N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated with Post-Stroke In-Hospital Complications
Abstract
1. Introduction
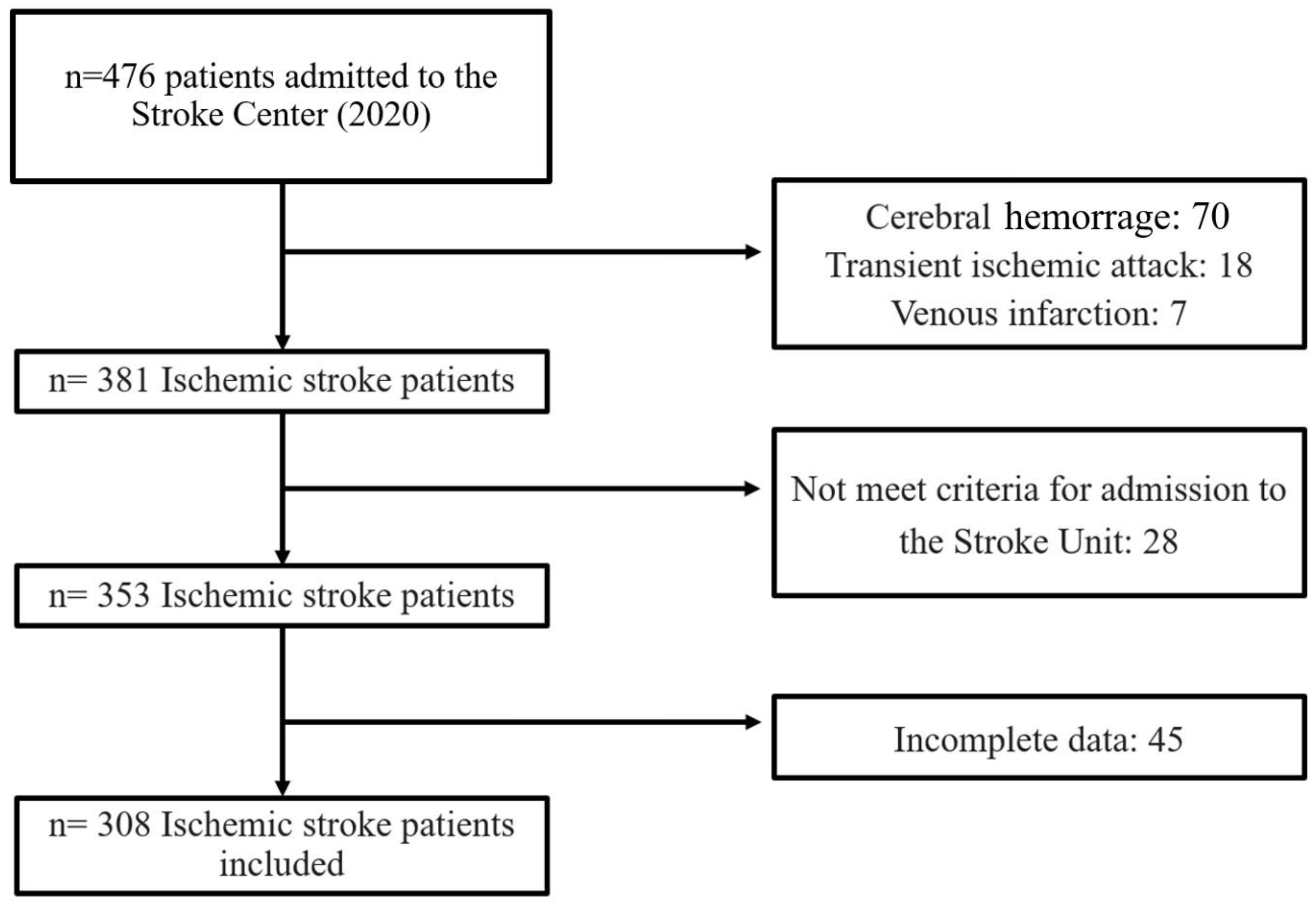
2. Materials and Methods
- − Systemic complications: RTI (2 symptoms: dyspnea, fever, or purulent expectoration and one sign: specific signs in lung auscultation or in a chest X-ray), other infections (urinary tract infection −1 symptom: dysuria or high urinary frequency and one sign: choluria, alterations in the urine analysis such as nitrites or urine culture, sepsis, change in mental status, systolic blood pressure less than or equal to 100 mm of mercury, respiratory rate higher than or equal to 22 breaths per minute, cutaneous herpes, compatible cutaneous lesion with response to adequate treatment, phlebitis, inflammation signs with or without fever and with adequate response to the venous line exchange, febrile syndrome, fever and symptoms such as headache, chills or muscle and joint pains) and other non-infective complications (DHF—defined as a new onset or rapidly or gradual worsening of heart failure symptoms that require urgent therapy, acute urinary retention and gout attack).
