Clinical Features and Outcomes of Japanese Patients with Giant Cell Arteritis: A Comparison with Takayasu Arteritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Evaluations
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with GCA and TA
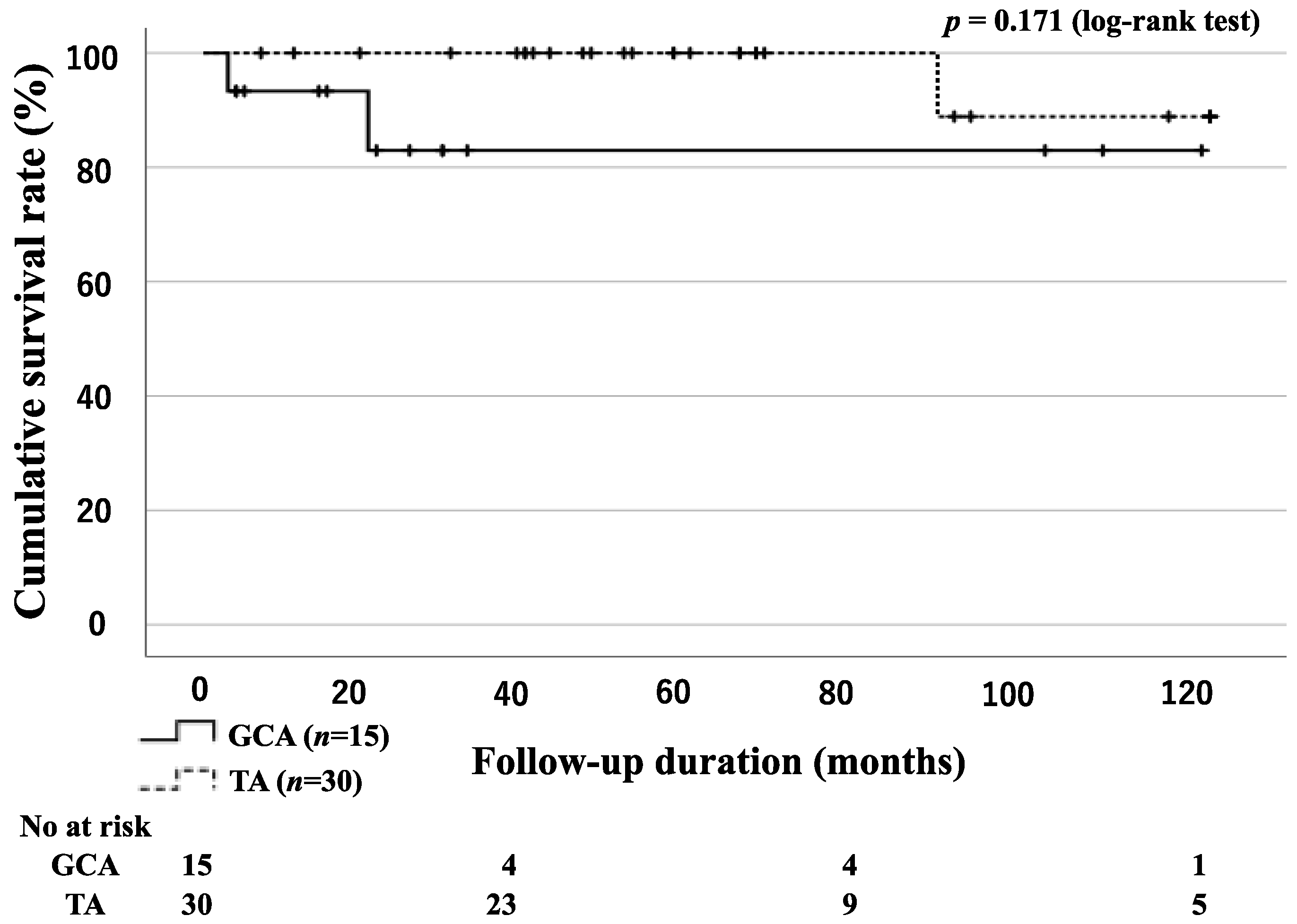
3.2. Survival Rates for Patients with GCA and TA
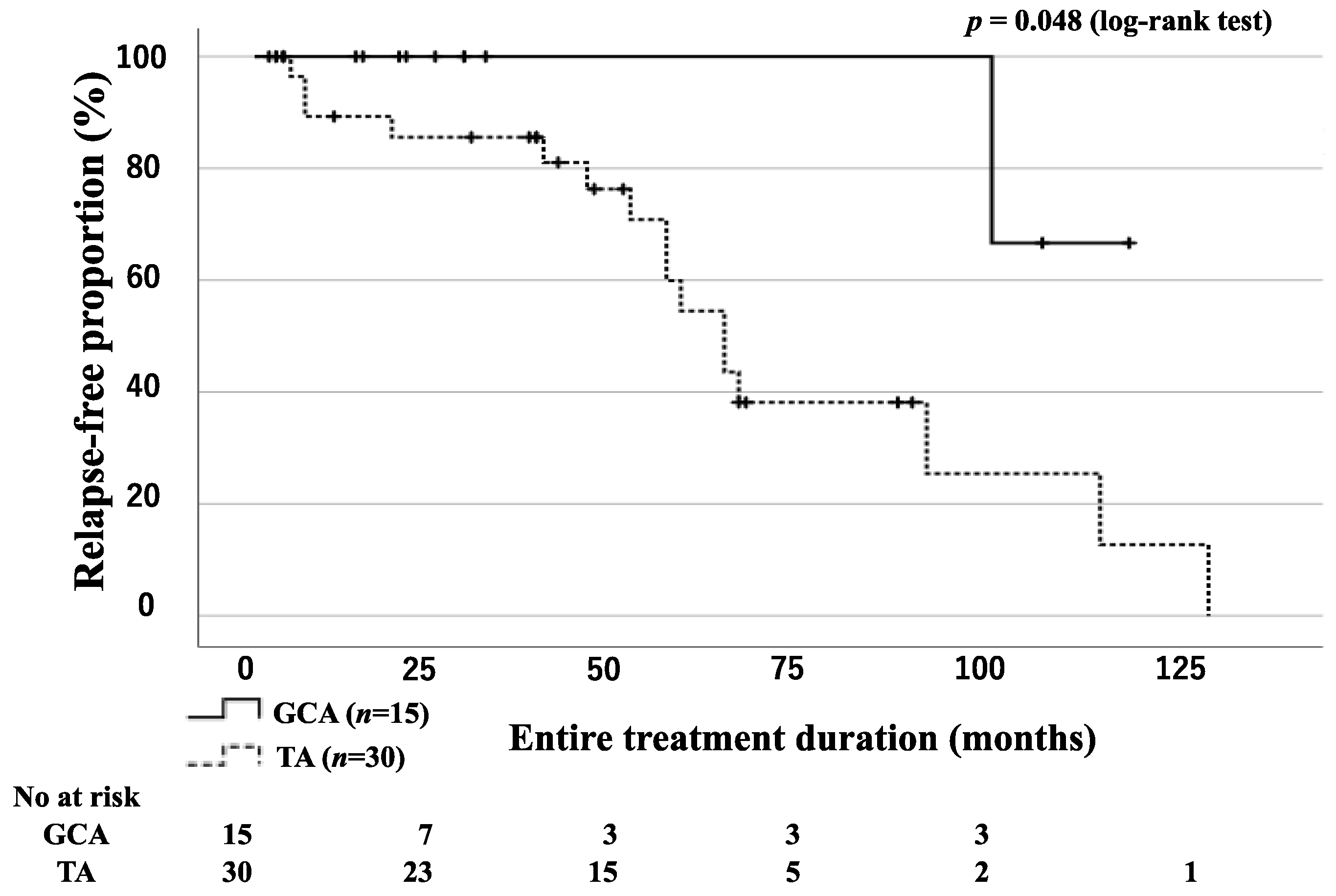
3.3. Relapse-Free Survival Rates for Patients with GCA and TA
3.4. Incidence of Cardiovascular Events in Patients with GCA and TA
3.5. Tapering of GC Dose
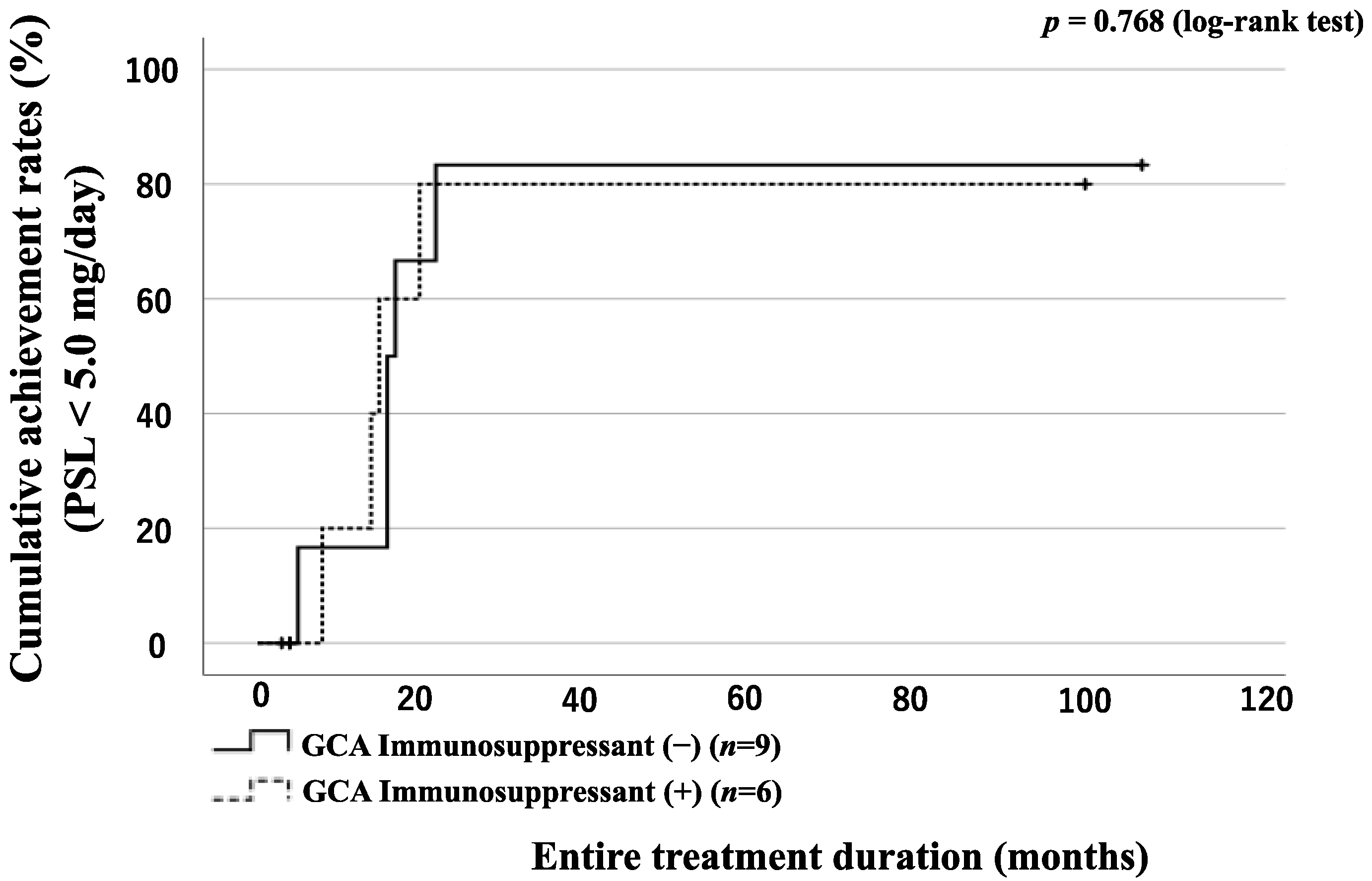
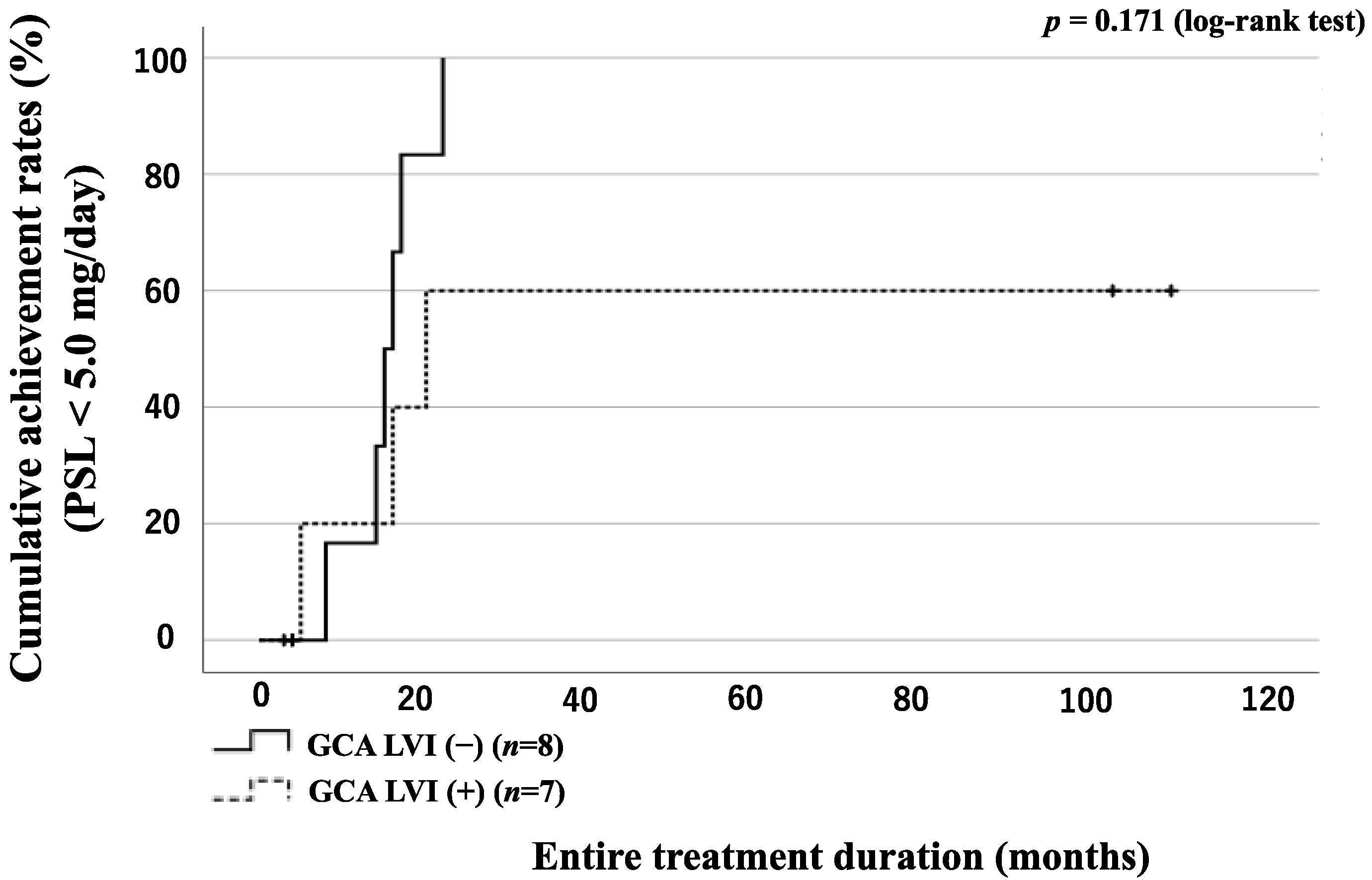
3.6. GC Tapering According to the Presence of LVI or Concomitant Use of Immunosuppressant in GCA Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GCA: | giant cell arteritis |
| TA: | Takayasu arteritis |
| LVI: | large vessel involvement |
| ACR: | American College of Rheumatology |
| JCS: | Japanese Circulation Society |
| ESR: | erythrocyte sedimentation rate |
| CRP: | C-reactive protein |
| EULAR: | European Alliance of Associations for Rheumatology |
| LVV: | large vessel vasculitis |
| GCs: | glucocorticoids |
| MTX: | methotrexate |
| TCZ: | tocilizumab |
| PSL: | prednisolone |
| GiACTA: | Giant Cell Arteritis ACTEMRA |
References
- Ness, T.; Bley, T.A.; Schmidt, W.A.; Lamprecht, P. The diagnosis and treatment of giant cell arteritis. Dtsch. Arztebl. Int. 2013, 110, 376–386. [Google Scholar] [CrossRef] [PubMed]
- de Boysson, H.; Daumas, A.; Vautier, M.; Parienti, J.J.; Liozon, E.; Lambert, M.; Samson, M.; Ebbo, M.; Dumont, A.; Sultan, A.; et al. Large-vessel involvement and aortic dilation in giant-cell arteritis. A multicenter study of 549 patients. Autoimmun. Rev. 2018, 17, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Garcia-Porrua, C.; Piñeiro, A.; Pego-Reigosa, R.; Llorca, J.; Hunder, G.G. