Clinical Applications of Three-Dimensional Printing in Upper Extremity Surgery: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Search Strategy
2.3. Article Selection
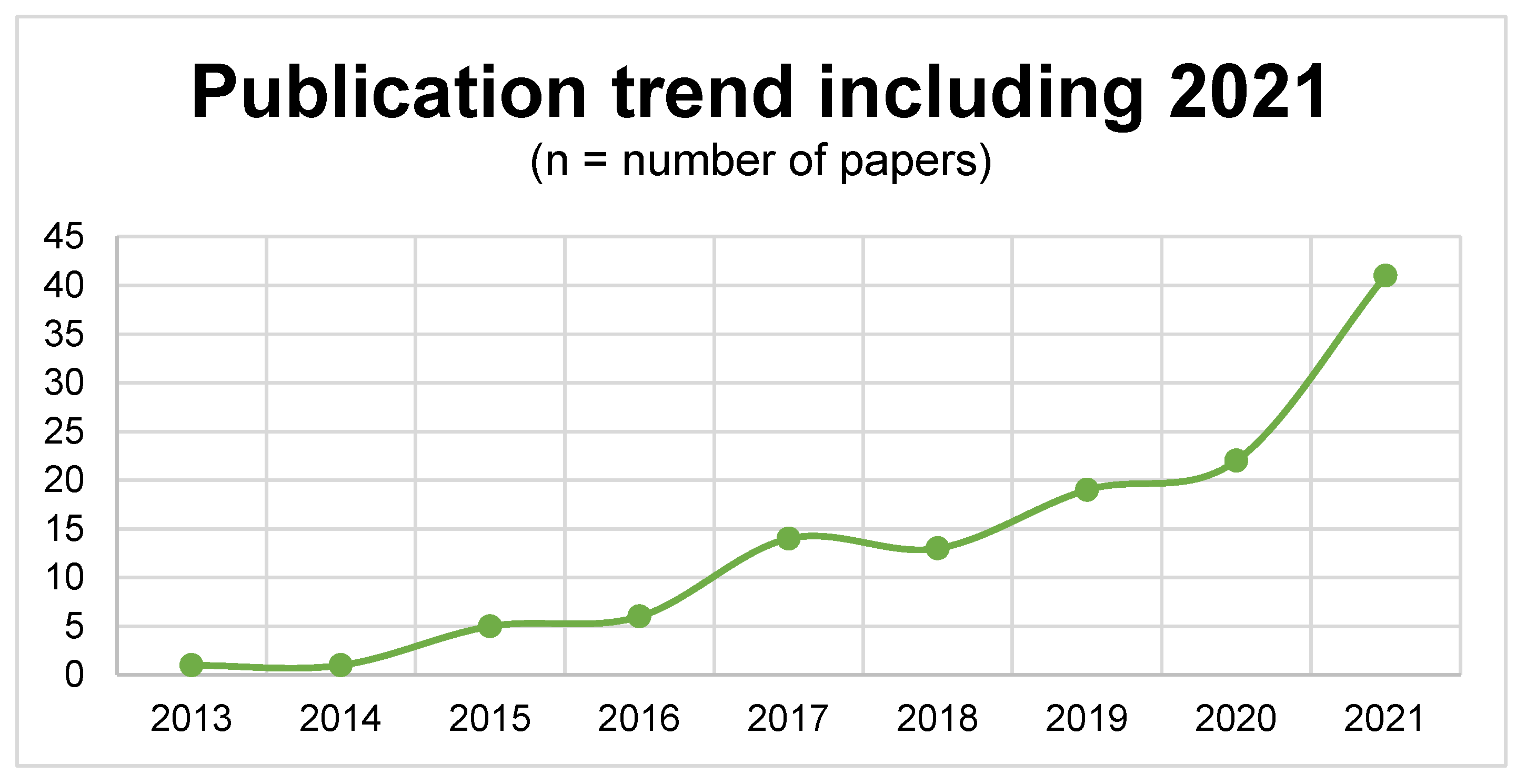
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Entities
3.4. Applications
3.5. Involved Anatomical Upper Extremity Structures
3.6. Reported Outcomes
3.7. Comparative Outcome Results (3D-Printed Group vs. Conventional Group)
3.8. Evidence Level
3.9. Language/Nation of Affiliated Institution
4. Discussion
4.1. Applications
4.2. Preoperative Planning
4.3. Intraoperative Support
4.4. Patient-Specific Implants, Prostheses, and Orthoses
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matter-Parrat, V.; Liverneaux, P. 3D printing in hand surgery. Hand Surg. Rehabil. 2019, 38, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Vujaklija, I.; Farina, D. 3D printed upper limb prosthetics. Expert. Rev. Med. Devices 2018, 15, 505–512. [Google Scholar] [CrossRef]
- Maroulakos, M.; Kamperos, G.; Tayebi, L.; Halazonetis, D.; Ren, Y. Applications of 3D printing on craniofacial bone repair: A systematic review. J. Dent. 2019, 80, 1–14. [Google Scholar] [CrossRef]
