Interdisciplinary Surgical Therapy of Extremity Soft-Tissue Sarcomas: A Personalized Resection and Reconstruction Algorithm
Abstract
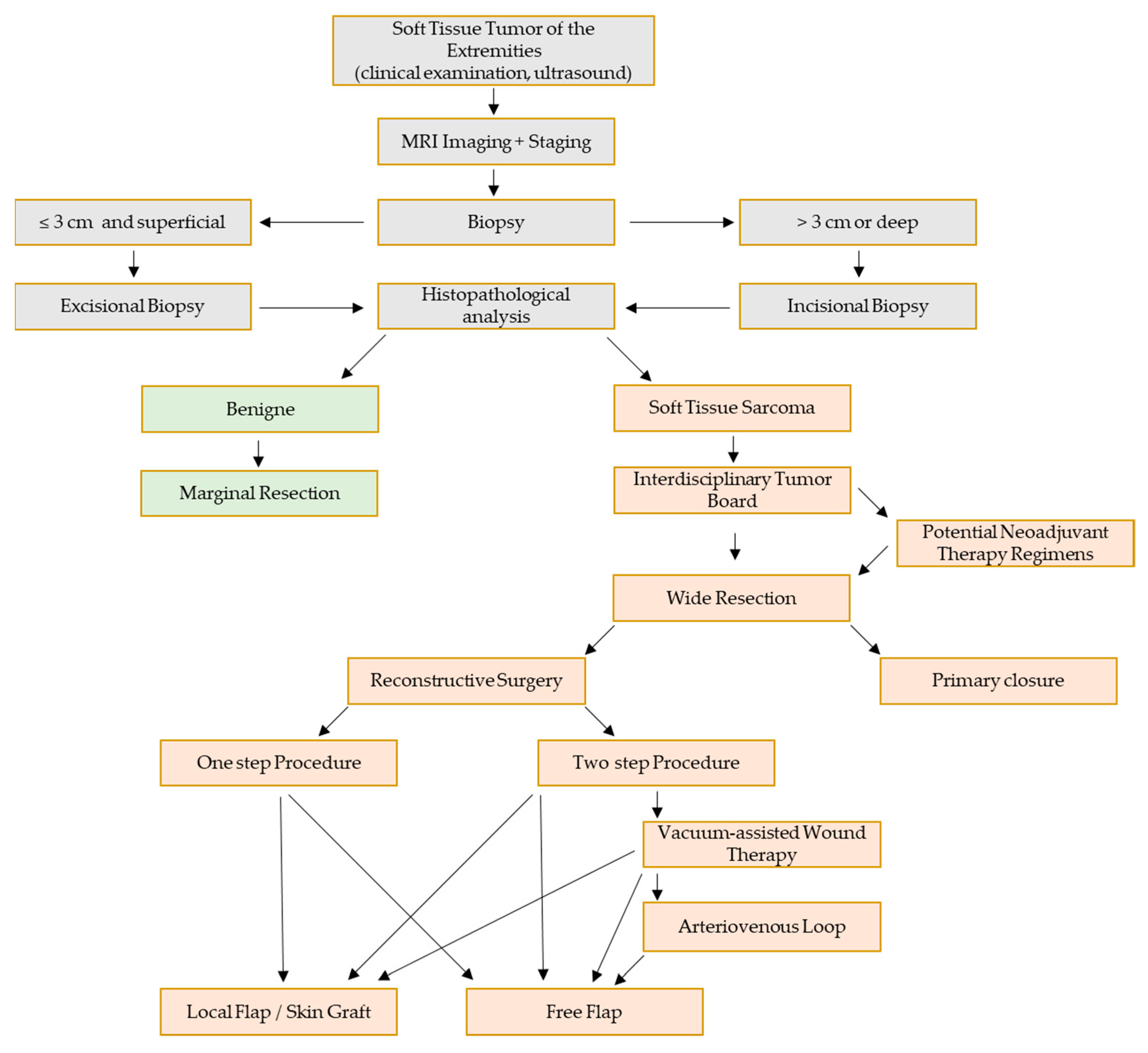
1. Introduction
2. Material and Methods
3. Results
4. Case Demonstrations
4.1. Case 1
4.2. Case 2
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, J.H.M.; Ro, J.Y.M. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv. Anat. Pathol. 2020, 28, 44–58. [Google Scholar] [CrossRef]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Paredes, T.; Pereira, M.; Moreira, H.; Simoes, M.; Canavarro, M.C. Quality of life of sarcoma patients from diagnosis to treatments: Predictors and longitudinal trajectories. Eur. J. Oncol. Nurs. 2011, 15, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma Epidemiology and Etiology: Potential Environmental and Genetic Factors. Surg. Clin. North Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Gerrand, C.H.; Wunder, J.S.; Kandel, R.A.; Catton, C.N.; Bell, R.S.; Griffin, A.; Davis, A.M.; O’Sullivan, B. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J. Bone Jt. Surg. 2001, 83, 1149–1155. [Google Scholar] [CrossRef]
- Blay, J.-Y.; Honoré, C.; Stoeckle, E.; Meeus, P.; Jafari, M.; Gouin, F.; Anract, P.; Ferron, G.; Rochwerger, A.; Ropars, M.; et al. Surgery in reference centers improves survival of sarcoma patients: A nationwide study. Ann. Oncol. 2019, 30, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Cramer, L.D.; Hoskins, W.J.; Brennan, M.F. Impact of Hospital Volume on Operative Mortality for Major Cancer Surgery. JAMA 1998, 280, 1747–1751. [Google Scholar] [CrossRef]
- Maurice, M.J.; Yih, J.M.; Ammori, J.B.; Abouassaly, R. Predictors of surgical quality for retroperitoneal sarcoma: Volume matters. J. Surg. Oncol. 2017, 116, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Crombé, A.; Marcellin, P.-J.; Buy, X.; Stoeckle, E.; Brouste, V.; Italiano, A.; Le Loarer, F.; Kind, M. Soft-Tissue Sarcomas: Assessment of MRI Features Correlating with Histologic Grade and Patient Outcome. Radiology 2019, 291, 710–721. [Google Scholar] [CrossRef]
- Hong, J.H.; Jee, W.-H.; Jung, C.-K.; Jung, J.-Y.; Shin, S.H.; Chung, Y.-G. Soft tissue sarcoma: Adding diffusion-weighted imaging improves MR imaging evaluation of tumor margin infiltration. Eur. Radiol. 2018, 29, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Birgin, E.; Yang, C.; Hetjens, S.; Reissfelder, C.; Hohenberger, P.; Rahbari, N.N. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: A systematic review and meta-analysis. Cancer 2020, 126, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, P.K.; Kasper, B.; Grünwald, V. S3-Leitlinie Adulte Weichgewebesarkome. Leitlinienprogramm 2021, 1, 56–57. [Google Scholar]
- Arbiser, Z.K.; Folpe, A.L.; Weiss, S.W. Consultative (Expert) Second Opinions in Soft Tissue Pathology. Am. J. Clin. Pathol. 2001, 116, 473–476. [Google Scholar] [CrossRef]