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: A population-based study. Medicine 2004, 83, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Kermani, T.A.; Warrington, K.J.; Crowson, C.S.; Ytterberg, S.R.; Hunder, G.G.; Gabriel, S.E.; Matteson, E.L. Large-vessel involvement in giant cell arteritis: A population-based cohort study of the incidence-trends and prognosis. Ann. Rheum. Dis. 2013, 72, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- de Boysson, H.; Liozon, E.; Espitia, O.; Daumas, A.; Vautier, M.; Lambert, M.; Parienti, J.J.; Granel, B.; Dumont, A.; Sultan, A.; et al. Different patterns and specific outcomes of large-vessel involvements in giant cell arteritis. J. Autoimmun. 2019, 103, 102283. [Google Scholar] [CrossRef]
- Proven, A.; Gabriel, S.E.; Orces, C.; O’Fallon, W.M.; Hunder, G.G. Glucocorticoid therapy in giant cell arteritis: Duration and adverse outcomes. Arthritis Rheum. 2003, 49, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Martinez-Dubois, C.; Agudo, M.; Pompei, O.; Blanco, R.; Llorca, J. Giant cell arteritis: Epidemiology, diagnosis, and management. Curr. Rheumatol. Rep. 2010, 12, 436–442. [Google Scholar] [CrossRef]
- Walker, B.R. Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 2007, 157, 545–559. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Loricera, J.; Blanco, R.; Hernández, J.L.; Castañeda, S.; Mera, A.; Pérez-Pampín, E.; Peiró, E.; Humbría, A.; Calvo-Alén, J.; Aurrecoechea, E.; et al. Tocilizumab in giant cell arteritis: Multicenter open-label study of 22 patients. Semin. Arthritis Rheum. 2015, 44, 717–723. [Google Scholar] [CrossRef]
- Calderón-Goercke, M.; Loricera, J.; Aldasoro, V.; Castañeda, S.; Villa, I.; Humbría, A.; Moriano, C.; Romero-Yuste, S.; Narváez, J.; Gómez-Arango, C.; et al. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin. Arthritis Rheum. 2019, 49, 126–135. [Google Scholar] [CrossRef]