- Tanaka, K.S.; Lightdale-Miric, N. Advances in 3D-Printed Pediatric Prostheses for Upper Extremity Differences. J. Bone Jt. Surg. 2016, 98, 1320–1326. [Google Scholar] [CrossRef]
- Hong, C.J.; Giannopoulos, A.A.; Hong, B.Y.; Witterick, I.J.; Irish, J.C.; Lee, J.; Vescan, A.; Mitsouras, D.; Dang, W.; Campisi, P.; et al. Clinical applications of three-dimensional printing in otolaryngology–head and neck surgery: A systematic review. Laryngoscope 2019, 129, 2045–2052. [Google Scholar] [CrossRef]
- ten Kate, J.; Smit, G.; Breedveld, P. 3D-printed upper limb prostheses: A review. Disabil. Rehabil. Assist. Technol. 2017, 12, 300–314. [Google Scholar] [CrossRef]
- Diment, L.E.; Thompson, M.S.; Bergmann, J.H.M. Three-dimensional printed upper-limb prostheses lack randomised controlled trials: A systematic review. Prosthet. Orthot. Int. 2018, 42, 7–13. [Google Scholar] [CrossRef]
- Krettek, C.; Bruns, N. Aktueller Stand und neue Entwicklungen des 3D-Drucks in der Unfallchirurgie. Der Unfallchirurg 2019, 122, 256–269. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- American Society of Plastic Surgeons. ASPS Evidence Rating Scales. 2011. Available online: www.plasticsurgery.org/Documents/medical-professionals/health-policy/evidence-practice/ASPS-Rating-Scale-March-2011.pdf (accessed on 30 August 2021).