- − Neurological complications: neurological impairment (worsening of previous cognitive level), hemorrhage (diagnosed with cerebral scan or magnetic resonance), malignant edema (diagnosed with cerebral scan or magnetic resonance with midline shift) or seizures (seizures after the stroke in patients without a previous history of epilepsy).
2.1. NT-ProBNP Measurements
2.2. Statistical Analysis
3. Results
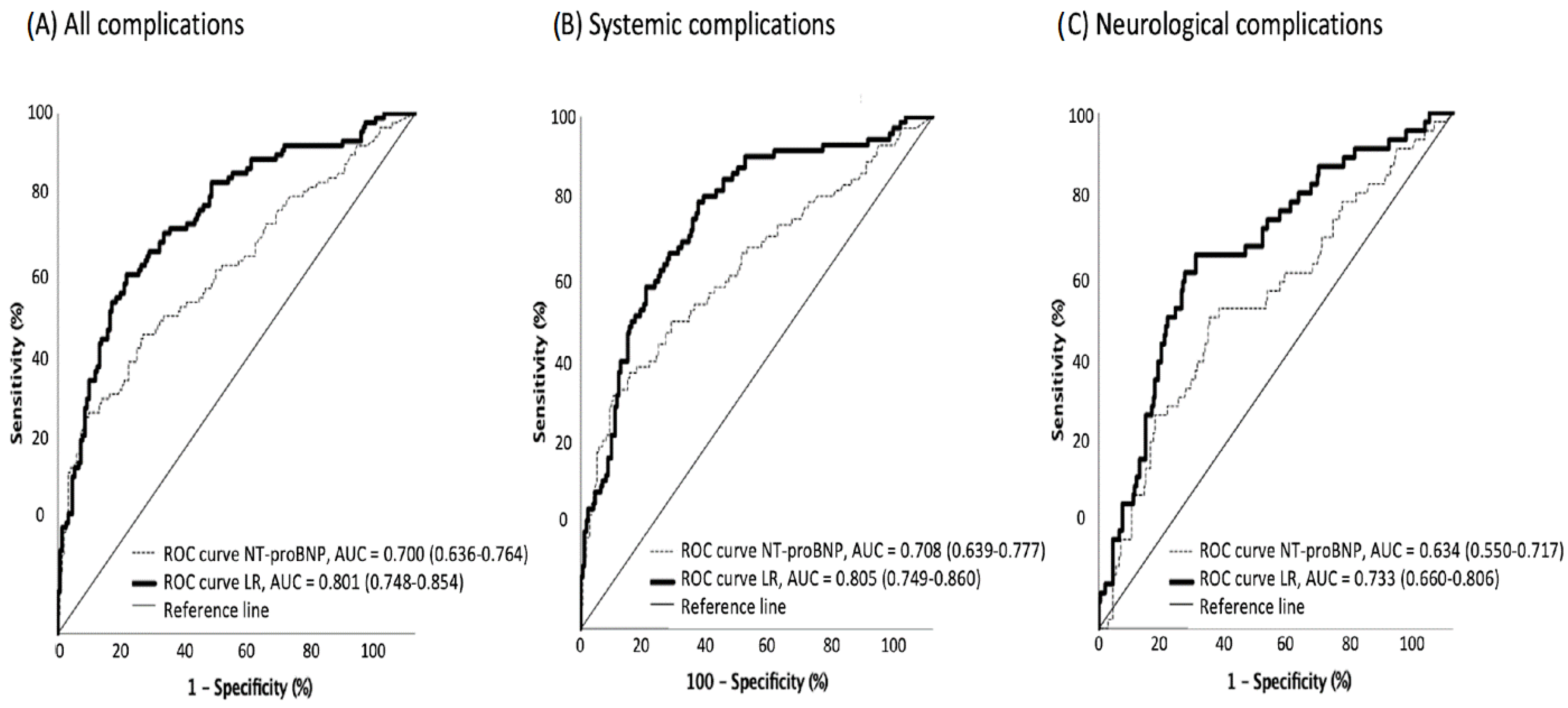
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Selim, M.H.; Caplan, L.R. Medical complications after stroke. Lancet Neurol. 2010, 9, 105–118. [Google Scholar] [CrossRef]
- Burkot, J.; Kopec, G.; Pera, J.; Slowik, A.; Dziedzic, T. Decompensated heart failure is a strong independent predictor of functional outcome after ischemic stroke. J. Card. Fail. 2015, 21, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Badve, M.S.; Zhou, Z.; van de Beek, D.; Anderson, C.S.; Hackett, M.L. Frequency of post-stroke pneumonia: Systematic review and metaanalysis of observational studies. Int. J. Stroke. 2019, 14, 125–136. [Google Scholar] [CrossRef]
- Teh, W.H.; Smith, C.J.; Barlas, R.S.; Wood, A.D.; Bettencourt-Silva, J.H.; Clark, A.B.; Metcalf, A.K.; Bowles, K.M.; Potter, J.F.; Myint, P.K. Impact of stroke associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol. Scand. 2018, 138, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Faura, J.; Bustamante, A.; Reverté, S.; García-Berrocoso, T.; Millán, M.; Castellanos, M.; Lara-Rodríguez, B.; Zaragoza, J.; Ventura, O.; Hernández-Pérez, M.; et al. Blood biomarker panels for the early prediction of stroke-associated complications. J. Am. Heart Assoc. 2021, 10, e018946. [Google Scholar] [CrossRef]
- De Lemos, J.A.; McGuire, D.K.; Drazner, M.H. B-type natriuretic peptide in cardiovascular disease. Lancet 2003, 362, 316–322. [Google Scholar] [CrossRef]
- Gopal, D.J.; Iqbal, M.N.; Maisel, A. Updating the role of natriuretic peptide levels in cardiovascular disease. Postgrad. Med. 2011, 123, 102–113. [Google Scholar] [CrossRef]
- Etgen, T.; Baum, H.; Sander, K.; Sander, D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke 2005, 36, 270–275. [Google Scholar] [CrossRef]
