Abstract
We aimed to investigate high-order aberration (HOA) change between topography-guided (TG) and wavefront-optimized (WFO) laser in situ keratomileusis (LASIK) in patients with different degrees of myopia. A non-randomized clinical trial was conducted, in which 40 eyes of 20 patients aged 20–50 years old were included. Participants received TG-LASIK in one eye and WFO-LASIK on the alternate eye. Corneal topography and HOAs including coma, trefoil, spherical aberration (SA), and contrast sensitivity (CS) were collected. Moreover, a quality of vision (QoV) questionnaire was completed by each participant. Non-parametric tests were used to infer the difference in HOAs and CS between the TG-LASIK and WFO-LASIK groups, and subgroup analyses stratified by myopia degree were performed. The high-myopia patients with TG-LASIK showed more coma and SA compared to low-myopia individuals (all 95% CI lower limits > 0), and subjects who received WFO-LASIK exhibited more SA in high-myopia status (both 95% CI lower limits > 0). The TG-LASIK group showed lower postoperative trefoil compared to the WFO-LASIK group in the high-myopia population (mean difference: −0.1267, 95% CI: −0.24 to −0.01). The TG-LASIK group yielded less surgically induced haze, better clarity at night, and better total quality scores (all p < 0.05). In conclusion, TG-LASIK might yield less postoperative trefoil in high-myopia patients and higher QoV in the general population compared to the WFO-LASIK procedure.
1. Introduction
Laser in situ keratomileusis (LASIK) has been established as an effective treatment for refractive errors such as myopia, hyperopia, and astigmatism in recent decades [1]. In a previous study, conventional LASIK was noted to produce high-order aberrations (HOA) after surgery, which cause post-surgical symptoms such as glare, halos, haze, light sensitivity, and loss of contrast sensitivity, which can influence the visual quality [2].
The field of refractive surgery has since shifted to custom correction tailored to the patient’s HOA refractive error, such as wavefront-optimized (WFO) LASIK [3]. However, as these corneal refractive surgery patients age, cataract surgery and intraocular lens calculation become a problem, as the lens aberration must be factored onto the cornea with WFG-LASIK. Changing to an aspherical intraocular lens would probably worsen the spherical aberration (SA) of the patient [4]; therefore, the refractive surgery community has returned to surface treatment, whereby customization occurs on the anterior surface of the cornea. Topography-guided (TG) LASIK customizes the laser treatment to the unique surface condition of the patient, with the target of annihilating HOA [5].
A review of the literature comparing the performance of different LASIK profiles reveals that most patients in these studies have low- or middle-level of myopia [6,7,8,9]. Taiwan’s population has nearly the highest myopia rate in the whole world and, in terms of severity, has the most severe high myopia, i.e., more than 6.0 diopters (D), with up to 22% suffering from this condition [10,11]. With the high amount of myopia, different HOA amounts may be yielded with different LASIK types, such as WFO-LASIK and TG-LASIK; however, this concept requires further study to validate the possibility.
Accordingly, the aim of our study is to analyze and compare outcomes between TG-LASIK and WFO-LASIK in terms of visual performance and the change in different types of HOA, including coma, trefoil, and SA, in patients with high myopia. We also administered a subjective quality of vision (QoV) questionnaire to confirm whether the alleged superiority of TG-LASIK has translated to real-life scenarios and is noticeable to patients.
2. Materials and Methods
2.1. Patients
The inclusion criteria for this study were as follows: age between 20 and 50 years old, corrected distance visual acuity (CDVA) of both eyes reaching a 0.1 logarithm of the minimum angle of resolution (log MAR), and eyes wit stable refractive errors of myopia and astigmatism within the last 6 months. The exclusion criteria were as follows: cataracts; corneal opacities or irregularities; dry eye (Schirmer’s test, I ≤ 5 mm); amblyopia; coexisting ocular pathologies; glaucoma; non-dilating pupils; history of intraocular surgery, laser therapy, or retinopathy; optic nerve or macular diseases; estimated postoperative cornea residual stromal thickness less than 250 μm; pregnancy or under lactation; uncontrolled diabetic mellitus or systemic immune disease; or refusal or inability to maintain follow-up. This study included 20 patients and 40 eyes, among which 20 eyes received TG ablation and the other contralateral 20 eyes received WFO ablation.