- Jain, S.; Xu, R.; Prieto, V.G.; Lee, P. Molecular classification of soft tissue sarcomas and its clinical applications. Int. J. Clin. Exp. Pathol. 2010, 3, 416–428. [Google Scholar]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.Y.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Casali, P.G. Adjuvant chemotherapy for soft tissue sarcoma. Am. Soc. Clin. Oncol. Educ. Book 2015, e629–e633. [Google Scholar] [CrossRef]
- Pervaiz, N.; Colterjohn, N.; Farrokhyar, F.; Tozer, R.; Figueredo, A.; Ghert, M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resec-table soft-tissue sarcoma. Cancer 2008, 113, 573–581. [Google Scholar] [CrossRef]
- Tsuchihashi, K.; Kusaba, H.; Yoshihiro, T.; Fujiwara, T.; Setsu, N.; Endo, M.; Matsumoto, Y.; Imajima, T.; Shinohara, Y.; Ito, M.; et al. Eribulin as a first-line treatment for soft tissue sarcoma patients with contraindications for doxorubicin. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, F.; Sayyed, R.; Jones, R.L.; Hayes, A.J. Risk factors for the development of local recurrence in extremity soft-tissue sarcoma. Expert Rev. Anticancer. Ther. 2021, 22, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Dadras, M.; Steinau, H.-U.; Goertz, O.; Lehnhardt, M.; Behr, B.; Harati, K. Limb preserving surgery for soft-tissue sarcoma in the hand: A retrospective study of 51 cases. J. Hand Surg. 2020, 45, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennan, M.; DeMoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, I.C.; Whitwell, D.J.; Battistuta, D.; Thompson, B.; Strobel, N.; Duggal, A.; Steadman, P. SURGICAL MARGIN AND ITS INFLUENCE ON SURVIVAL IN SOFT TISSUE SARCOMA. ANZ J. Surg. 2006, 76, 104–109. [Google Scholar] [CrossRef]
- Kannan, S.; Chong, H.H.; Chew, B.; Ferguson, J.D.; Galloway, E.; McCulloch, T.; Rankin, K.S.; Ashford, R.U. Leiomyosarcoma in the extremities and trunk wall: Systematic review and meta-analysis of the oncological outcomes. World J. Surg. Oncol. 2022, 20, 1–11. [Google Scholar] [CrossRef]
- Clarkson, P.W.; Griffin, A.; Catton, C.N.; O’Sullivan, B.; Ferguson, P.C.; Wunder, J.S.; Bell, R.S. Epineural Dissection Is a Safe Technique That Facilitates Limb Salvage Surgery. Clin. Orthop. Relat. Res. 2005, 438, 92–96. [Google Scholar] [CrossRef]
- Ghert, M.A.; Abudu, A.; Driver, N.; Davis, A.M.; Griffin, A.M.; Pearce, D.; White, L.; O’Sullivan, B.; Catton, C.N.; Bell, R.S.; et al. The Indications for and the Prognostic Significance of Amputation as the Primary Surgical Procedure for Localized Soft Tissue Sarcoma of the Extremity. Ann. Surg. Oncol. 2004, 12, 10–17. [Google Scholar] [CrossRef]
- Götzl, R.; Sterzinger, S.; Arkudas, A.; Boos, A.M.; Semrau, S.; Vassos, N.; Grützmann, R.; Agaimy, A.; Hohenberger, W.; Horch, R.E.; et al. The Role of Plastic Reconstructive Surgery in Surgical Therapy of Soft Tissue Sarcomas. Cancers 2020, 12, 3534. [Google Scholar] [CrossRef]
- Koulaxouzidis, G.; Simunovic, F.; Bannasch, H. Soft Tissue Sarcomas of the Arm–Oncosurgical and Reconstructive Principles within a Multimodal, Interdisciplinary Setting. Front. Surg. 2016, 3, 12. [Google Scholar] [CrossRef]