- Chang, K.-W.; Hsu, J.C.; Toomu, A.; Fox, S.; Maisel, A.S. Clinical applications of biomarkers in atrial fibrillation. Am. J. Med. 2017, 130, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Gao, H.; Pan, Z.Y.; Li, J.; Huang, W.H.; Wang, Z.F.; Li, Z.Q. Prognostic Value of NT-proBNP After Ischemic Stroke: A Systematic Review and Meta-analysis of Prospective Cohort Studies. J. Stroke Cerebrovasc. Dis. 2020, 29, 104659. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sayan, S.; Kotan, D. Levels of brain natriuretic peptide as a marker for the diagnosis and prognosis of acute ischemic stroke. Arch. Med. Sci. Atheroscler. Dis. 2016, 1, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Shibazaki, K.; Kimura, K.; Okada, Y.; Iguchi, Y.; Uemura, J.; Terasawa, Y.; Aoki, J. Plasma brain natriuretic peptide as an independent predictor of in-hospital mortality after acute ischemic stroke. Intern. Med. 2009, 48, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Naveen, V.; Vengamma, B.; Mohan, A.; Vanajakshamma, V. N-terminal pro-brain natriuretic peptide levels and short-term prognosis in acute ischemic stroke. Ann. Indian Acad. Neurol. 2015, 18, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Feng, K.; Fu, M.; Ge, W.; Zhou, C. Correlation between the B-type Natriuretic Peptide before thrombolysis and prognosis in patients with ischemic stroke. Clin. Neurol. Neurosurg. 2021, 211. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhan, X.; Chen, M.; Lei, H.; Wang, Y.; Wei, D.; Jiang, X. The prognostic value of combined NT-pro-BNP levels and NIHSS scores in patients with acute ischemic stroke. Intern. Med. 2012, 51, 2887–2892. [Google Scholar] [CrossRef]
- Hajdinjak, E.; Klemen, P.; Grmec, S. Prognostic value of a single prehospital measurement of N-terminal pro-brain natriuretic peptide and troponin T after acute ischaemic stroke. J. Int. Med. Res. 2012, 40, 768–776. [Google Scholar] [CrossRef]
- Otaki, Y.; Watanabe, T.; Sato, N.; Shirata, T.; Tsuchiya, H.; Wanezaki, M.; Tamura, H.; Nishiyama, S.; Arimoto, T.; Takahashi, H.; et al. Direct comparison of prognostic ability of cardiac biomarkers for cardiogenic stroke and clinical outcome in patients with stroke. Heart Vessel. 2019, 34, 1178–1186. [Google Scholar] [CrossRef]
- Battaglini, D.; Robba, C.; Da Silva, A.L.; Samary, C.D.S.; Silva, P.L.; Pizzol, F.D.; Pelosi, P.; Rocco, P.R.M. Brain–heart interaction after acute ischemic stroke. Crit. Care 2020, 24, 163. [Google Scholar] [CrossRef]
- Sander, D.; Winbeck, K.; Klingelhofer, J.; Etgen, T.; Conrad, B. Prognostic relevance of pathological sympathetic activation after acute thromboembolic stroke. Neurology 2001, 57, 833–838. [Google Scholar] [CrossRef]