- Stamatis, P. Giant Cell Arteritis versus Takayasu Arteritis: An Update. Mediterr. J. Rheumatol. 2020, 31, 174–182. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Gomez-Acebo, I.; Pego-Reigosa, R.; Lopez-Diaz, M.J.; Vazquez-Triñanes, M.C.; Miranda-Filloy, J.A.; Blanco, R.; Dierssen, T.; Gonzalez-Juanatey, C.; et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine 2009, 88, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R.; Malmvall, B.E.; Bengtsson, B.A. Long-term survival in giant cell arteritis including temporal arteritis and polymyalgia rheumatica. A follow-up study of 90 patients treated with corticosteroids. Acta Med. Scand. 1986, 220, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Matteson, E.L.; Gold, K.N.; Bloch, D.A.; Hunder, G.G. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am. J. Med. 1996, 100, 193–196. [Google Scholar] [CrossRef]
- Tomasson, G.; Peloquin, C.; Mohammad, A.; Love, T.J.; Zhang, Y.; Choi, H.K.; Merkel, P.A. Risk for cardiovascular disease early and late after a diagnosis of giant-cell arteritis: A cohort study. Ann. Intern. Med. 2014, 160, 73–80. [Google Scholar] [CrossRef]
- Mainbourg, S.; Addario, A.; Samson, M.; Puéchal, X.; François, M.; Durupt, S.; Gueyffier, F.; Cucherat, M.; Durieu, I.; Reynaud, Q.; et al. Prevalence of Giant Cell Arteritis Relapse in Patients Treated With Glucocorticoids: A Meta-Analysis. Arthritis Care Res. 2020, 72, 838–849. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; García-Porrúa, C. Epidemiology of the vasculitides. Rheum. Dis. Clin. N. Am. 2001, 27, 729–749. [Google Scholar] [CrossRef]
- Konda, N.; Sakai, R.; Saeki, K.; Matsubara, Y.; Nakamura, Y.; Miyamae, T.; Nakaoka, Y.; Harigai, M. Nationwide clinical and epidemiological study of large-vessel vasculitis in Japan in 2017. Mod. Rheumatol. 2023, road019. [Google Scholar] [CrossRef]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef]