2.2. Surgical Technique and Preoperative Assessment
All LASIK surgeries were performed by one of two experienced surgeons (C.K.C. and E.L.C.M.) using an identical technique. In all the operated eyes, a 9.0 mm diameter flap was created using a femtosecond laser (WaveLight FS200, Alcon, Fort Worth, TX, USA) with a hinge on the superior and flap the thickness set to 110 μm; refractive laser ablation was performed utilizing an excimer laser WaveLight EX500 with Contoura Vision software input (both Alcon Laboratories, Fort Worth, TX, USA) in all cases. We designated a Contoura-based TG ablation profile to the dominant eye and a WFO ablation profile to the non-dominant eye in all patients. The treatment target was based on manifest refraction with adjustment using Alcon’s nomogram of LASIK, with both eyes corrected to emmetropia. In the TG group, adjusted manifest refractions were used, which were based on corneal topography images obtained from a WaveLight Topolyzer VARIO device and then transferred to Contoura Vision software to correct the HOA; at least three consistent images were acquired for all eyes according to the manufacturer’s protocol.
2.3. Ophthalmic Examinations and Postoperative Assessment
The patients were examined preoperatively, as well as on post-op day 1, the 1st week, 1st month, and 3rd month after surgery. During each visit, we performed a thorough ophthalmologic examination that included tests for UCVA and CDVA, manifest refraction, biomicroscopy, and pneumotonometry. Fundus examination and cycloplegic refraction were performed before surgery. Ocular dominance was evaluated preoperatively by the hole-in-the-card method described in the literature [12]. The following four types of postoperative measurements were used to compare the performance between TG and WFO ablation: (i) ophthalmic examinations, including uncorrected distance visual acuity (UDVA) and CDVA; (ii) cornea wavefront examination, including coma, trefoil, and SA; (3) contrast sensitivity (CS), including 3 cycles per degree (CPD), 6 CPD, 12 CPD, and 18 CPD with glare off and on under photopic and mesopic lighting conditions; and (iv) a QoV questionnaire, including a score of 11 questions and mean total score. Corneal topography and corneal wavefront analyses were performed preoperatively and during the 1st week, 1st month, and 3rd month postoperative visits with a Placido disc system (WaveLight Allegro Topolyzer Vario, Alcon, Fort Worth, TX, USA). Higher-order aberrations were assessed, including the RMS errors of coma (Z 31), trefoil (Z 33), and spherical aberration (Z 40). CS was measured preoperatively and during the 1st week, 1st month, and 3rd month postoperative visits using Vector Vision CSV-1000 (Greenville, OH, USA) chart. All subjects were tested at the recommended distance of 8 feet. CSV-1000 consists of a series of circular achromatic sine-wave patches 1.5 inch in diameter and comprising four rows, each corresponding to one of four spatial frequencies, i.e., 3, 6, 12, or 18 CPD. The QoV questionnaire was administered during the 1st week, 1st month, and 3rd month postoperative visits. Subjective QoV was evaluated using an 11-item questionnaire adopted from a previous study [13]. Our questionnaire contained multiple subscales, including glare at night and glare during the day; haze, halos, and clarity at night; clarity during the day; problems with dry eyes; severity of dry eyes; gritty or sandy feelings; fluctuating vision; and double vision or ghost images. The item scores ranged from 0 to 10, and higher scores on the questionnaire indicated more difficulty in achieving specific visual tasks and subsequent poor QoV. Rasch scaling was used to convert the logistic scale to a linear scale ranging from 0 to 100, with higher scores indicating poorer QoV in the patients.
2.4. Statistical Analysis
Rasch analysis was used to evaluate the QoV questionnaire and determine whether all items measured a single underlying construct [14]. The raw ordinal scores were then converted to interval scores, which were used in parametric statistical tests. We also used Rasch analysis to assess item hierarchy (items were ordered from least to most difficult) and person separation statistics (distinction between groups of participants based on the extent of the underlying construct). The person separation index was set at 2.0. The mean square outfit statistics of each questionnaire item were set as 0.80 to 1.20. We transferred items that fit the Rasch model from the ordinal data to numerical data ranging from 0 to 100. Questionnaire data were analyzed using WINSTEPS Version 4.8.2. Other statistical tests included the Kruskal–Wallis rank test for continuous data, Pearson’s chi-squared test for ordinal data, and Wilcoxon matched-pair signed rank test for matched continuous data. Under the study design, all factors were matched between each pair of TG eye and WFO eye, except for the level of myopia, in which high myopia was defined by a cutoff of more than −6.0D spherical equivalent (SE). We drew a forest plot to show the mean difference of HOAs between the TG-LASIK and WFO-LASIK groups, as well as the low-myopia population and high-myopia population, with a 95% confidence interval (CI). Statistical significance was set at p < 0.05.