- Ozdemir, O.; Hachinski, V. Brain lateralization and sudden death: Its role in the neurogenic heart syndrome. J. Neurol. Sci. 2008, 268, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, R.; Ayya, S.S. Neurogenic stress cardiomyopathy: What do we need to know. Ann. Card. Anaesth. 2018, 21, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Laredo, C.; Zhao, Y.; Rudilosso, S.; Renú, A.; Pariente, J.C.; Chamorro, A.; Urra, X. Prognostic significance of infarct size and location: The case of insular stroke. Sci. Rep. 2018, 8, 9498. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-heart interaction: Cardiac complications after stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef]
- Shibazaki, K.; Kimura, K.; Aoki, J.; Sakai, K.; Saji, N.; Uemura, J. Brain natriuretic eptide level on dmission predicts recurrent stroke after discharge in stroke survivors with atrial fibrillation. Clin. Neurol. Neurosurg. 2014, 127, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Shiga, T.; Iijima, M.; Moriya, S.; Mizuno, S.; Toi, S.; Arai, K.; Ashihara, K.; Abe, K.; Uchiyama, S. Brain natriuretic peptide in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Llombart, V.; Antolin-Fontes, A.; Bustamante, A.; Giralt, D.; Rost, N.S.; Furie, K.; Shibazaki, K.; Biteker, M.; Castillo, J.; Rodríguez-Yáñez, M.; et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis:pooled data meta-analysis. Stroke 2015, 46, 1187–1195. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Zhong, W.; Yu, Y.; Lou, M. Early NT-ProBNP (N-terminal probrain natriuretic peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients. Stroke 2021, 52, 537–542. [Google Scholar] [CrossRef]
- Shibazaki, K.; Kimura, K.; Fujii, S.; Sakai, K.; Iguchi, Y. Brain natriuretic peptide levels as a predictor for new atrial fibrillation during hospitalization in patients with acute ischemic stroke. Am. J. Cardiol. 2012, 109, 1303–1307. [Google Scholar] [CrossRef]
- Muscari, A.; Bianchi, G.; Forti, P.; Magalotti, D.; Pandolfi, P.; Zoli, M.; Pianoro Study Group. N-terminal pro B-type natriuretic peptide (NT-proBNP): A possible surrogate of biological age in the elderly people. Geroscience 2021, 43, 845–857. [Google Scholar] [CrossRef]
- Biscetti, F.; Giovannini, S.; Straface, G.; Bertucci, F.; Angelini, F.; Porreca, C.; Landolfi, R.; Flex, A. RANK/RANKL/OPG pathway: Genetic association with history of ischemic stroke in Italian population. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4574–4580. [Google Scholar] [PubMed]