- Yang, L.; Grottkau, B.; He, Z.; Ye, C. Three dimensional printing technology and materials for treatment of elbow fractures. Int. Orthop. 2017, 41, 2381–2387. [Google Scholar] [CrossRef]
- Chen, C.; Cai, L.; Zheng, W.; Wang, J.; Guo, X.; Chen, H. The efficacy of using 3D printing models in the treatment of fractures: A randomised clinical trial. BMC Musculoskelet. Disord. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Kong, L.; Yang, G.; Yu, J.; Zhou, Y.; Li, S.; Zheng, Q.; Zhang, B. Surgical treatment of intra-articular distal radius fractures with the assistance of three-dimensional printing technique. Medicine 2020, 99, e19259. [Google Scholar] [CrossRef]
- Zheng, W.; Su, J.; Cai, L.; Lou, Y.; Wang, J.; Guo, X.; Tang, J.; Chen, H. Application of 3D-printing technology in the treatment of humeral intercondylar fractures. Orthop. Traumatol. Surg. Res. 2018, 104, 83–88. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Xiao, X.; Gao, W.-C.; Xiao, Y.; Zhang, S.-L.; Ni, W.-Y.; Deng, L. Efficacy evaluation of three-dimensional printing assisted osteotomy guide plate in accurate osteotomy of adolescent cubitus varus deformity. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Yin, H.-W.; Feng, J.-T.; Yu, B.-F.; Shen, Y.-D.; Gu, Y.-D.; Xu, W.-D. 3D printing-assisted percutaneous fixation makes the surgery for scaphoid nonunion more accurate and less invasive. J. Orthop. Transl. 2020, 24, 138–143. [Google Scholar] [CrossRef]
- Schweizer, A.; Mauler, F.; Vlachopoulos, L.; Nagy, L.; Fürnstahl, P. Computer-Assisted 3-Dimensional Reconstructions of Scaphoid Fractures and Nonunions With and Without the Use of Patient-Specific Guides: Early Clinical Outcomes and Postoperative Assessments of Reconstruction Accuracy. J. Hand. Surg. 2016, 41, 59–69. [Google Scholar] [CrossRef]
- Bauer, D.E.; Zimmermann, S.; Aichmair, A.; Hingsammer, A.; Schweizer, A.; Nagy, L.; Fürnstahl, P. Conventional Versus Computer-Assisted Corrective Osteotomy of the Forearm: A Retrospective Analysis of 56 Consecutive Cases. J. Hand. Surg. 2017, 42, 447–455. [Google Scholar] [CrossRef]
- Wang, Y.; Min, L.; Lu, M.; Zhou, Y.; Wang, J.; Zhang, Y.; Yu, X.; Tang, F.; Luo, Y.; Duan, H.; et al. The functional outcomes and complications of different reconstruction methods for Giant cell tumor of the distal radius: Comparison of Osteoarticular allograft and three-dimensional-printed prosthesis. BMC Musculoskelet. Disord. 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Yu, Q.; Zhang, X.; Wang, D.; Shi, L.; Huang, W.; Zhong, S. Application of 3D-Printed Orthopedic Cast for the Treatment of Forearm Fractures: Finite Element Analysis and Comparative Clinical Assessment. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.J.; Cha, Y.H.; Lee, K.H.; Kwon, J.Y. Effect of personalized wrist orthosis for wrist pain with three-dimensional scanning and printing technique: A preliminary, randomized, controlled, open-label study. Prosthet. Orthot. Int. 2018, 42, 636–643. [Google Scholar] [CrossRef]
- Beliën, H.; Biesmans, H.; Steenwerckx, A.; Bijnens, E.; Dierickx, C. Prebending of osteosynthesis plate using 3D printed models to treat symptomatic os acromiale and acromial fracture. J. Exp. Orthop. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Kataoka, T.; Oka, K.; Miyake, J.; Omori, S.; Tanaka, H.; Murase, T. 3-Dimensional Prebent Plate Fixation in Corrective Osteotomy of Malunited Upper Extremity Fractures Using a Real-Sized Plastic Bone Model Prepared by Preoperative Computer Simulation. J. Hand. Surg. 2013, 38, 909–919. [Google Scholar] [CrossRef]