- Japanese Circulation Society (JCS) Joint Working Group. Guideline for management of vasculitis syndrome (JCS 2008). Circ. J. 2011, 75, 474–503. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Cid, M.C.; Hellmann, D.B.; Guillevin, L.; Stone, J.H.; Schousboe, J.; Cohen, P.; Calabrese, L.H.; Dickler, H.; Merkel, P.A.; et al. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002, 46, 1309–1318. [Google Scholar] [CrossRef]
- Hoffman, G.S.; Cid, M.C.; Rendt-Zagar, K.E.; Merkel, P.A.; Weyand, C.M.; Stone, J.H.; Salvarani, C.; Xu, W.; Visvanathan, S.; Rahman, M.U. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: A randomized trial. Ann. Intern. Med. 2007, 146, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Jover, J.A.; Hernández-García, C.; Morado, I.C.; Vargas, E.; Bañares, A.; Fernández-Gutiérrez, B. Combined treatment of giant-cell arteritis with methotrexate and prednisone. a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2001, 134, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G. Takayasu’s arteritis. Curr. Opin. Rheumatol. 1994, 6, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Watanabe, R.; Ishii, T.; Machiyama, T.; Akita, K.; Fujita, Y.; Shirota, Y.; Sugimura, K.; Fujii, H.; Shimokawa, H.; et al. Retrospective analysis of 95 patients with large vessel vasculitis: A single center experience. Int. J. Rheum. Dis. 2016, 19, 87–94. [Google Scholar] [CrossRef]