- Zhang, K.J.; Jin, H.; Xu, R.; Zhang, P.; Guo, Z.N.; Yang, Y. N-Terminal Pro-brain Natriuretic Peptide Is Associated With Hemorrhagic Transformation and Poor Outcomes in Patients With Stroke Treated With Intravenous Thrombolysis. Front. Mol. Neurosci. 2021, 14, 758915. [Google Scholar] [CrossRef] [PubMed]

| All Complications | Systemic Complications | Neurological Complications | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 121) | No (n = 187) | p Value | Yes (n = 96) | No (n = 212) | p Value | Yes (n = 62) | No (n = 246) | p Value | |
| Demographic data and comorbidities | |||||||||
| Male sex, n (%) | 77 (63.6) | 137 (73.3) | 0.073 | 60 (62.5) | 154 (72.6) | 0.073 | 38 (61.3) | 176 (71.5) | 0.117 |
| Age, median (IQR), years | 74 (26) | 59 (19) | <0.001 | 74 (26) | 60 (21) | <0.001 | 73 (26) | 63 (22) | <0.001 |
| Previous mRS < 2, n (%) | 93 (76.9) | 168 (89.9) | 0.002 | 73 (76%) | 188 (88.7) | 0.004 | 48 (77.4) | 213 (86.6) | 0.073 |
| Arterial hypertension, n (%) | 68 (56.2) | 108 (57.8) | 0.788 | 52 (54.2) | 124 (58.5) | 0.478 | 34 (54.8) | 142 (57.7) | 0.682 |
| Dyslipidemia, n (%) | 54 (44.6) | 71 (38) | 0.245 | 45 (46.9) | 80 (37.7) | 0.130 | 28 (45.2) | 97 (39.4) | 0.412 |
| Diabetes mellitus, n (%) | 37 (30.6) | 40 (21.4) | 0.069 | 39 (31.3) | 47 (22.2) | 0.088 | 15 (24.2) | 62 (25.2) | 0.870 |
| Smoking, n (%) | 47 (38.8) | 98 (52.4) | 0.020 | 38 (39.6) | 107 (50.5) | 0.076 | 22 (35.5) | 123 (50) | 0.041 |
| Alcohol abuse, n (%) | 14 (11.6) | 36 (19.3) | 0.074 | 11 (11.5) | 39 (18.4) | 0.126 | 5 (8.1) | 45 (18.3) | 0.051 |
| Atrial fibrillation, n (%) | 31 (25.6) | 22 (11.8) | 0.002 | 26 (27.1) | 27 (12.7) | 0.002 | 17 (27.4) | 36 (14.6) | 0.017 |
| Coronary heart disease, n (%) | 21 (17.4) | 15 (8) | 0.013 | 17 (17.7) | 19 (9) | 0.027 | 8 (12.9) | 28 (11.4) | 0.739 |
| Valvular Heart Disease, n (%) | 0 (0) | 6 (3.2) | 0.085 | 0 (0) | 6 (2.8) | 0.182 | 0 (0) | 6 (2.4) | 0.604 |
| Renal dysfunction, n (%) | 27 (23.1) | 19 (10.6) | 0.004 | 25 (26.6) | 21 (10.3) | <0.001 | 9 (15.5) | 37 (15.5) | 0.995 |
| Previous stroke, n (%) | 21 (17.4) | 21 (11.3) | 0.131 | 14 (14.6) | 28 (13.3) | 0.756 | 11 (17.7) | 31 (12.7) | 0.298 |
| Prior treatments | |||||||||
| Antiplatelet Agents, n (%) | 15 (12.4) | 21 (11.2) | 0.756 | 13 (13.5) | 23 (10.8) | 0.496 | 5 (8.1) | 31 (12.6) | 0.320 |