3. Results
3.1. Demographic Data and Visual Outcome
Table 1 presents preoperative demographic data and postoperative data. Because of the contralateral eye design of the study, there were no significant differences in gender, CDVA, manifest refraction, CS, or corneal HOA before surgery among the study population (Table 1). In the two stratified groups, neither the refractive spherical nor cylinder were found to have significant differences. The postoperative visual acuity UDVA, CDVA, and SE between the TG-LASIK and WFO-LASIK groups were not significantly different (Table 2).

Table 1.
Preoperative demographics, visual acuity, contrast sensitivity, and corneal wavefront aberration between the two groups.

Table 2.
Postoperative visual acuity and spherical equivalent at 3 months.
3.2. HOAs and CS Outcome
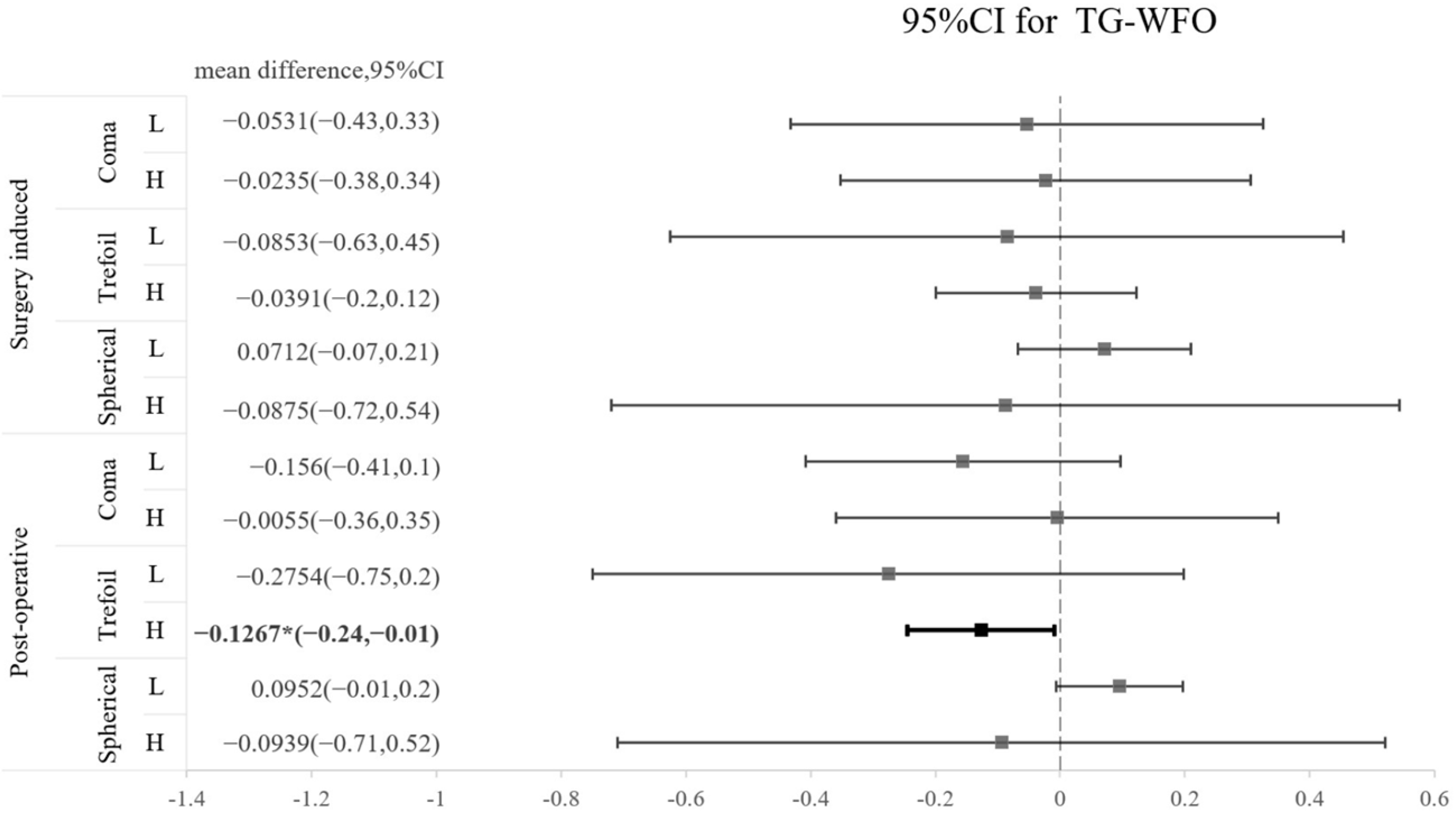
The SA and coma in both groups were significantly increased after operation, whereas trefoil aberration did not show a significant increase after operation in either type of surgery (Table 3). A comparison of the three types of HOA between TG-LASIK and WFO-LASIK is presented as a forest plot in Figure 1. We found that trefoil was marginally lower by TG-LASIK ablation in high-myopia patients than in the WFO-LASIK ablation group, with a mean difference of −0.1267 (CI −0.24 to −0.01); however, this result was not observed in the surgically induced trefoil (Figure 1). The CS 3 months after the LASIK procedure did not reveal a significant difference between the TG-LASIK and WFO-LASIK groups (all p > 0.05) (Table 4).