- Brekke, L.K.; Fevang, B.S.; Diamantopoulos, A.P.; Assmus, J.; Esperø, E.; Gjesdal, C.G. Survival and death causes of patients with giant cell arteritis in Western Norway 1972–2012: A retrospective cohort study. Arthritis Res. Ther. 2019, 21, 154. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Blanco, R.; Abraira, V.; García-Porrúa, C.; Ibáñez, D.; García-Pais, M.J.; Rigueiro, M.T.; Sánchez-Andrade, A.; Guerrero, J.; Casariego, E. Giant cell arteritis in Lugo, Spain, is associated with low longterm mortality. J. Rheumatol. 1997, 24, 2171–2176. [Google Scholar]
- Gran, J.T.; Myklebust, G.; Wilsgaard, T.; Jacobsen, B.K. Survival in polymyalgia rheumatica and temporal arteritis: A study of 398 cases and matched population controls. Rheumatology 2001, 40, 1238–1242. [Google Scholar] [CrossRef]
- Maksimowicz-McKinnon, K.; Clark, T.M.; Hoffman, G.S. Takayasu arteritis and giant cell arteritis: A spectrum within the same disease? Medicine 2009, 88, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Kadoba, K.; Watanabe, R.; Iwasaki, T.; Kitagori, K.; Akizuki, S.; Murakami, K.; Nakashima, R.; Hashimoto, M.; Tanaka, M.; et al. Clinical Profile and Outcome of Large-Vessel Giant Cell Arteritis in Japanese Patients: A Single-Center Retrospective Cohort Study. Mod. Rheumatol. 2023, 33, 175–181. [Google Scholar] [CrossRef]
- González-Gay, M.A.; Prieto-Peña, D.; Martínez-Rodríguez, I.; Calderon-Goercke, M.; Banzo, I.; Blanco, R.; Castañeda, S. Early large vessel systemic vasculitis in adults. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101424. [Google Scholar] [CrossRef] [PubMed]
- Mahr, A.D.; Jover, J.A.; Spiera, R.F.; Hernández-García, C.; Fernández-Gutiérrez, B.; Lavalley, M.P.; Merkel, P.A. Adjunctive methotrexate for treatment of giant cell arteritis: An individual patient data meta-analysis. Arthritis Rheum. 2007, 56, 2789–2797. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | GCA (n = 15) | TA (n = 30) | p-Value |
|---|---|---|---|
| Male, n (%) | 4 (26.7) | 4 (13.3) | 0.270 |
| Age at onset (years), median (range) | 77 (57–89) | 24 (16–72) | <0.001 * |