| Anticoagulants, n (%) | 15 (12.4) | 11 (5.9) | 0.045 | 13 (13.5) | 13 (6.1) | 0.030 | 8 (12.9) | 18 (7.3) | 0.157 |
| Statins, n (%) | 41 (33.9) | 54 (28.9) | 0.353 | 31 (32.3) | 64 (30.2) | 0.711 | 24 (38.7) | 71 (28.9) | 0.133 |
| Antihypertensives, n (%) | 70 (57.9) | 89 (47.6) | 0.079 | 54 (56.3) | 105 (49.5) | 0.274 | 37 (59.7) | 122 (49.6) | 0.156 |
| Oral antidiabetics, n (%) | 15 (12.4) | 14 (7.5) | 0.154 | 14 (14.6) | 15 (7.1) | 0.037 | 4 (6.5) | 25 (10.2) | 0.371 |
| Insulin, n (%) | 9 (7.4) | 9 (4.8) | 0.337 | 9 (9.4) | 9 (4.2) | 0.075 | 2 (3.2) | 16 (6.5) | 0.544 |
| Stroke severity | |||||||||
| NIHSS, median (IQR) | 9 (12) | 3 (5) | <0.001 | 10 (12) | 3 (5) | <0.001 | 8 (12) | 4 (7) | <0.001 |
| Acute phase treatments | |||||||||
| Thrombolysis, n (%) | 35 (28.9) | 41 (21.9) | 0.164 | 27(28.1) | 49 (23.1) | 0.345 | 20 (32.3) | 56 (22.8) | 0.121 |
| Mechanical thrombectomy, n (%) | 30 (24.8) | 24 (12.8) | 0.007 | 26 (27.1) | 28 (13.2) | 0.003 | 11 (17.7) | 43 (17.5) | 0.961 |
| Stroke etiology (TOAST), n (%) | |||||||||
| Large-vessel occlusive | 25 (20.7) | 31 (16.6) | <0.001 | 20 (20.8) | 36 (17) | <0.001 | 12 (19.4) | 44 (17.9) | 0.014 |
| Small-vessel occlusive | 10 (8.3) | 58 (31) | 8 (8.3) | 69 (28.3) | 4 (6.5) | 64 (26) | |||
| Cardioembolic | 50 (41.3) | 47 (25.1) | 42 (43.8) | 55 (25.9) | 27 (43.5) | 70 (28.5) | |||
| Other | 10 (8.3) | 15 (8) | 8 (8.3) | 17(8) | 5 (8.1) | 20 (8.1) | |||
| Unknown | 26 (21.5) | 36 (19.3) | 18 (18.8) | 44 (20.8) | 14 (22.6) | 48 (19.5) | |||
| Stroke unit length of stay, median (IQR), days | 2 (2) | 2 (2) | 0.622 | 2 (3) | 2 (2) | <0.976 | 2 (3) | 2 (2) | 0.549 |
| Blood pressure | |||||||||
| Systolic, median (IQR), mmHg | 152 (46) | 160 (42) | 0.052 | 152.5 (48) | 160 (42) | 0.140 | 151.5 (40) | 160 (40) | 0.251 |
| Diastolic, median (IQR), mmHg | 85 (20) | 93 (25) | <0.001 | 85 (19) | 92 (24) | 0.002 | 87.5 (25) | 90 (20) | 0.040 |
| Pulse pressure, median (IQR), mmHg | 66 (34.2) | 68,5 (31.2) | 0.889 | 66 (36) | 68 (31) | 0.995 | 65.5 (36.2) | 68.5 (32.2) | 0.993 |
| Biochemical markers | |||||||||
| NT-proBNP, median (IQR) pg/mL | 864 (2556) | 142 (623) | <0.001 | 1144.5 (2781.5) | 142 (663) | <0.001 | 847 (2453) | 199 (906) | 0.001 |
| Creatinine, median (IQR), mg/dL | 0.82 (0.45) | 0.82 (0.24) | 0.754 | 0.82 (0.5) | 0.82 (0.24) | 0.941 | 0.78 (0.3) | 0.83 (0.32) | 0.178 |
| Glicemia, median (IQR), mg/dL | 129(87.5) | 115 (49.8) | 0.003 | 131(90.3) | 115 (50) | 0.004 | 133.5(88.8) | 118 (53) | 0.034 |