Table 3.
Postoperative change in corneal wavefront aberration for the TG and WFO-LASIK groups after 3 months.
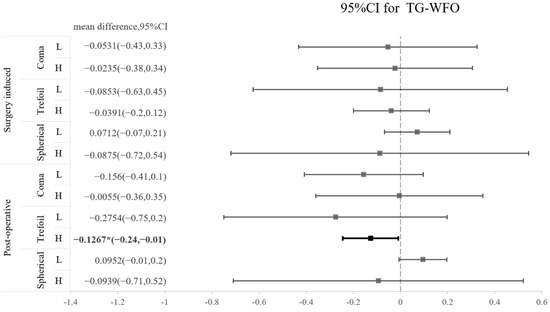
Figure 1.
Forest plot of the standardized mean difference in surgically induced HOA or postoperative HOA between TG and WFO on coma, trefoil, and spherical aberration with 95% confidence intervals stratified by level of myopia (L: low; H: high).

Table 4.
Preoperative and postoperative contrast sensitivity between TG-LASIK groups after 3 months.
3.3. High-Myopia vs. Low-Myopia Analysis for HOAs and CS
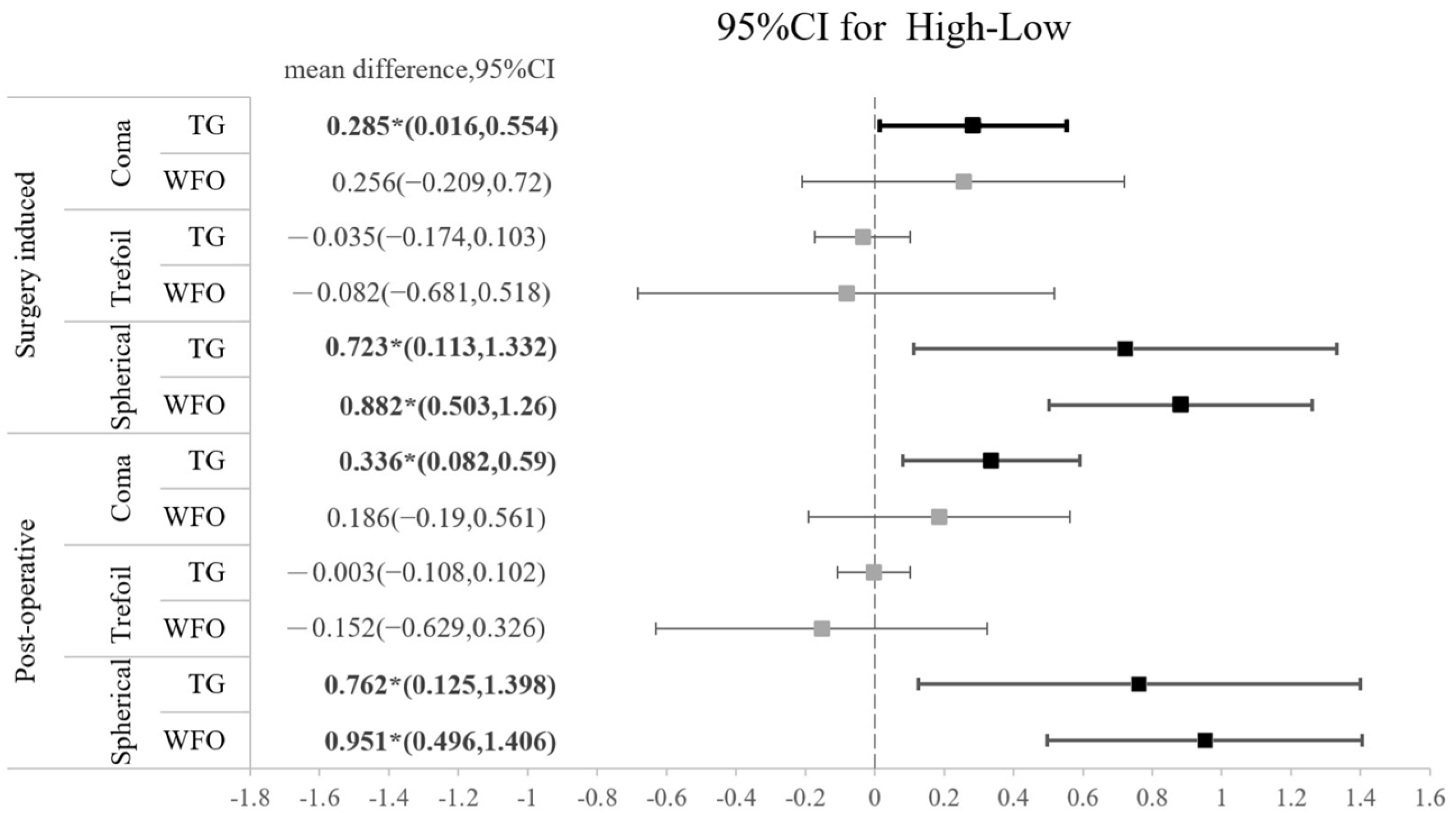
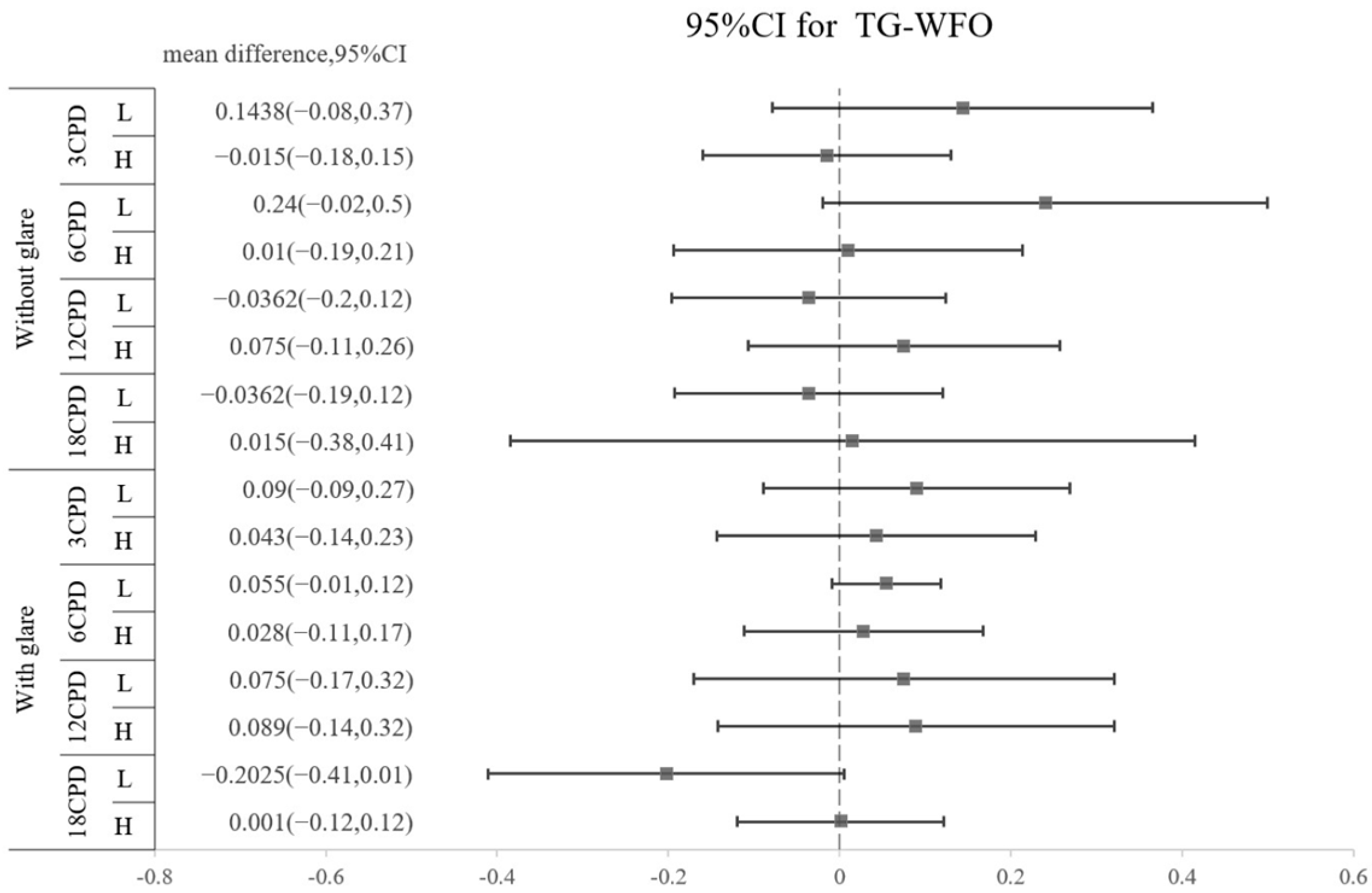
The amounts of all HOAs, including the surgically induced and postoperative types, are shown in Table 5. No significant difference was found regarding the amount of any HOA between the TG-LASIK and WFO-LASIK groups (all p > 0.05). As shown in Figure 2, high-myopia patients had higher SA than low-myopia patients, regardless of the TG or WFO ablation method. The surgically induced mean difference for SA is 0.8235 (95% CI: 0.518 to 1.216) for TG and 0.838 (95% CI: 0.473 to 1.204) for WFO in the surgically induced group. In the postoperative group, the SA standardized mean difference is 0.7955 (95% CI: 0.329 to 1.358) in TG and 0.996 (95% CI: 0.508 to 1.377) in WFO, and both are statistically significant. However, the number of refractive