- Ludolph, I.; Arkudas, A.; Müller-Seubert, W.; Cai, A.; Horch, R.E. Komplikationen und deren Management nach axillärer, inguinaler und iliakaler Lymphknotendissektion. Die Chir. 2022, 1–8. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Meroni, M.; Fritsche, E.; Fuchs, B. Lymphatic Complications Prevention and Soft Tissue Reconstruction after Soft Tissue Sarcoma Resec-tion in the Limbs. Medicina 2022, 58, 67. [Google Scholar] [CrossRef]
- Wan, R.; Hussain, A.; Kuruoglu, D.; Houdek, M.T.; Moran, S.L. Prophylactic lymphaticovenous anastomosis ( LVA ) for preventing lymphedema after sarcoma resection in the lower limb: A report of three cases and literature review. Microsurgery 2022, 1–8. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, N.; Kageyama, T.; Sakai, H.; Fuse, Y.; Tsukuura, R. Lymph-interpositional-flap transfer (LIFT) based on lymph-axiality concept: Simultaneous soft tissue and lymphatic reconstruction without lymph node transfer or lymphatic anastomosis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2604–2612. [Google Scholar] [CrossRef]
- Scaglioni, M.F.; Meroni, M.; Fritsche, E.; Fuchs, B. Combined pedicled superficial circumflex iliac artery perforator (SCIP) flap with lymphatic tissue preservation and lymphovenous anastomosis (LVA) for defect reconstruction and lymphedema–lymphocele prevention in thigh sarcoma surgery: Preliminary results. J. Surg. Oncol. 2020, 123, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Fuse, Y.; Hayashi, A.; Yamamoto, N. Lymph Flow Restoration after Tissue Replantation and Transfer: Importance of Lymph Axiality and Possibility of Lymph Flow Reconstruction without Lymph Node Transfer or Lymphatic Anastomosis. Plast. Reconstr. Surg. 2018, 142, 796–804. [Google Scholar] [CrossRef]
- Guillier, D.; Guiotto, M.; Cherix, S.; Raffoul, W.; di Summa, P.G. Lymphatic flow through (LyFT) ALT flap: An original solution to reconstruct soft tissue loss with lymphatic leakage or lower limb lymphedema. J. Plast. Surg. Hand Surg. 2022, 1–9. [Google Scholar] [CrossRef]
- Ludolph, I.; Horch, R.E.; Arkudas, A.; Schmitz, M. Enhancing Safety in Reconstructive Microsurgery Using Intraoperative Indocyanine Green Angiography. Front. Surg. 2019, 6, 39. [Google Scholar] [CrossRef]
- Ishiura, R.; Fujita, M.; Furuya, M.; Banda, C.; Narushima, M. Visualization of lymphatic ducts with preoperative ICG lymphography prevents donor-site lymphedema following PAP flap. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Bedi, M.; King, D.M.; DeVries, J.; Hackbarth, D.A.; Neilson, J.C. Does Vacuum-assisted Closure Reduce the Risk of Wound Complications in Patients with Lower Extremity Sarcomas Treated With Preoperative Radiation? Clin. Orthop. Relat. Res. 2019, 477, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.; Ströbel, A.; Ludolph, I.; Hauck, T.; May, M.S.; Beier, J.P.; Horch, R.E.; Arkudas, A. Improving the Safety of DIEP Flap Transplantation: Detailed Perforator Anatomy Study Using Preoperative CTA. J. Pers. Med. 2022, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Geierlehner, A.; Horch, R.E.; Ludolph, I.; Arkudas, A. Intraoperative