- Exner, G.U.; Dumont, C.E.; Walker, J.; Fürnstahl, P. Cement Spacer Formed in a 3D-Printed Mold for Endoprosthetic Reconstruction of an Infected Sarcomatous Radius. J. Bone Jt. Surg. 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaren, I.A.; Vreeken, R.D.; Verhulst, A.C.; Ulrich, D.J.O.; Maal, T.J.J.; Wagner, T. Virtual Incision Pattern Planning using Three-Dimensional Images for Optimization of Syndactyly Surgery. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1694. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Zhang, J.; Meng, Z.; Liu, L.; Zhang, W.; Chen, Y.; Cong, R. 3D Printing Technology in Planning Thumb Reconstructions with Second Toe Transplant. Orthop. Surg. 2017, 9, 215–220. [Google Scholar] [CrossRef]
- Schreiner, A.J.; Schmidutz, F.; Ateschrang, A.; Ihle, C.; Stöckle, U.; Ochs, B.G.; Gonser, C. Periprosthetic tibial fractures in total knee arthroplasty—An outcome analysis of a challenging and underreported surgical issue. BMC Musculoskelet. Disord. 2018, 19, 323. [Google Scholar] [CrossRef]
- Oka, K.; Tanaka, H.; Okada, K.; Sahara, W.; Myoui, A.; Yamada, T.; Yamamoto, M.; Kurimoto, S.; Hirata, H.; Murase, T. Three-Dimensional Corrective Osteotomy for Malunited Fractures of the Upper Extremity Using Patient-Matched Instruments. J. Bone Jt. Surg. 2019, 101, 710–721. [Google Scholar] [CrossRef]
- Ribak, S.; Medina, C.E.G.; Mattar, R.; Ulson, H.J.R.; De Resende, M.R.; Etchebehere, M. Treatment of scaphoid nonunion with vascularised and nonvascularised dorsal bone grafting from the distal radius. Int. Orthop. 2009, 34, 683–688. [Google Scholar] [CrossRef]
- Michielsen, M.; Van Haver, A.; Vanhees, M.; van Riet, R.; Verstreken, F. Use of three-dimensional technology for complications of upper limb fracture treatment. EFORT Open Rev. 2019, 4, 302–312. [Google Scholar] [CrossRef]
- Fillat-Gomà, F.; Marcano-Fernández, F.A.; Coderch-Navarro, S.; Martínez-Carreres, L.; Berenguer, A. 3D printing innovation: New insights into upper extremity surgery planning. Injury 2021, 52, S117–S124. [Google Scholar] [CrossRef]
- Kunz, M.; Ma, B.; Rudan, J.F.; Ellis, R.E.; Pichora, D.R. Image-Guided Distal Radius Osteotomy Using Patient-Specific Instrument Guides. J. Hand Surg. 2013, 38, 1618–1624. [Google Scholar] [CrossRef]
- Temmesfeld, M.J.; Hauksson, I.T.; Mørch, T. Intra-Articular Osteotomy of the Distal Radius with the Use of Inexpensive In-House 3D Printed Surgical Guides and Arthroscopy: A Case Report. JBJS Case Connect. 2020, 10, e0424. [Google Scholar] [CrossRef]
- Honigmann, P.; Thieringer, F.; Steiger, R.; Haefeli, M.; Schumacher, R.; Henning, J. A Simple 3-Dimensional Printed Aid for a Corrective Palmar Opening Wedge Osteotomy of the Distal Radius. J. Hand Surg. 2016, 41, 464–469. [Google Scholar] [CrossRef]
- Miyake, J.; Murase, T.; Moritomo, H.; Sugamoto, K.; Yoshikawa, H. Distal Radius Osteotomy with Volar Locking Plates Based on Computer Simulation. Clin. Orthop. Relat. Res. 2011, 469, 1766–1773. [Google Scholar] [CrossRef]