| Variable | Multivariate Analysis Dependent Variable: All In-Hospital Complications * | Multivariate Analysis Dependent Variable: Systemic Complications † | Multivariate Analysis Dependent Variable: Neurological Complications ‡ | |||
|---|---|---|---|---|---|---|
| Adjusted OR ** (95% CI ***) | p Values | Adjusted OR (95% CI) | p Values | Adjusted OR (95% CI) | p Values | |
| Age, years | - | - | - | - | 1.027 (1.005–1.048) | 0.015 |
| NT-proBNP > 326, pg/mL | 2.282 (1.269–4.104) | <0.005 | 2.336 (1.259–4.335) | 0.007 | - | - |
| NIHSS | 1.203 (1.128–1.283) | <0.001 | 1.193 (1.120–1.271) | <0.001 | 1.090 (1.042–1.141) | <0.001 |
| Glycaemia, g/dL | 1.005 (1.001–1.009) | 0.007 | 1.005 (1.002–1.009) | 0.005 | 1.004 (1.000–1.007) | 0.066 |
| Diastolic blood pressure, mmHg | 0.973 (0.956–0.991) | 0.003 | 0.973 (0.955–0.992) | 0.024 | - | - |
| Mechanical thrombectomy | 0.297 (0.113–0.781) | 0.014 | 0.425 (0.168–1.073) | 0.070 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Franco, M.L.; Guevara-Sánchez, E.; Amaya-Pascasio, L.; Quesada-López, M.; Arjona-Padillo, A.; García-Torrecillas, J.M.; Martínez-Sánchez, P. N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated with Post-Stroke In-Hospital Complications. J. Pers. Med. 2023, 13, 474. https://doi.org/10.3390/jpm13030474
Ruiz-Franco ML, Guevara-Sánchez E, Amaya-Pascasio L, Quesada-López M, Arjona-Padillo A, García-Torrecillas JM, Martínez-Sánchez P. N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated with Post-Stroke In-Hospital Complications. Journal of Personalized Medicine. 2023; 13(3):474. https://doi.org/10.3390/jpm13030474
Chicago/Turabian StyleRuiz-Franco, María Luisa, Eva Guevara-Sánchez, Laura Amaya-Pascasio, Miguel Quesada-López, Antonio Arjona-Padillo, Juan Manuel García-Torrecillas, and Patricia Martínez-Sánchez. 2023. "N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated with Post-Stroke In-Hospital Complications" Journal of Personalized Medicine 13, no. 3: 474. https://doi.org/10.3390/jpm13030474
APA StyleRuiz-Franco, M. L., Guevara-Sánchez, E., Amaya-Pascasio, L., Quesada-López, M., Arjona-Padillo, A., García-Torrecillas, J. M., & Martínez-Sánchez, P. (2023). N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated with Post-Stroke In-Hospital Complications. Journal of Personalized Medicine, 13(3), 474. https://doi.org/10.3390/jpm13030474