| Follow-up period (months), median (range) | 25 (3–121) | 58 (7–122) | 0.073 |
| Entire treatment period (months), median (range) | 25 (3-121) | 58 (7-222) | 0.073 |
| Hypertension, n (%) | 9 (60) | 2 (6.7) | <0.001 * |
| Arterial stenosis, n (%) | 0 (0) | 8 (26.6) | 0.027 * |
| Aneurysm, n (%) | 2 (13.3) | 2 (6.7) | 0.459 |
| Arterial involvement, n (%) | 7 (46.7) | 30 (100) | <0.001 * |
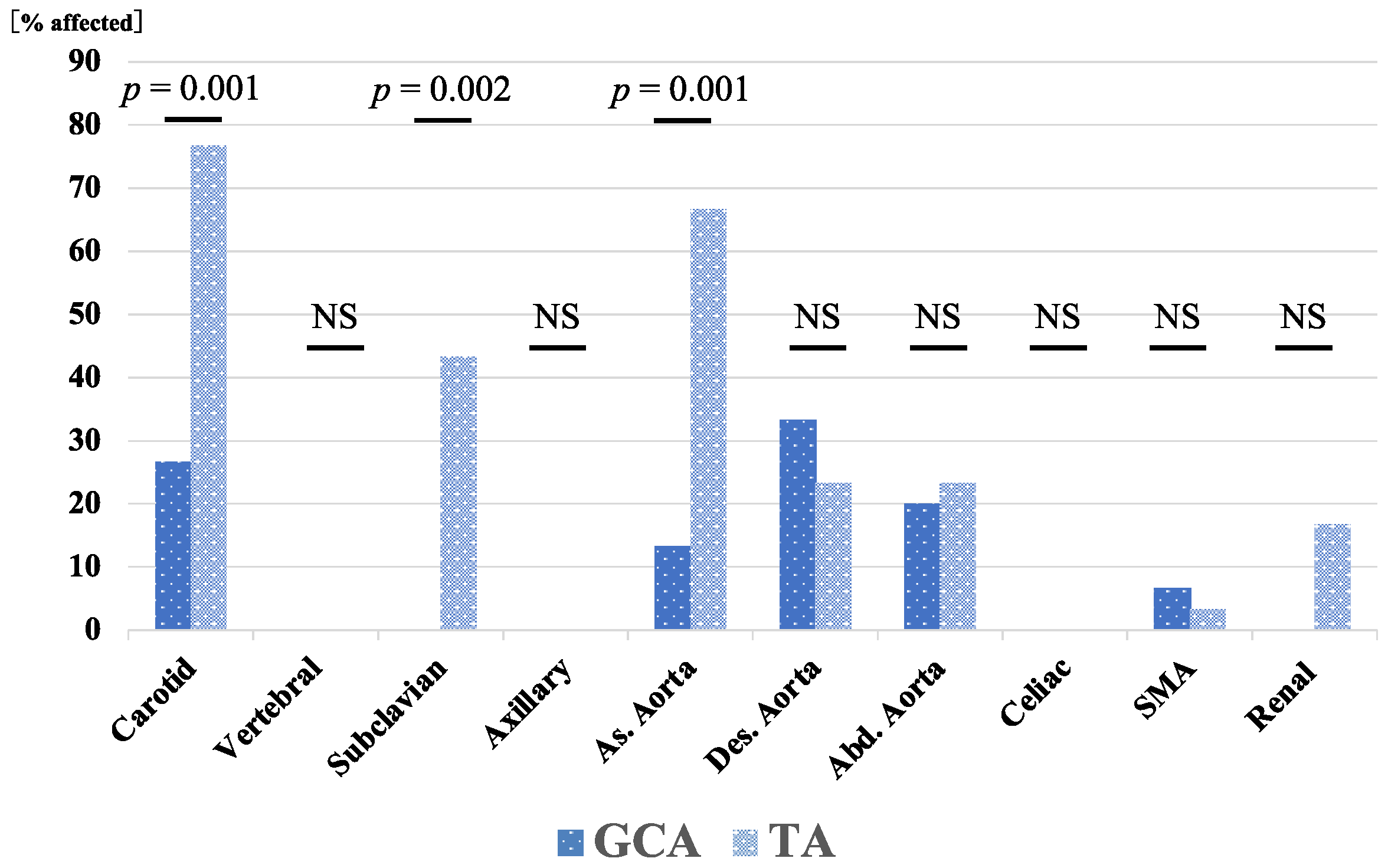
| Carotid artery, n (%) | 4 (26.7) | 23 (76.7) | 0.001 * |
| Vertebral artery, n (%) | 0 (0) | 0 (0) | |
| Subclavian artery, n (%) | 0 (0) | 13 (43.3) | 0.002 * |
| Axillary artery, n (%) | 0 (0) | 0 (0) | |
| Ascending aorta, n (%) | 2 (13.3) | 20 (66.7) | 0.001 * |
| Descending aorta, n (%) | 5 (33.3) | 7 (23.3) | 0.475 |
| Abdominal aorta, n (%) | 3 (20) | 7 (23.3) | 0.8 |
| Celiac artery, n (%) | 0 (0) | 0 (0) | |
| Superior mesenteric artery, n (%) | 1 (6.7) | 1 (3.3) | 0.609 |
| Renal artery, n (%) | 0 (0) | 5 (16.7) | 0.094 |
| Initial dose of prednisolone (mg/day), median (range) | 30 (10–40) | 40 (0–60) | 0.187 |
| Steroid pulse therapy, n (%) | 1 (6.7) | 9 (30) | 0.076 |
| Autoimmune comorbidities, n (%) | 1 (6.7) | 6 (20) | 0.245 |
| Immunosuppressants, n (%) | 2 (13.3) | 7 (23.3) | 0.429 |
| TCZ, n (%) | 4 (26.6) | 15 (50) | 0.135 |
| Death, n (%) | 2 (13.3) | 1 (3.3) | 0.205 |
| ESR (mm/1st hour), median (range) | 65 (22-95) | 56 (27–111) | 0.488 |
| CRP (mg/dl), median (range) | 6.09 (0.33–21.31) | 6.30 (0.2–35.09) | 0.951 |
| Relapse during the entire treatment period, n (%) | 1 (6.7) | 16 (53.3) | 0.002 * |
| Relapse rate (per 100 person years), n | 1.88 | 14.49 | |
| Rate ratio, (95% CI) | 6.78 (1.39-162.93) | 0.013 * | |
| Characteristics | Value |
|---|---|
| Number, n | 15 |
| ACR criteria for active disease, n (%) | |
| Age at disease onset ≥ 50 years | 15 (100) |
