Micha, R.; Khatibzadeh, S.; Shi, P.; Ezzati, M.; Engell, R.E.; Lim, S.S.; Danaei, G.; Mozaffarian, D. Global, regional and national sodium intakes in 1990 and 2010: A systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 2013, 3, e003733. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Li, T.; Lou, P.; Chang, G.; Zhang, P.; Chen, P.; Qiao, C.; Dong, Z. Salt intake, knowledge of salt intake, and blood pressure control in Chinese hypertensive patients. J. Am. Soc. Hypertens. 2014, 8, 909–914. [Google Scholar] [CrossRef]
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. Adv. Exp. Med. Biol. 2017, 956, 61–84. [Google Scholar]
- Qi, H.; Liu, Z.; Cao, H.; Sun, W.P.; Peng, W.J.; Liu, B.; Dong, S.J.; Xiang, Y.T.; Zhang, L. Comparative Efficacy of Antihypertensive Agents in Salt-Sensitive Hypertensive Patients: A Network Meta-Analysis. Am. J. Hypertens. 2018, 31, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, X.; Huo, Y.; Li, N.; Song, L.; Sun, Y.; Shi, Z.; Wang, B.; Yang, X.; Xie, L.; et al. Expert consensus on the diagnosis and treatment of H-type hypertension. Chin. J. Hypertens. 2016, 24, 123–127. (In Chinese) [Google Scholar]
- Cui, H.; Wang, F.; Fan, L.; Hu, Y.X.; Hu, G.L.; Liu, L.; Hong, C.M. Association factors of target organ damage: Analysis of 17,682 elderly hypertensive patients in China. Chin. Med. J. 2011, 124, 3676–3681. [Google Scholar] [PubMed]
- Xu, X.; Li, J.; Sheng, W.; Liu, L. Meta-analysis of genetic studies from journals published in China of ischemic stroke in the Han Chinese population. Cerebrovasc. Dis. 2008, 26, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Sutton-Tyrrell, K.; Bostom, A.; Selhub, J.; Zeigler-Johnson, C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation 1997, 96, 1745–1749. [Google Scholar] [CrossRef]
- Boers, G.H. Mild hyperhomocysteinemia is an independent risk factor of arterial vascular disease. Semin. Thromb. Hemost. 2000, 26, 291–295. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar]
- Rodrigo, R.; Passalacqua, W.; Araya, J.; Orellana, M.; Rivera, G. Homocysteine and essential hypertension. J. Clin. Pharmacol. 2003, 43, 1299–1306. [Google Scholar] [CrossRef]
- Rodrigo, R.; Passalacqua, W.; Araya, J.; Orellana, M.; Rivera, G. Implications of oxidative stress and homocysteine in the pathophysiology of essential hypertension. J. Cardiovasc. Pharmacol. 2003, 42, 453–461. [Google Scholar] [CrossRef]
- Zhong, F.; Zhuang, L.; Wang, Y.; Ma, Y. Homocysteine levels and risk of essential hypertension: A meta-analysis of published epidemiological studies. Clin. Exp. Hypertens. 2017, 39, 160–167. [Google Scholar] [CrossRef]
- Wu, H.; Wang, B.; Ban, Q.; Chen, L.; Yan, D.; Yu, Y.; Song, Y.; Liu, C.; Cao, J.; Zhang, J.; et al. Association of total homocysteine with blood pressure in a general population of Chinese adults: A cross-sectional study in Jiangsu province, China. BMJ Open 2018, 8, e021103. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Zhang, J.; Yu, S.; Zhao, S.; Tang, J.; Zheng, Y.; Meng, W.; Xu, C.; Zhang, Y.; Xu, Y. Hypertension-Mediated Organ Damage Correlates with Serum Homocysteine Level in Community-Dwelling Elderly Chinese: The North Shanghai Study. Front. Cardiovasc. Med. 2021, 8, 662741. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ma, C.; Zhang, J.; Wang, J.; Jiang, L. Relationship between serum Hcy, urine microalbumin/creatinine ratio and blood pressure variability and target organ dysfunction in patients with H-type hypertension. Chin. J. Evid.-Based Cardiovasc. Med. 2020, 12, 333–336. (In Chinese) [Google Scholar]
- Sun, Y.; Chien, K.L.; Hsu, H.C.; Su, T.C.; Chen, M.F.; Lee, Y.T. Use of serum homocysteine to predict stroke, coronary heart disease and death in ethnic Chinese. 12-year prospective cohort study. Circ. J. Off. J. Jpn. Circ. Soc. 2009, 73, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Liu, Y.; Wang, C.; Tang, L.; Feng, X.; Astell-Burt, T.; Wen, Q.; Duan, D.; Lu, N.; Xu, G.; et al. Determinants of hyperhomocysteinemia in healthy and hypertensive subjects: A population-based study and systematic review. Clin. Nutr. 2017, 36, 1215–1230. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef] [Green Version]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef] [Green Version]

