Microvascular Alterations of Peripapillary Choriocapillaris in Young Adult High Myopia Detected by Optical Coherence Tomography Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
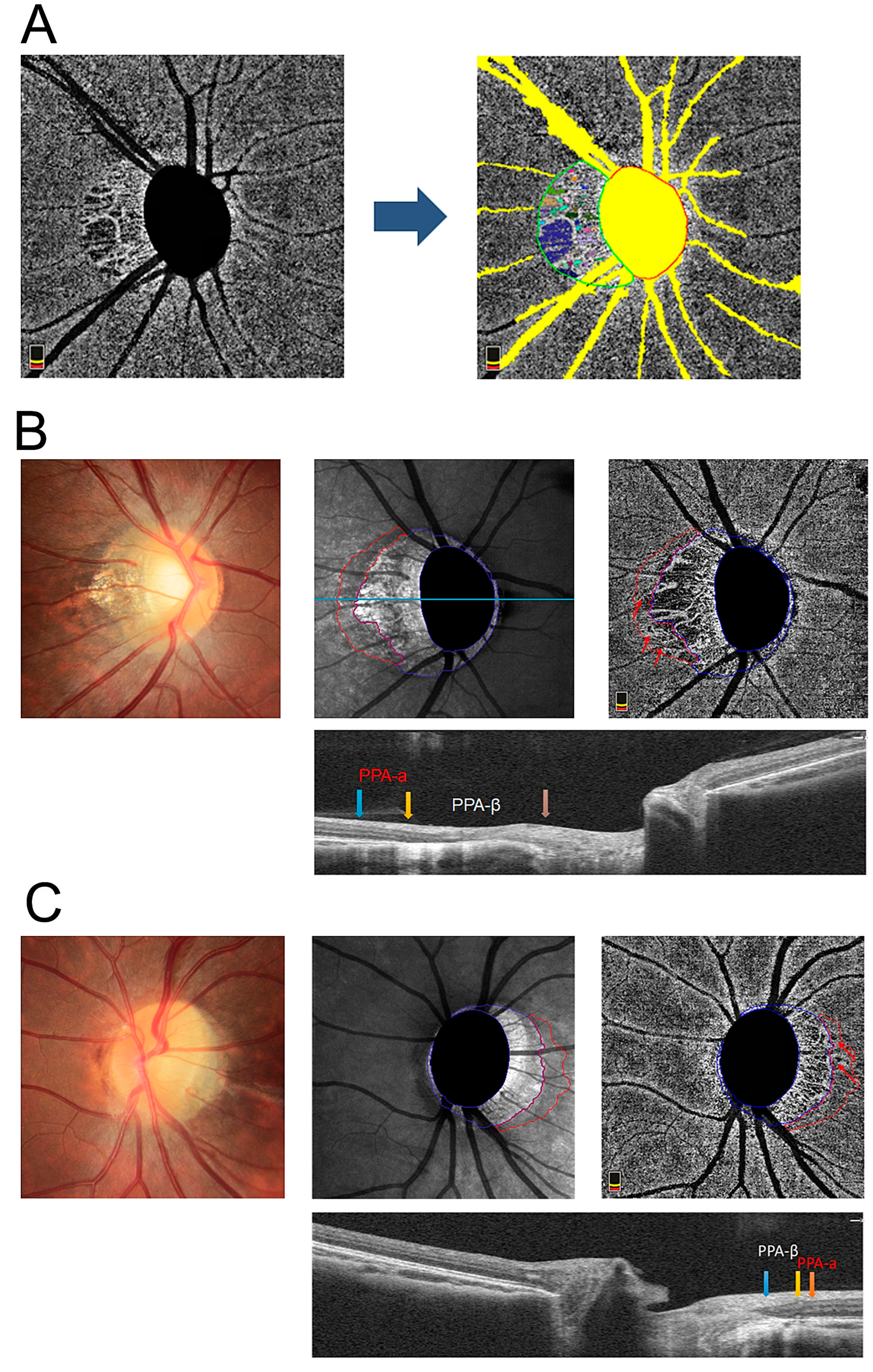
2.2. OCTA and Determination of the Presence of a MvD
2.3. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, Y. Overview of the complications of high myopia. Retina 2017, 37, 2347–2351. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, S.; Ambati, B.; Hunter, A.A. Pathogenesis and Prevention of Worsening Axial Elongation in Pathological Myopia. Clin. Ophthalmol. 2020, 14, 853–873. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Jiang, H.; Gregori, G.; Roisman, L.; Zheng, F.; Ke, B.; Qu, D.; Wang, J. Retinal Microvascular Network and Microcirculation Assessments in High Myopia. Am. J. Ophthalmol. 2017, 174, 56–67. [Google Scholar] [CrossRef]
- Sung, M.S.; Lee, T.H.; Heo, H.; Park, S.W. Association Between Optic Nerve Head Deformation and Retinal Microvasculature in High Myopia. Am. J. Ophthalmol. 2018, 188, 81–90. [Google Scholar] [CrossRef]
- Sung, M.S.; Heo, H.; Park, S.W. Microstructure of Parapapillary Atrophy Is Associated With Parapapillary Microvasculature in Myopic Eyes. Am. J. Ophthalmol. 2018, 192, 157–168. [Google Scholar] [CrossRef]
- Van Alphen, G. Choroidal stress and emmetropization. Vis. Res. 1986, 26, 723–734. [Google Scholar] [CrossRef]
- Stübinger, K.; Brehmer, A.; Neuhuber, W.L.; Reitsamer, H.; Nickla, D.; Schrödl, F. Intrinsic choroidal neurons in the chicken eye: Chemical coding and synaptic input. Histochem. Cell Biol. 2010, 134, 145–157. [Google Scholar] [CrossRef]
- Junghans, B.M.; Liang, H.; Crewther, S.G.; Crewther, D.P. A role for choroidal lymphatics during recovery from form deprivation myopia? Optom. Vis. Sci. 1999, 76, 796–803. [Google Scholar] [CrossRef]
- Spaide, R.F. Choriocapillaris Flow Features Follow a Power Law Distribution: Implications for Characterization and Mechanisms of Disease Progression. Am. J. Ophthalmol. 2016, 170, 58–67. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef]
- Borrelli, E.; Sarraf, D.; Freund, K.B.; Sadda, S.R. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res. 2018, 67, 30–55. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, K.M.; Lee, S.H.; Kim, T.-W. Parapapillary Choroidal Microvasculature Dropout in Glaucoma: A Comparison between Optical Coherence Tomography Angiography and Indocyanine Green Angiography. Ophthalmology 2017, 124, 1209–1217. [Google Scholar] [CrossRef]
- Sayanagi, K.; Ikuno, Y.; Uematsu, S.; Nishida, K. Features of the choriocapillaris in myopic maculopathy identified by optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 1524–1529. [Google Scholar] [CrossRef]
- Ye, J.; Wang, M.; Shen, M.; Huang, S.; Xue, A.; Lin, J.; Fan, Y.; Wang, J.; Lu, F.; Shao, Y. Deep Retinal Capillary Plexus Decreasing Correlated With the Outer Retinal Layer Alteration and Visual Acuity Impairment in Pathological Myopia. Investig. Ophthalmol. Vis. Sci. 2020, 61, 45. [Google Scholar] [CrossRef]