Blood Flow Analysis of DIEP vs. ms-TRAM Flap Breast Reconstruction Combining Transit-Time Flowmetry and Microvascular Indocyanine Green Angiography. J. Pers. Med. 2022, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Müller-Seubert, W.; Ostermaier, P.; Horch, R.E.; Distel, L.; Frey, B.; Cai, A.; Arkudas, A. Intra- and Early Postoperative Evaluation of Malperfused Areas in an Irradiated Random Pattern Skin Flap Model Using Indocyanine Green Angiography and Near-Infrared Reflectance-Based Imaging and Infrared Ther-mography. J. Pers. Med. 2022, 12, 237. [Google Scholar] [CrossRef]
- Karakawa, R.; Yoshimatsu, H.; Fuse, Y.; Tanakura, K.; Imai, T.; Sawaizumi, M.; Yano, T. Immediate tendon transfer for functional reconstruction of a dorsal forearm defect after sarcoma resection. J. Plast. Surg. Hand Surg. 2022, 1–6. [Google Scholar] [CrossRef]
- Krauss, S.; Goertz, O.; Pakosch-Nowak, D.; Daigeler, A.; Harati, K.; Lehnhardt, M.; Held, M.; Kolbenschlag, J. Microvascular tissue transfer after the resection of soft tissue sarcomas. J. Plast. Reconstr. Aesthetic Surg. 2020, 74, 995–1003. [Google Scholar] [CrossRef]
- Horch, R.E.; Ludolph, I.; Arkudas, A.; Cai, A. Personalized Reconstruction of Genital Defects in Complicated Wounds with Vertical Rectus Abdominis Myocutaneous Flaps including Urethral Neo-Orifice. J. Pers. Med. 2021, 11, 1076. [Google Scholar] [CrossRef]
- Bigdeli, A.K.; Didzun, O.; Thomas, B.; Harhaus, L.; Gazyakan, E.; Horch, R.E.; Kneser, U. Combined versus Single Perforator Propeller Flaps for Reconstruction of Large Soft Tissue Defects: A Retrospective Clinical Study. J. Pers. Med. 2022, 12, 41. [Google Scholar] [CrossRef]
- Momeni, A.; Kalash, Z.; Stark, G.B.; Bannasch, H. The use of the anterolateral thigh flap for microsurgical reconstruction of distal extremities after on-cosurgical resection of soft-tissue sarcomas. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 643–648. [Google Scholar] [CrossRef]
- Penna, V.; Iblher, N.; Momeni, A.; Stark, G.B.; Bannasch, H. Free tissue transfer in reconstruction following soft tissue sarcoma resection. Microsurgery 2011, 31, 434–440. [Google Scholar] [CrossRef]
- Müller-Seubert, W.; Scheibl, K.; Bührer, G.; Möbius, C.; Ludolph, I.; Horch, R.E.; Arkudas, A. Less is more–retrospective comparison of shoulder strength and range of motion between con-ventional and muscle-sparing harvesting technique of a latissimus dorsi flap. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2527–2536. [Google Scholar] [CrossRef]
- Geierlehner, A.M.; Ludolph, I.; Arkudas, A.; Horch, R.E. A Myocutaneous Latissimus Dorsi Propeller Flap Based on a Single Dorsal Intercostal Perforator. Plast. Reconstr. Surg. - Glob. Open 2021, 9, e3881. [Google Scholar] [CrossRef] [PubMed]


| Patient/Treatment Characteristics | Values/Numbers | ||
|---|---|---|---|
| Median Age (Years) | 54.7 (20–86) | ||
| Sex | Male | 16 | 51.6% |
| Female | 15 | 48.4%% | |
| Neoadjuvant RT | Yes | 7 | 22.6% |
| No | 24 | 77.4% | |