- Byrne, A.-M.; Impelmans, B.; Bertrand, V.; Van Haver, A.; Verstreken, F. Corrective Osteotomy for Malunited Diaphyseal Forearm Fractures Using Preoperative 3-Dimensional Planning and Patient-Specific Surgical Guides and Implants. J. Hand Surg. 2017, 42, 836.e1–836.e12. [Google Scholar] [CrossRef]
- Gauci, M.-O.; Chelli, M.; Fernandez, J.; Bronsard, N. Patient-Specific Three-Dimensional-printed Instrumentation for Radius Lengthening Osteotomy by a Volar Approach in Epiphysiodesis Sequelae: A Case Report. J. Orthop. 2020, 10, 21–24. [Google Scholar]
- Mueller, S.; Kahrs, L.A.; Gaa, J.; Ortmaier, T.; Clausen, J.-D.; Krettek, C. Patient specific pointer tool for corrective osteotomy: Quality of symmetry based planning and case study of ulnar reconstruction surgery. Injury 2017, 48, 1325–1330. [Google Scholar] [CrossRef]
- Haefeli, M.; Schaefer, D.J.; Schumacher, R.; Müller-Gerbl, M.; Honigmann, P. Titanium template for scaphoid reconstruction. J. Hand Surg. 2014, 40, 526–533. [Google Scholar] [CrossRef]
- Katt, B.; Imbergamo, C.; Seigerman, D.; Rivlin, M.; Beredjiklian, P.K. The use of 3D printed customized casts in children with upper extremity fractures: A report of two cases. Arch. Bone Jt. Surg. 2021, 9, 126–130. [Google Scholar]
- Lu, M.; Min, L.; Xiao, C.; Li, Y.; Luo, Y.; Zhou, Y.; Zhang, W.; Tu, C. Uncemented three-dimensional-printed prosthetic replacement for giant cell tumor of distal radius: A new design of prosthesis and surgical techniques. Cancer Manag. Res. 2018, 10, 265–277. [Google Scholar] [CrossRef]
- Beltrami, G. Custom 3D-printed finger proximal phalanx as salvage of limb function after aggressive recurrence of giant cell tumour. BMJ Case Rep. 2018, 2018, 226007. [Google Scholar] [CrossRef] [PubMed]
- Ackmann, T.; Klingebiel, S.; Gosheger, G.; Rachbauer, A.; Theil, C.; Andreou, D. Reconstruction of Total Bone Defects following Resection of Malignant Tumors of the Upper Extremity with 3D Printed Prostheses: Presentation of Two Patients with a Follow-Up of Three Years. Case Rep. Orthop. 2020, 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, H.W.; Jang, W.Y. The usefulness of a three-dimensional printed segmental scapula prosthesis for recovering shoulder function in a patient with scapula chondrosarcoma: A case report. Medicine 2021, 100, e24817. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhao, X.; Wei, C.; Qu, W.; Yu, L.; Zhu, S. Application of a three-dimensional printed segmental scapula prosthesis in the treatment of scapula tumors. J. Int. Med. Res. 2019, 47, 5873–5882. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, Y.; Han, Q.; Yang, K.; Zhang, K.; Wang, J.; Zou, Y. Novel exploration of customized 3D printed shoulder prosthesis in revision of total shoulder arthroplasty A case report. Medicine 2018, 97, e13282. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Shi, Q.; Li, T.; Liu, Z.; Yang, Z.; Liu, Y.; Xu, Y.; Dai, K.; Yu, C. Varisized 3D-Printed Lunate for Kienböck’s Disease in Different Stages: Preliminary Results. Orthop. Surg. 2020, 12, 792–801. [Google Scholar] [CrossRef]
- Xie, M.-M.; Tang, K.-L.; Yuan, C.-S. 3D printing lunate prosthesis for stage IIIc Kienböck’s disease: A case report. Arch. Orthop. Trauma Surg. 2018, 138, 447–451. [Google Scholar] [CrossRef]