- Lee, S.S.; Lingham, G.; Alonso-Caneiro, D.; Chen, F.; Yazar, S.; Hewitt, A.; Mackey, D.A. Choroidal Thickness in Young Adults and its Association with Visual Acuity. Am. J. Ophthalmol. 2020, 214, 40–51. [Google Scholar] [CrossRef]
- Nagiel, A.; Sadda, S.R.; Sarraf, D. A Promising Future for Optical Coherence Tomography Angiography. JAMA Ophthalmol. 2015, 133, 629–630. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M.; Cooney, M.J. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Kubota, T.; Jonas, J.B.; Naumann, G.O. Direct clinico-histological correlation of parapapillary chorioretinal atrophy. Br. J. Ophthalmol. 1993, 77, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Fantes, F.E.; Anderson, D.R. Clinical histologic correlation of human peripapillary anatomy. Ophthalmology 1989, 96, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Jiang, R.; Wang, N.L.; Xu, L.; Jonas, J.B. Acute Peripapillary Retinal Pigment Epithelium Changes Associated with Acute Intraocular Pressure Elevation. Ophthalmology 2015, 122, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B. Clinical implications of peripapillary atrophy in glaucoma. Curr. Opin. Ophthalmol. 2005, 16, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Nguyen, X.N.; Gusek, G.C.; Naumann, G.O. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Investig. Ophthalmol. Vis. Sci. 1989, 30, 908–918. [Google Scholar]
- Lee, E.J.; Kim, T.-W.; Kim, J.-A.; Kim, J.-A. Parapapillary Deep-Layer Microvasculature Dropout in Primary Open-Angle Glaucoma Eyes With a Parapapillary γ-Zone. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5673–5680. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, E.J.; Kim, T.-W. Topographic correlation between juxtapapillary choroidal thickness and parapapillary deep-layer microvasculature dropout in primary open-angle glaucoma. Br. J. Ophthalmol. 2018, 102, 1134–1140. [Google Scholar] [CrossRef]
- Marmor, M.F.; Ravin, J.G. Fluorescein angiography: Insight and serendipity a half century ago. Arch. Ophthalmol. 2011, 129, 943–948. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Horng, I.-H.; Yang, C.-H.; Lin, L.L.-K.; Peng, Y.; Hung, P.-T. Ocular pulse amplitude in myopia. J. Ocul. Pharmacol. 1991, 7, 83–87. [Google Scholar] [CrossRef]
- Yang, Y.S.; Koh, J.W. Choroidal Blood Flow Change in Eyes with High Myopia. Korean J. Ophthalmol. 2015, 29, 309–314. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, E.J.; Kim, J.-A.; Kim, H.; Kim, T.-W. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br. J. Ophthalmol. 2021, 105, 674–680. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.X.; Zhang, Q.; Bin Wei, W.; Xu, L.; Jonas, J.B. Macular Choroidal Small-Vessel Layer, Sattler’s Layer and Haller’s Layer Thicknesses: The Beijing Eye Study. Sci. Rep. 2018, 8, 4411. [Google Scholar] [CrossRef]