| Etiological Subtype | Clinical Clues | Screening Method | Further Diagnostic Tests | Treatment |
|---|---|---|---|---|
| Nephrogenous hypertension | Personal/family history of chronic kidney disease; frequent urination, nocturia. | Urine routine; serum creatinine. | renal ultrasonography | Drug therapy: ACEIs or ARBs (first-line medication); ACEIs or ARBs + dihydropyridine CCB, ACEIs or ARBs + thiazide diuretics and dihydropyridine CCB + thiazide diuretics (combined therapy). |
| Renovascular hypertension | Murmurs in the abdomen or in other arteries; moderate-to-severe hypertension (grade 2 or above) and unilateral small kidney/transient pulmonary edema; young (<30 years) hypertensive patients with fibromuscular dysplasia; atherosclerosis. | Serum creatinine; renal artery ultrasound. | CT angiography; gadolinium contrast agent + MRA; renal angiography | Drug therapy: antihypertensive drugs, antiplatelet drugs and lipid-lowering drugs; non-drug treatment: percutaneous stent implantation, balloon dilatation. |
| Primary aldosteronism | Symptoms of hypokalemia;refractory hypertension;adrenal adenoma;family history of primary aldosteronism and/or early-onset hypertension or cerebrovascular accident (< 40 years old.) | Serum potassium; Aldosterone/renin ratio (ARR). | Saline load test; captopril test; adrenal CT scan; blood was collected from bilateral adrenal veins | Drug therapy: spironolactone is the first choice for bilateral lesions and eplerenone can be used for intolerance; non-drug treatment: surgical resection is the first choice for unilateral lesions. |
| Pheochromocytoma | Headache, palpitations, sweating, fainting; unstable blood pressure; paroxysmal hypertension. | Blood and urine catecholamine levels; blood and urine 3-Methoxyadrenaline. | CT/MRI; nuclear medicine imaging genetic testing | First-line treatment: surgical resection; second-line treatment: radionuclide therapy, radiotherapy or chemotherapy. |
| Cushing’s syndrome | History of osteoporosis at a young age; central obesity, purple skin lines, full-moon face, buffalo back. | 24 h urine-free cortisol measurement; midnight salivary-cortisol measurement; adrenocorticotropic hormone. | Low-dose dexamethasone suppression test; large dose dexamethasone suppression test; CRH stimulation test; CT or MRI | Surgical resection; radiotherapy or chemotherapy; adjuvant therapy with drugs. |
| Aortic stenosis | The systolic blood pressure of upper limb is 20 mmHg more than lower limb; headache; delay or disappearance of femoral beats; arterial murmur. | Echocardiography | CTA or MRA | Endovascular aortic intervention. |
| Sleepapnea syndrome | Snoring; daytime sleepiness; asthma, asphyxia or apnea during sleep at night. | Sleepiness scale; history of snoring | Polysomnography | First-line treatment: non-invasive positive airway pressure ventilation; second-line treatment: oral orthotics, surgical treatment; etiology and lifestyle intervention: weight loss, smoking cessation, etc. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Li, M.; Meng, W.; Han, J.; Zhao, S.; Tang, J.; Yang, H.; Maimaitiaili, R.; Teliewubai, J.; Yu, S.; et al. Etiological Diagnosis and Personalized Therapy for Hypertension: A Hypothesis of the REASOH Classification. J. Pers. Med. 2023, 13, 261. https://doi.org/10.3390/jpm13020261
Xu C, Li M, Meng W, Han J, Zhao S, Tang J, Yang H, Maimaitiaili R, Teliewubai J, Yu S, et al. Etiological Diagnosis and Personalized Therapy for Hypertension: A Hypothesis of the REASOH Classification. Journal of Personalized Medicine. 2023; 13(2):261. https://doi.org/10.3390/jpm13020261
Chicago/Turabian StyleXu, Chong, Moran Li, Weilun Meng, Jun Han, Song Zhao, Jiamin Tang, Haotian Yang, Rusitanmujiang Maimaitiaili, Jiadela Teliewubai, Shikai Yu, and et al. 2023. "Etiological Diagnosis and Personalized Therapy for Hypertension: A Hypothesis of the REASOH Classification" Journal of Personalized Medicine 13, no. 2: 261. https://doi.org/10.3390/jpm13020261