corrections does not affect trefoil aberration; the mean difference for the high-myopia minus low-myopia group is −0.035 (95% CI: −0.174 to 0.103) for the TG-LASIK group with surgically induced trefoil and −0.082 (95% CI: −0.681 to 0.518) for the WFO group. Similar findings were found in postoperative trefoil (mean difference: −0.003; 95% CI: −0.108 to 0.102). For surgically induced coma aberration, an intriguing trend was observed in the high-myopic patients that marginally increased after TG-LASIK and decreased after WFO-LASIK, although this was not statistically significant, as shown in Table 5. Moreover, the CS did not illustrate any difference between the TG-LASIK and WFO-LASIK groups in any CPDs, whether with low or high myopia (Figure 3).

Table 5.
Comparison of clinical characteristics between high- and low-myopia groups by TG and WFO ablation after 3 months.
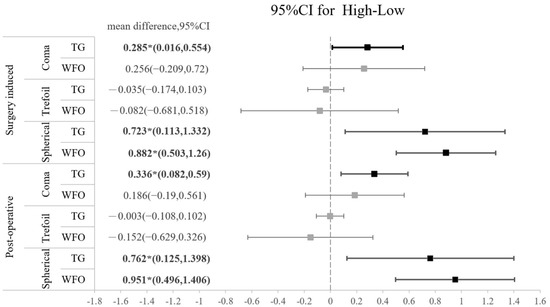
Figure 2.
Forest plot of the standardized mean difference of surgically induced HOA or postoperative HOA between high myopia and low myopia on coma, trefoil, and spherical aberration with 95% confidence intervals stratified by TG vs. WFO.
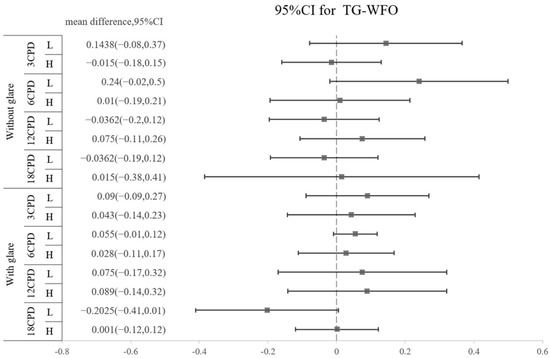
Figure 3.
Difference in postoperative contrast sensitivity between TG and WFO stratified by cycles per degree (3, 6, 12, and 18 CPD), glare (off and on) and level of myopia (high and low).