- He, J.; Chen, Q.; Yin, Y.; Zhou, H.; Fan, Y.; Zhu, J.; Zou, H.; Xu, X. Association between retinal microvasculature and optic disc alterations in high myopia. Eye 2019, 33, 1494–1503. [Google Scholar] [CrossRef]
- Baird, P.N.; Saw, S.-M.; Lanca, C.; Guggenheim, J.A.; Smith, E.L., III.; Zhou, X.; Matsui, K.-O.; Wu, P.-C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Prim. 2020, 6, 99. [Google Scholar] [CrossRef]
- Rao, H.L.; Sreenivasaiah, S.; Riyazuddin, M.; Dasari, S.; Dixit, S.; Venugopal, J.P.; Pradhan, Z.S.; Puttaiah, N.K.; Devi, S.; Weinreb, R.N.; et al. Choroidal Microvascular Dropout in Primary Angle Closure Glaucoma. Am. J. Ophthalmol. 2019, 199, 184–192. [Google Scholar] [CrossRef]
- Jo, Y.H.; Sung, K.R.M.; Shin, J.W. Comparison of Peripapillary Choroidal Microvasculature Dropout in Primary Open-angle, Primary Angle-closure, and Pseudoexfoliation Glaucoma. J. Glaucoma 2020, 29, 1152–1157. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.H.; Kim, J.-A.; Kim, T.-W. Parapapillary Deep-Layer Microvasculature Dropout in Glaucoma: Topographic Association With Glaucomatous Damage. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3004–3010. [Google Scholar] [CrossRef]
- Rao, H.L.; Sreenivasaiah, S.; Dixit, S.; Riyazuddin, M.; Dasari, S.; Venugopal, J.P.; Pradhan, Z.S.; Puttaiah, N.K.; Devi, S.; Mansouri, K.; et al. Choroidal Microvascular Dropout in Primary Open-angle Glaucoma Eyes With Disc Hemorrhage. J. Glaucoma 2019, 28, 181–187. [Google Scholar] [CrossRef]
- Kim, J.-A.; Son, D.H.; Lee, E.J.; Kim, H.; Kim, T.-W. Intereye Comparison of the Characteristics of the Peripapillary Choroid in Patients with Unilateral Normal-Tension Glaucoma. Ophthalmol. Glaucoma 2021, 4, 512–521. [Google Scholar] [CrossRef]
- Kim, J.-A.; Lee, E.J.; Kim, T.-W. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal Nerve Fiber Layer Thinning in Patients With Glaucoma. JAMA Ophthalmol. 2019, 137, 810–816. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, H.; Zhang, S.; Ye, C.; Pan, X.; Tao, A.; Xu, X.; Qu, J.; Liang, Y. Parapapillary Choroidal Microvasculature Dropout Is Associated with the Decrease in Retinal Nerve Fiber Layer Thickness: A Prospective Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 838–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, M.W.; de Vries, M.M.; Montolio, F.G.J.; Jansonius, N.M. Myopia as a risk factor for open-angle glaucoma: A systematic review and meta-analysis. Ophthalmology 2011, 118, 1989–1994.e2. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shang, K.; Chen, X.; Sun, X.; Dai, Y. Clinical features of microvasculature in subzones of parapapillary atrophy in myopic eyes: An OCT-angiography study. Eye 2021, 35, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Parver, L.M.; Auker, C.; Carpenter, D.O. Choroidal blood flow as a heat dissipating mechanism in the macula. Am. J. Ophthalmol. 1980, 89, 641–646. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The multifunctional choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef]