- Rossello, M.I. A case of total scaphoid titanium custom-made 3D-printed prostheses with one-year follow-up. Case Rep. Plast. Surg. Hand Surg. 2020, 7, 7–12. [Google Scholar] [CrossRef]
- Wu, N.; Li, S.; Liu, Y.; Zhang, A.; Chen, B.; Han, Q.; Wang, J. Novel exploration of 3D printed personalized total elbow arthroplasty to solve the severe bone defect after internal fixation failure of comminuted distal humerus fracture: A case report. Medicine 2020, 99, E21481. [Google Scholar] [CrossRef]
- Schmauder, P.; Kraus, T.; Küper, M.A.; Ziegler, P.; Ateschrang, A.; Stöckle, U.; Freude, T. Custom-made glenoid component via 3D print: A rescue prosthetic option for patients with massive glenoid destruction and simultaneous cuff-arthropathy. Orthopade 2020, 49, 267–272. [Google Scholar] [CrossRef]
- Luenam, S.; Kosiyatrakul, A.; Hansudewechakul, C.; Phakdeewisetkul, K.; Lohwongwatana, B.; Puncreobutr, C. The Patient-Specific Implant Created with 3D Printing Technology in Treatment of the Irreparable Radial Head in Chronic Persistent Elbow Instability. Case Rep. Orthop. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Yin, Y.; Xu, H.; Li, Z.; Wang, F.; Chen, G. Personalized three-dimensional printed polyether-ether-ketone prosthesis for reconstruction after subtotal removal of chronic clavicle osteomyelitis: A case report. Medicine 2021, 100, e25703. [Google Scholar] [CrossRef]
- Cabibihan, J.-J. Patient-Specific Prosthetic Fingers by Remote Collaboration–A Case Study. PLoS ONE 2011, 6, e19508. [Google Scholar] [CrossRef]
- Young, K.J.; Pierce, J.E.; Zuniga, J.M. Assessment of body-powered 3D printed partial finger prostheses: A case study. 3D Print. Med. 2019, 5, 7–8. [Google Scholar] [CrossRef]
- Xu, G.; Gao, L.; Tao, K.; Wan, S.; Lin, Y.; Xiong, A.; Kang, B.; Zeng, H. Three-dimensional-printed upper limb prosthesis for a child with traumatic amputation of right wrist. Medicine 2017, 96, e9426. [Google Scholar] [CrossRef]
- Alvial, P.; Bravo, G.; Bustos, M.P.; Moreno, G.; Alfaro, R.; Cancino, R.; Zagal, J.C. Quantitative functional evaluation of a 3D–printed silicone-embedded prosthesis for partial hand amputation: A case report. J. Hand Ther. 2018, 31, 129–136. [Google Scholar] [CrossRef]
- Zuniga, J.; Katsavelis, D.; Peck, J.; Stollberg, J.; Petrykowski, M.; Carson, A.; Fernandez, C. Cyborg beast: A low-cost 3d-printed prosthetic hand for children with upper-limb differences. BMC Res. Notes 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Young, K.J.; Peck, J.L.; Srivastava, R.; Pierce, J.E.; Dudley, D.R.; Salazar, D.A.; Bergmann, J. Remote fitting procedures for upper limb 3d printed prostheses. Expert Rev. Med. Devices 2019, 16, 257–266. [Google Scholar] [CrossRef]
- Dote, J.; Nahuelhual, P.; Cubillos, R.; Fuentes, G.; Zuniga, J. 3D-printed hand prostheses function in adolescents with congenital hand amputation: A case series. Rev. Chil. Pediatr. 2020, 91, 410–416. [Google Scholar] [CrossRef]


| Author | Study | Indication | Number of Patients (3D: Conventional) | Clinical Application | 3D Group (Mean Operative Time in Minutes; Mean Intraoperative Blood Loss in mL) | Conventional Group (Operative Time in Minutes) | p-Value |
|---|---|---|---|---|---|---|---|
| Yang et al. [11] | RCT | Elbow fracture | 40 (20:20) | Preoperative planning | 61 35.6 | 82 52.1 | 0.023 <0.001 |