3.4. Subjective Quality of Vision (QoV) Result
Table 6 presents the subjective optical quality data obtained from the questionnaire responses. For vision tasks, patients with TG-LASIK ablation were more satisfied with respect to haze (35.3 ± 6.7 vs. 37.8 ± 5.4, p = 0.0215), clarity at night (34.6 ± 9.1 vs. 38.4 ± 6.4, p = 0.0386), and total scores (35.8 ± 6.1 vs. 37.3 ± 4.6, p = 0.0225) than patients who received WFO-LASIK ablation.

Table 6.
Subjective quality of vision results obtained from questionnaire between TG-LASIK and WFO-LASIK groups after 3 months.
4. Discussion
The current study shows similar results with respect to UDVA, CDVA, and SE between the TG-LASIK group and the WFO-LASIK group. TG-LASIK induced less trefoil than WFO-LASIK in the high-myopia group, while other HOAs were similar between the two groups. Moreover, the TG-LASIK group reported higher postoperative satisfaction and QoV.
The overall rating of TG-LASIK on the postoperative adverse symptom questionnaire was more satisfactory than that of WFO-LASIK, particularly for the items “haze” and “clarity at night”. A previous study showed that postoperative higher-order aberrations could negatively affect QoV and may cause visual disturbance, especially with coma and spherical aberration [15]. This is in line with our results indicating less postoperative and surgically induced trefoil from TG-LASIK.