- Wangsa-Wirawan, N.D.; Linsenmeier, R.A. Retinal oxygen: Fundamental and clinical aspects. Arch. Ophthalmol. 2003, 121, 547–557. [Google Scholar] [CrossRef]
- Rensch, F.; Jonas, J.B. Direct microperimetry of alpha zone and beta zone parapapillary atrophy. Br. J. Ophthalmol. 2008, 92, 1617–1619. [Google Scholar] [CrossRef]
- Meyer, J.H.; Guhlmann, M.; Funk, J. Blind spot size depends on the optic disc topography: A study using SLO controlled scotometry and the Heidelberg retina tomograph. Br. J. Ophthalmol. 1997, 81, 355–359. [Google Scholar] [CrossRef]
- Jonas, J.B.; Gusek, G.C.; Fernández, M.C. Correlation of the blind spot size to the area of the optic disk and parapapillary atrophy. Am. J. Ophthalmol. 1991, 111, 559–565. [Google Scholar] [CrossRef]
- Lal, B.; Alonso-Caneiro, D.; Read, S.A.; Carkeet, A. Induced refractive error changes the optical coherence tomography angiography transverse magnification and vascular indices. Am. J. Ophthalmol. 2021, 229, 230–241. [Google Scholar] [CrossRef]


| Group | N (Eyes) | Female | Age | SE 1 | AL 2 |
|---|---|---|---|---|---|
| High myopia group | 95 | 32 | 36.21 ± 14.28 | −7.14 ± 0.927 | 26.65 ± 0.88 |
| Mild to moderate myopia group | 110 | 31 | 32.30 ± 12.84 | −2.51 ± 2.10 | 24.36 ± 1.15 |
| p value | - | 0.873 | 0.517 | 0.000 | 0.012 |
| Group | N | n (Eyes with MvD in PPA-α Zone) | MvD 2 Area (mm2) | PPA-α Area (mm2) | Mean Density of Choroidal Flow in PPA-α Zone |
|---|---|---|---|---|---|
| High myopia group | 95 | 21 (22.1%) | 0.068 ± 0.050 | 0.336 ± 0.188 | 428.89 ± 312.20 |
| Mild-moderate myopia group | 110 | 63 (57.3%) | 0.089 ± 0.082 | 0.235 ± 122 | 404.88 ± 432.47 |
| p value | - | 0.000 | 0.210 | 0.012 | 0.159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, J.; Fan, Y.; Wu, Y.; Yuan, S.; Ye, Y.; Huang, K.; Chen, Q.; Yang, B.; Xie, P. Microvascular Alterations of Peripapillary Choriocapillaris in Young Adult High Myopia Detected by Optical Coherence Tomography Angiography. J. Pers. Med. 2023, 13, 289. https://doi.org/10.3390/jpm13020289
Lei J, Fan Y, Wu Y, Yuan S, Ye Y, Huang K, Chen Q, Yang B, Xie P. Microvascular Alterations of Peripapillary Choriocapillaris in Young Adult High Myopia Detected by Optical Coherence Tomography Angiography. Journal of Personalized Medicine. 2023; 13(2):289. https://doi.org/10.3390/jpm13020289
Chicago/Turabian StyleLei, Jie, Yuanyuan Fan, Yan Wu, Songtao Yuan, Yurong Ye, Kun Huang, Qiang Chen, Bin Yang, and Ping Xie. 2023. "Microvascular Alterations of Peripapillary Choriocapillaris in Young Adult High Myopia Detected by Optical Coherence Tomography Angiography" Journal of Personalized Medicine 13, no. 2: 289. https://doi.org/10.3390/jpm13020289