| New headache | 15 (100) |
| Temporal artery abnormality | 11 (73.3) |
| Elevated ESR | 15 (100) |
| Abnormal temporal artery biopsy | 4 (26.7) |
| PMR involvement, n (%) | 7 (46.7) |
| Aches or pain in shoulders, n/7 (%) | 5/7 (71.4) |
| Aches or pain in the neck, upper arms, buttocks, hips, or thighs, n (%) | 6/7 (85.7) |
| Stiffness in affected areas, n (%) | 5/7 (71.4) |
| Limited range of motion in affected areas, n (%) | 2/7 (28.6) |
| Mild fever, n (%) | 5/7 (71.4) |
| Fatigue, n (%) | 4/7 (57.1) |
| Unintended weight loss, n (%) | 6/7 (85.7) |
| Depression, n (%) | 4/7 (57.1) |
| Ischemic optic neuropathy, n (%) | 0 (0) |
| Aortic regurgitation, n (%) | 1 (6.7) |
| Malignancy, n (%) | 2 (13.3) |
| Characteristics | Value |
|---|---|
| Number, n | 30 |
| HLA typing | |
| HLA-B52 positive, n/patients HLA tested (%) | 7/25 (28.0) |
| Classification of Takayasu arteritis, n (%) | |
| I | 8 (26.7) |
| IIA | 6 (20) |
| IIB | 6 (20) |
| III | 0 (0) |
| IV | 0 (0) |
| V | 10 (33.3) |
| Characteristics | LVI(+) (n = 7) | LVI(−) (n = 8) | p-Value |
|---|---|---|---|
| Male, n (%) | 2 (28.6) | 2 (25.0) | 0.876 |
| Age at onset (years), median (range) | 80 (57–89) | 76 (65–86) | 0.598 |
| Entire treatment period (months), median (range) | 29 (15–121) | 17 (3–109) | 0.619 |
| ACR criteria for active disease | |||
| Age at disease onset ≧ 50 years | 7 (100) | 8 (100) | |
| New headache | 7 (100) | 8 (100) | |
| Temporal artery abnormality | 4 (57.1) | 7 (87.5) | 0.185 |
| Elevated ESR | 7 (100) | 8 (100) | |
| Abnormal temporal artery biopsy | 2 (28.6) | 2 (25.0) | 0.876 |
| PMR, n (%) | 2 (28.6) | 5 (62.5) | 0.189 |
| Hypertension, n (%) | 6 (85.7) | 3 (37.5) | 0.057 |
| Malignancy, n (%) | 0 (0) | 2 (25.0) | 0.155 |
| Steroid pulse therapy, n (%) | 1 (14.3) | 0 (0) | 0.268 |