| Chen et al. [12] | RCT | Intraarticular radius fracture | 48 (23:25) | Preoperative planning | 66.5 41.1 | 75.4 54.2 | <0.001 <0.001 |
| Kong et al. [13] | RCT | Intraarticular radius fracture | 32 (16:16) | Preoperative planning | 51.4 52.3 | 63.5 74.2 | <0.001 <0.001 |
| Zheng et al. [14] | RCT | Intraarticular humerus fracture | 91 (43:48) | Preoperative planning | 76.6 231.1 | 92.0 278.6 | <0.0001 <0.0001 |
| Zhang et al. [15] | Retrospective | Cubitus varus deformity | 25 (14:11) | Intraoperative aid | 48.3 35.6 | 73.5 52.1 | <0.001 <0.001 |
| Yin et al. [16] | RCT | Scaphoid fracture/nonunions | 16 (8:8) | Intraoperative aid | 69.4 | 94.1 | 0.012 |
| Schweizer et al. [17] | Prospective | Scaphoid fracture/nonunions | 22 (9:13) | Intraoperative aid | 118 | 150 | 0.01 |
| Bauer et al. [18] | Retrospective | Forearm malunions | 56 (25:31) | Intraoperative aid | 108 | 140 | <0.05 |
| Author | Study | Indication | Number of Patients (3D: Conventional) | Clinical Application | Test | 3D Group | Conventional Group | p-Value |
|---|---|---|---|---|---|---|---|---|
| Yang et al. [11] | RCT | Elbow fracture | 40 (20:20) | Preoperative planning | MEFS | 88.0 | 82 | 0.001 |
| Wang et al. [19] | Retrospective | Carcinoma (giant cell tumor) | 30 (15:15) | Body implant | MWS VAS | 65 1.2 | 71.0 1.3 | 0.013 0.806 |
| Kong et al. [13] | RCT | Intraarticular radius fracture | 32 (16:16) | Preoperative planning | DASH VAS | 23.8 0.9 | 24.5 0.9 | 0.80 0.91 |
| Zheng et al. [14] | RCT | Intraarticular humerus fracture | 91 (43:48) | Preoperative planning | MEFS | 85.2 | 83.1 | 0.448 |
| Chen et al. [20] | RCT | Forearm fractures | 60 (20:20:20) | Orthosis | GOBS | 85 | 65.0/70 | 0.014/0.035 |
| Yin et al. [16] | RCT | Scaphoid fracture/nonunions | 16 (8:8) | Intraoperative aid | MMS PRWE VAS | 9.4 −11.6 −4.2 | 5.6 −16.7 −4.17 | 0.52 0.52 0.98 |
| Kim et al. [21] | RCT | Wrist pain | 22 (11:11) | Orthosis | JHFT PRWE | 4.3 19.2 | 1.0 23.4 | 0.101 0.109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hecker, A.; Tax, L.; Giese, B.; Schellnegger, M.; Pignet, A.-L.; Reinbacher, P.; Watzinger, N.; Kamolz, L.-P.; Lumenta, D.B. Clinical Applications of Three-Dimensional Printing in Upper Extremity Surgery: A Systematic Review. J. Pers. Med. 2023, 13, 294. https://doi.org/10.3390/jpm13020294
Hecker A, Tax L, Giese B, Schellnegger M, Pignet A-L, Reinbacher P, Watzinger N, Kamolz L-P, Lumenta DB. Clinical Applications of Three-Dimensional Printing in Upper Extremity Surgery: A Systematic Review. Journal of Personalized Medicine. 2023; 13(2):294. https://doi.org/10.3390/jpm13020294
Chicago/Turabian StyleHecker, Andrzej, Lukas Tax, Barbara Giese, Marlies Schellnegger, Anna-Lisa Pignet, Patrick Reinbacher, Nikolaus Watzinger, Lars-Peter Kamolz, and David Benjamin Lumenta. 2023. "Clinical Applications of Three-Dimensional Printing in Upper Extremity Surgery: A Systematic Review" Journal of Personalized Medicine 13, no. 2: 294. https://doi.org/10.3390/jpm13020294
APA StyleHecker, A., Tax, L., Giese, B., Schellnegger, M., Pignet, A.-L., Reinbacher, P., Watzinger, N., Kamolz, L.-P., & Lumenta, D. B. (2023). Clinical Applications of Three-Dimensional Printing in Upper Extremity Surgery: A Systematic Review. Journal of Personalized Medicine, 13(2), 294. https://doi.org/10.3390/jpm13020294