| Neoadjuvant CT | Yes | 8 | 25.8% |
| No | 23 | 74.2% | |
| Adjuvant RT | Yes | 9 | 29.0% |
| No | 22 | 71.0% | |
| Adjuvant CT | Yes | 5 | 16.1% |
| No | 26 | 83.9% | |
| Localization | Upper Extremity | 7 | 22.6% |
| Lower Extremity | 24 | 77.4% | |
| Tumor size (cm, n = 23) | 6.5 (1.5–17.5) | ||
| STS Subtype | Undifferentiated pleomorphic sarcoma | 10 | 32.2% |
| Leiomyosarcoma | 4 | 12.9% | |
| Liposarcoma | 4 | 12.9% | |
| Synovial sarcoma | 3 | 9.7% | |
| Myxofibrosarcoma | 3 | 9.7% | |
| Myxoinflammatory fibroblastic sarcoma | 2 | 6.5% | |
| Chondrosarcoma | 1 | 3.2% | |
| Fibromyxoid sarcoma | 1 | 3.2% | |
| Other unclassified sarcoma | 3 | 9.7% | |
| Follow up (Month) | 12.03 (0–22) | ||
| Surgical Treatment after Tumor Resection (n = 31) | Procedure | Flap |
|---|---|---|
| Primary Wound Closure (n = 21) | Secondary Flap Reconstruction (n = 5) | Free VRAM Flap (n = 3) Free Lat. Dorsi Flap (n = 1) Pedicled VRAM Flap (n = 1) |
| No Primary Wound Closure (n = 10) | Primary Flap Reconstruction (n = 7) | Free ALT Flap (n = 2) Free TFL Flap (n = 1) Free VRAM Flap (n = 1) Peroneus Brevis Flap (n = 1) Pedicled VRAM Flap (n = 1) LICAP Propeller Flap (n = 1) |
| NPWT + Skin Graft (n = 2) | ||
| NPWT without Reconstruction (n = 1) |
| Subgroup | Complications |
|---|---|
| Primary Wound Closure (n = 21) | Infected Seroma (n = 1) Wound Healing Disturbance and Seroma (n = 1) Wound Healing Disturbance and Hematoma (n = 1) Lymphedema (n = 1) Hematoma and Infection (n = 1) Skin Necrosis (n = 1) |
| Primary Flap Reconstruction (n = 7) | Seroma (n = 1) |
| Secondary Flap Reconstruction (n = 5) | Wound Healing Disturbance (n = 1) Seroma (n = 1) Wound Healing Disturbance and Infection (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osterloh, J.; Ludolph, I.; Grützmann, R.; Meyer, A.; Lang, W.; Horch, R.E.; Fechner, K.; Arkudas, A. Interdisciplinary Surgical Therapy of Extremity Soft-Tissue Sarcomas: A Personalized Resection and Reconstruction Algorithm. J. Pers. Med. 2023, 13, 262. https://doi.org/10.3390/jpm13020262
Osterloh J, Ludolph I, Grützmann R, Meyer A, Lang W, Horch RE, Fechner K, Arkudas A. Interdisciplinary Surgical Therapy of Extremity Soft-Tissue Sarcomas: A Personalized Resection and Reconstruction Algorithm. Journal of Personalized Medicine. 2023; 13(2):262. https://doi.org/10.3390/jpm13020262
Chicago/Turabian StyleOsterloh, Justus, Ingo Ludolph, Robert Grützmann, Alexander Meyer, Werner Lang, Raymund E. Horch, Katja Fechner, and Andreas Arkudas. 2023. "Interdisciplinary Surgical Therapy of Extremity Soft-Tissue Sarcomas: A Personalized Resection and Reconstruction Algorithm" Journal of Personalized Medicine 13, no. 2: 262. https://doi.org/10.3390/jpm13020262
APA StyleOsterloh, J., Ludolph, I., Grützmann, R., Meyer, A., Lang, W., Horch, R. E., Fechner, K., & Arkudas, A. (2023). Interdisciplinary Surgical Therapy of Extremity Soft-Tissue Sarcomas: A Personalized Resection and Reconstruction Algorithm. Journal of Personalized Medicine, 13(2), 262. https://doi.org/10.3390/jpm13020262