| Initial dose of prednisolone (mg/day), median (range) | 30 (20–40) | 30 (10–40) | 0.875 |
| Immunosuppressants, n (%) | 0 (0) | 2 (25.0) | 0.155 |
| TCZ, n (%) | 4 (57.1) | 0 (0) | 0.013 * |
| Death, n (%) | 0 (0) | 2 (25.0) | 0.155 |
| Relapse, n (%) | 0 (0) | 1 (12.5) | 0.333 |
| ESR (mm/1st hour), median (range) | 59 (22–95) | 70.5 (36–88) | 0.557 |
| CRP (mg/dl), median (range) | 3.77 (0.99–9.76) | 13.3 (0.3321.34) | 0.009 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, S.; Suzuki, E.; Sumichika, Y.; Saito, K.; Matsumoto, H.; Temmoku, J.; Fujita, Y.; Matsuoka, N.; Asano, T.; Sato, S.; et al. Clinical Features and Outcomes of Japanese Patients with Giant Cell Arteritis: A Comparison with Takayasu Arteritis. J. Pers. Med. 2023, 13, 387. https://doi.org/10.3390/jpm13030387
Yoshida S, Suzuki E, Sumichika Y, Saito K, Matsumoto H, Temmoku J, Fujita Y, Matsuoka N, Asano T, Sato S, et al. Clinical Features and Outcomes of Japanese Patients with Giant Cell Arteritis: A Comparison with Takayasu Arteritis. Journal of Personalized Medicine. 2023; 13(3):387. https://doi.org/10.3390/jpm13030387
Chicago/Turabian StyleYoshida, Shuhei, Eiji Suzuki, Yuya Sumichika, Kenji Saito, Haruki Matsumoto, Jumpei Temmoku, Yuya Fujita, Naoki Matsuoka, Tomoyuki Asano, Shuzo Sato, and et al. 2023. "Clinical Features and Outcomes of Japanese Patients with Giant Cell Arteritis: A Comparison with Takayasu Arteritis" Journal of Personalized Medicine 13, no. 3: 387. https://doi.org/10.3390/jpm13030387
APA StyleYoshida, S., Suzuki, E., Sumichika, Y., Saito, K., Matsumoto, H., Temmoku, J., Fujita, Y., Matsuoka, N., Asano, T., Sato, S., Watanabe, H., & Migita, K. (2023). Clinical Features and Outcomes of Japanese Patients with Giant Cell Arteritis: A Comparison with Takayasu Arteritis. Journal of Personalized Medicine, 13(3), 387. https://doi.org/10.3390/jpm13030387