The postoperative SA in both groups significantly increased (p = 0.0001), and TG-LASIK yielded more surgically induced comas in the high-myopia group. The induction of SA could be due to the ablation-induced change in corneal asphericity and may be positively correlated with the amount of refraction correction. The induction of coma aberration may also result from subclinical decentrations or asymmetry from wound healing [16]. In a study by Alio, patients with high myopia who received WFO-LASIK had spherical aberration that increased from 0.22 ± 0.11 μm to 0.57 ± 0.15 μm, as well as coma that increased from 0.22 ± 0.15 μm to 0.58 ± 0.28 μm [17]. In another retrospective case series, significant induction of coma aberration and SA after 3 months were also observed in high-myopia patients after refractive correction (coma aberration: 0.21 ± 0.17 μm to 0.58 ± 0.37 μm; and SA: 0.22 ± 0.09 μm to 0.59 ± 0.14 μm) [18], and a similar trend was observed in high-astigmatism patients receiving LASIK treatment [19]. The magnitude of induced SA may be correlated with the amount of achieved correction of SE and positively correlated with corneal asphericity [20].
The postoperative HOA of trefoil was noticeably lower following TG-LASIK in high-myopia patients than following WFO-LASIK; however, this result was not observed in terms of surgically induced difference. We think this may be a relatively novel finding to compare the postoperative HOA with different degrees of myopia between TG-LASIK and WFO-LASIK. Although the surgically induced difference in trefoil between the TG-LASIK and WFO-LASIK groups seems to be more prominent in the low-myopia group, statistical analysis revealed that TG-LASIK exhibited no superiority in terms of surgically induced trefoil reduction in the low-myopia population. The reason for these conflicting results may be the prominent standard deviation of surgically induced trefoil in the WFO-LASIK population, which can cause insignificant differences between the two groups. Furthermore, a trend of better TG results can be observed, with a mean difference leaning towards less HOA in coma and trefoil. The superiority of TG-LASIK in trefoil was also noted in study by Kim et al. (2019) in which the ocular trefoil was reported to be significantly lower after topography-guided ablation than at baseline [7]. However, in the same study, the magnitude of surgically induced ocular coma and SA did not differ significantly between the two groups. We think TG might induce less trefoil aberration in highly myopic patients, but further studies with larger samples are needed to clarify this concept. A major study carried out in 2001 showed that in virgin, unoperated eyes, the first surface of the cornea and internal optics partially compensate for each other’s aberrations to produce an improved retinal image [4]. Thus, it is not always beneficial to remove corneal aberrations. Because there is very little irregularity in virgin eyes, we look specifically into those high myopic patients with more aberration over the cornea. High-myopia patients may benefit from the use of TG-LASIK with better HOA and postoperative QoV, while most patients have regular corneas that are easily treated with WFO-LASIK.
This study is subject to several limitations. First, the number of patients included was limited, with less than 30 eyes in each group, which may have contributed to statistical bias; however, the contralateral eye design with pairwise data comparison might overcomes this shortcoming. Second, the design of the ablation profile was based on ocular dominance but not randomized, which may have caused bias in estimating subjective questionnaire outcomes. Moreover, different wavefront programs may have different effects on HOAs, some of which may have showed better HOA reductions than the TG program. Nevertheless, we cannot compare the TG program of our device to the wavefront program in other devices since we have only one excimer laser device in our institution.
5. Conclusions
In conclusion, TG-LASIK might contribute to less trefoil in high-myopia patients compared to WFO-LASIK. Our study shows that high-myopia patients correlate exhibit numerically higher SA and coma, regardless of TG or WFO-LASIK profiles, as compared to low-myopia patients. Moreover, our subjective QoV questionnaire shows higher patient satisfaction in the TG-LASIK group.
Author Contributions
Conceptualization, C.-K.C. and C.-C.C.; methodology, C.-C.C.; software, I.-B.L.; formal analysis, I.-B.L.; investigation, E.L.-C.M.; data curation, C.-C.C. and C.-K.C.; writing—original draft preparation, C.-C.C. and C.-Y.L.; writing—review and editing, E.L.-C.M. and C.-K.C.; visualization, C.-K.C. and C.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted from 2019 to 2020 at the Taipei Nobel Eye Clinic. All procedures were conducted in accordance with the tenets of the Declaration of Helsinki, and this clinical trial conducted in our institution was approved by the Ethics Committee of Changhua Christian Hospital (IRB no.190601) and registered on ClinicalTrials.gov (identifier NCT04919291; date of approval: 9 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon reasonable request to the corresponding author.
Acknowledgments
We thank Jie Yang for technical support, Yan-Ni Jhan for their assistance with statistical software, and Justina Ying-Zu Wang for their assistance in data acquisition.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sutton, G.; Lawless, M.; Hodge, C. Laser in situ keratomileusis in 2012: A review. Clin. Exp. Optom. 2014, 97, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barriuso, E.; Lloves, J.M.; Marcos, S.; Navarro, R.; Llorente, L.; Barbero, S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1396–1403. [Google Scholar]
- Schallhorn, S.C.; Farjo, A.A.; Huang, D.; Boxer Wachler, B.S.; Trattler, W.B.; Tanzer, D.J.; Majmudar, P.A.; Sugar, A. Wavefront-guided LASIK for the correction of primary myopia and astigmatism a report by the American Academy of Ophthalmology. Ophthalmology 2008, 115, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Artal, P.; Guirao, A.; Berrio, E.; Williams, D.R. Compensation of corneal aberrations by the internal optics in the human eye. J. Vis. 2001, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, T.; Krueger, R. Topography-guided laser refractive surgery. Curr. Opin. Ophthalmol. 2012, 23, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Stonecipher, K.; Parrish, J.; Stonecipher, M. Comparing wavefront-optimized, wavefront-guided and topography-guided laser vision correction: Clinical outcomes using an objective decision tree. Curr. Opin. Ophthalmol. 2018, 29, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, S.H.; Lim, D.H.; Yang, C.M.; Yoon, G.J.; Chung, T.Y. Topography-guided versus wavefront-optimized laser in situ keratomileusis for myopia: Surgical outcomes. J. Cataract. Refract. Surg. 2019, 45, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, S.H.; Lim, D.H.; Yoon, G.J.; Chung, T.Y. Comparison of outcomes after topography-modified refraction versus wavefront-optimized versus manifest topography-guided LASIK. BMC Ophthalmol. 2020, 20, 192. [Google Scholar] [CrossRef]
- Ozulken, K.; Yuksel, E.; Tekin, K.; Kiziltoprak, H.; Aydogan, S. Comparison of Wavefront-Optimized Ablation and Topography-Guided Contoura Ablation With LYRA Protocol in LASIK. J. Refract. Surg. 2019, 35, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Shih, Y.F.; Hsiao, C.K.; Chen, C.J.; Lee, L.A.; Hung, P.T. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J. Formos. Med. Assoc. 2001, 100, 684–691. [Google Scholar] [PubMed]
- Lin, L.L.; Shih, Y.F.; Hsiao, C.K.; Chen, C.J. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann. Acad. Med. Singap. 2004, 33, 27–33. [Google Scholar] [PubMed]
- Pointer, J.S. Sighting dominance, handedness, and visual acuity preference: Three mutually exclusive modalities? Ophthalmic. Physiol. Opt. 2001, 21, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Manche, E.E. Effect of preoperative pupil size on quality of vision after wavefront-guided LASIK. Ophthalmology 2011, 118, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Aryadoust, V.; Tan, H.A.H.; Ng, L.Y. A Scientometric Review of Rasch Measurement: The Rise and Progress of a Specialty. Front. Psychol. 2019, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Gutiérrez, R.; Jiménez, J.R.; González-Méijome, J.M. Night vision disturbances after successful LASIK surgery. Br. J. Ophthalmol. 2007, 91, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, P.; Basuthkar, S.S.; Joseph, R. Ocular aberrations after wavefront optimized LASIK for myopia. Indian J. Ophthalmol. 2010, 58, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Vega-Estrada, A.; Piñero, D.P. Laser-assisted in situ keratomileusis in high levels of myopia with the amaris excimer laser using optimized aspherical profiles. Am. J. Ophthalmol. 2011, 152, 954–963.e951. [Google Scholar] [CrossRef] [PubMed]
- Vega-Estrada, A.; Alió, J.L.; Arba Mosquera, S.; Moreno, L.J. Corneal higher order aberrations after LASIK for high myopia with a fast repetition rate excimer laser, optimized ablation profile, and femtosecond laser-assisted flap. J. Refract. Surg. 2012, 28, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Al-Zeraid, F.M.; Osuagwu, U.L. Induced Higher-order aberrations after Laser In Situ Keratomileusis (LASIK) Performed with Wavefront-Guided IntraLase Femtosecond Laser in moderate to high Astigmatism. BMC Ophthalmol. 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Goyal, J.L.; Garg, A.; Arora, R.; Jain, P.; Goel, Y. Comparative evaluation of higher-order aberrations and corneal asphericity between wavefront-guided and aspheric LASIK for myopia. J. Refract. Surg. 2014, 30, 777–784. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).