Cortical Astrocyte Progenitors and Astrocytes from Human Pluripotent Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cortical Neural Progenitors, Cortical Astrocyte Progenitors and Astrocyte Differentiation
2.3. Immunofluorescence Assay
2.4. Gene Expression Analysis
2.5. Statistical Analyses
3. Results
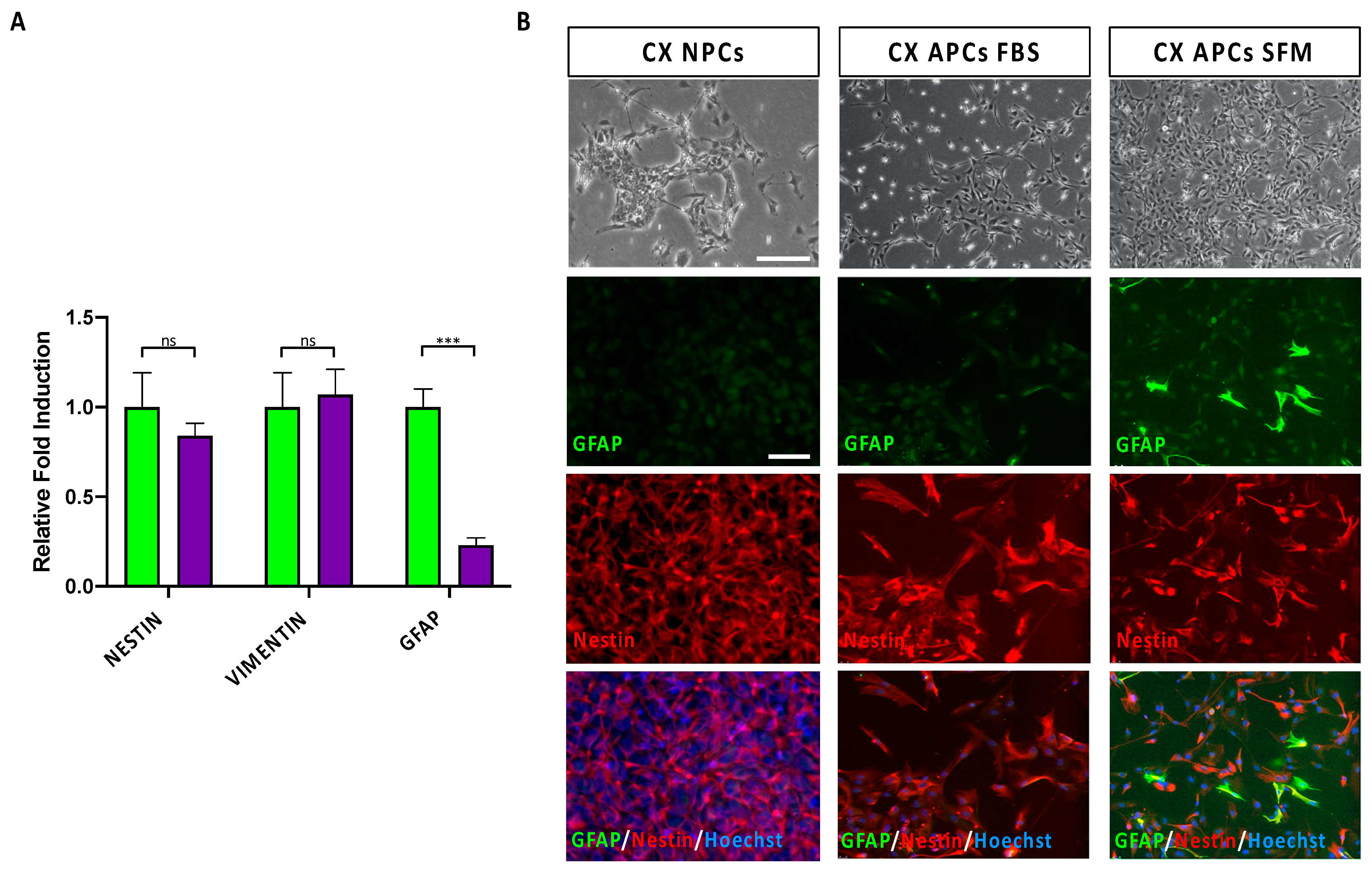
3.1. Specification of Cortical Astrocyte Progenitor Cells from hiPSCs
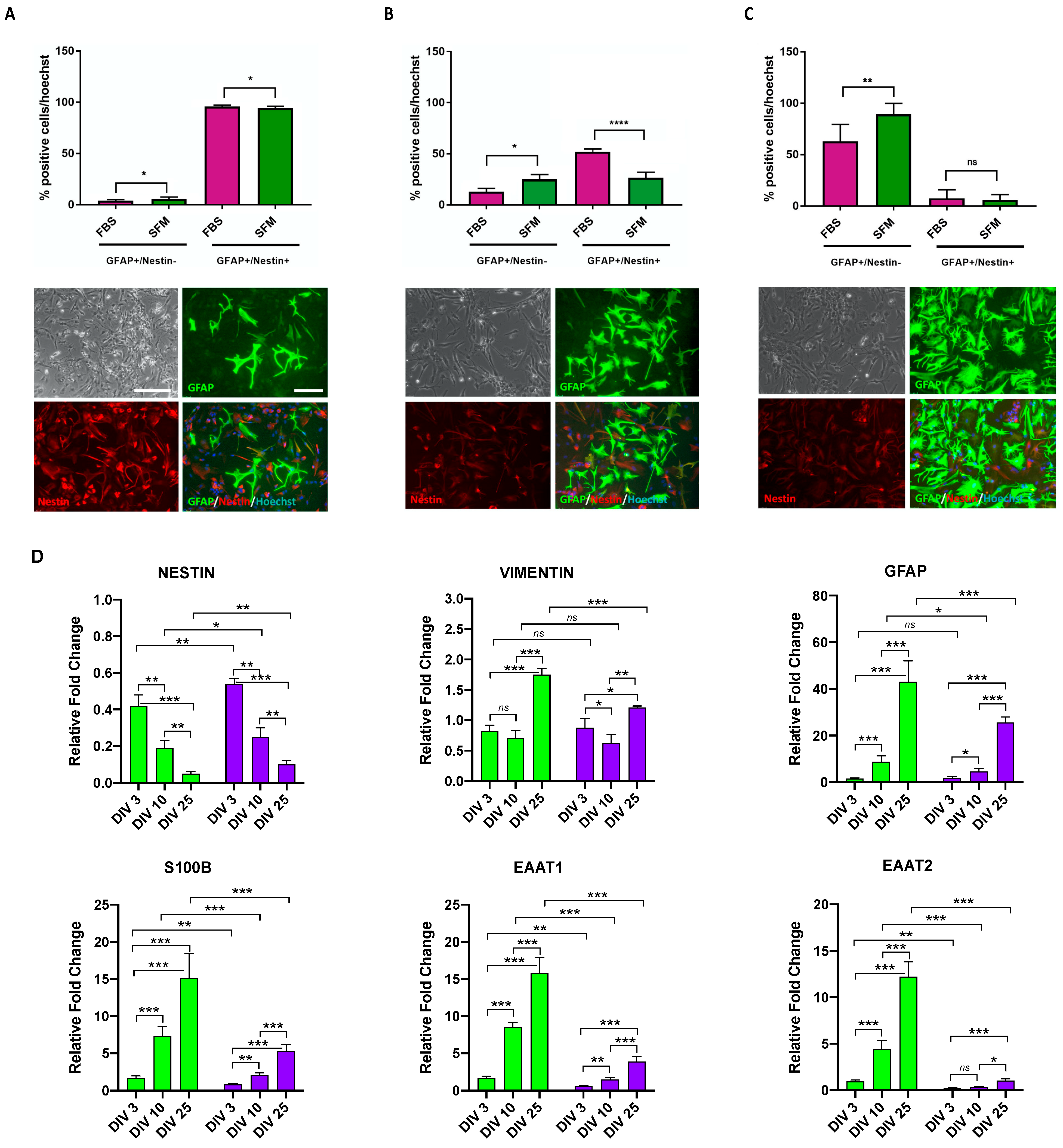
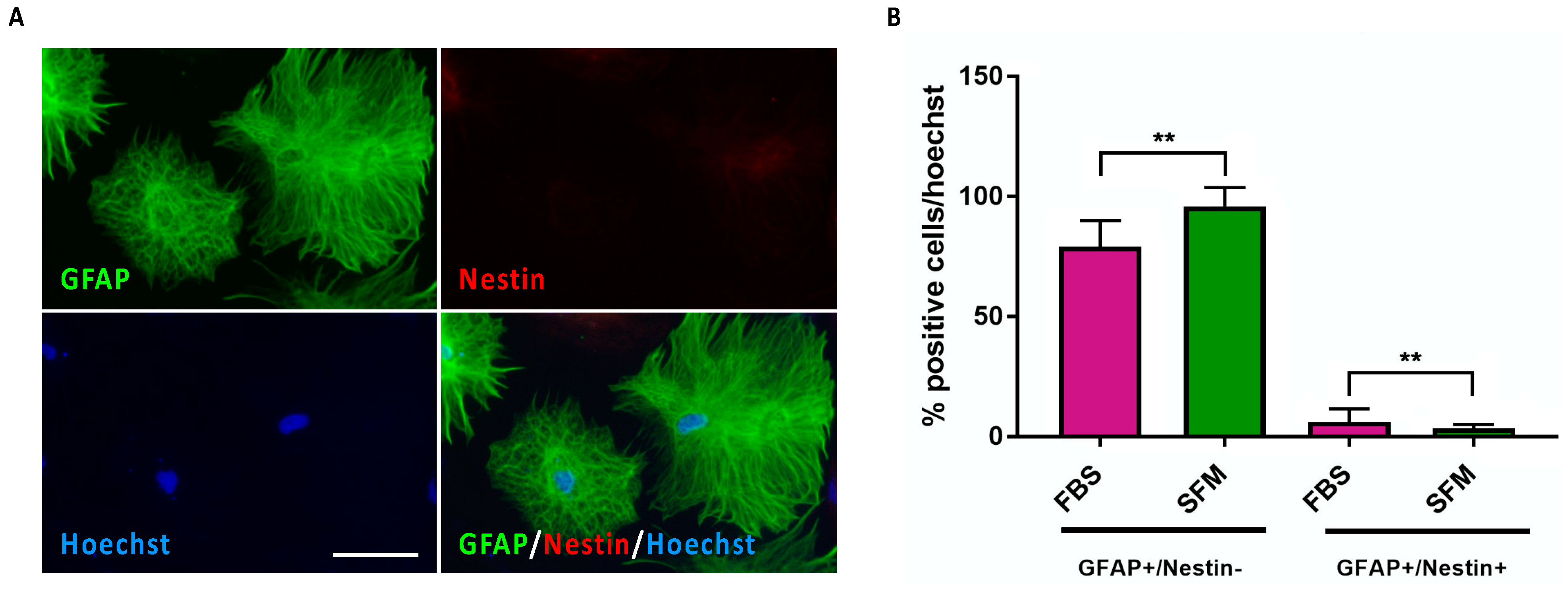
3.2. Astrocyte Commitment and Maturation of hiPSC-Derived CX APCs
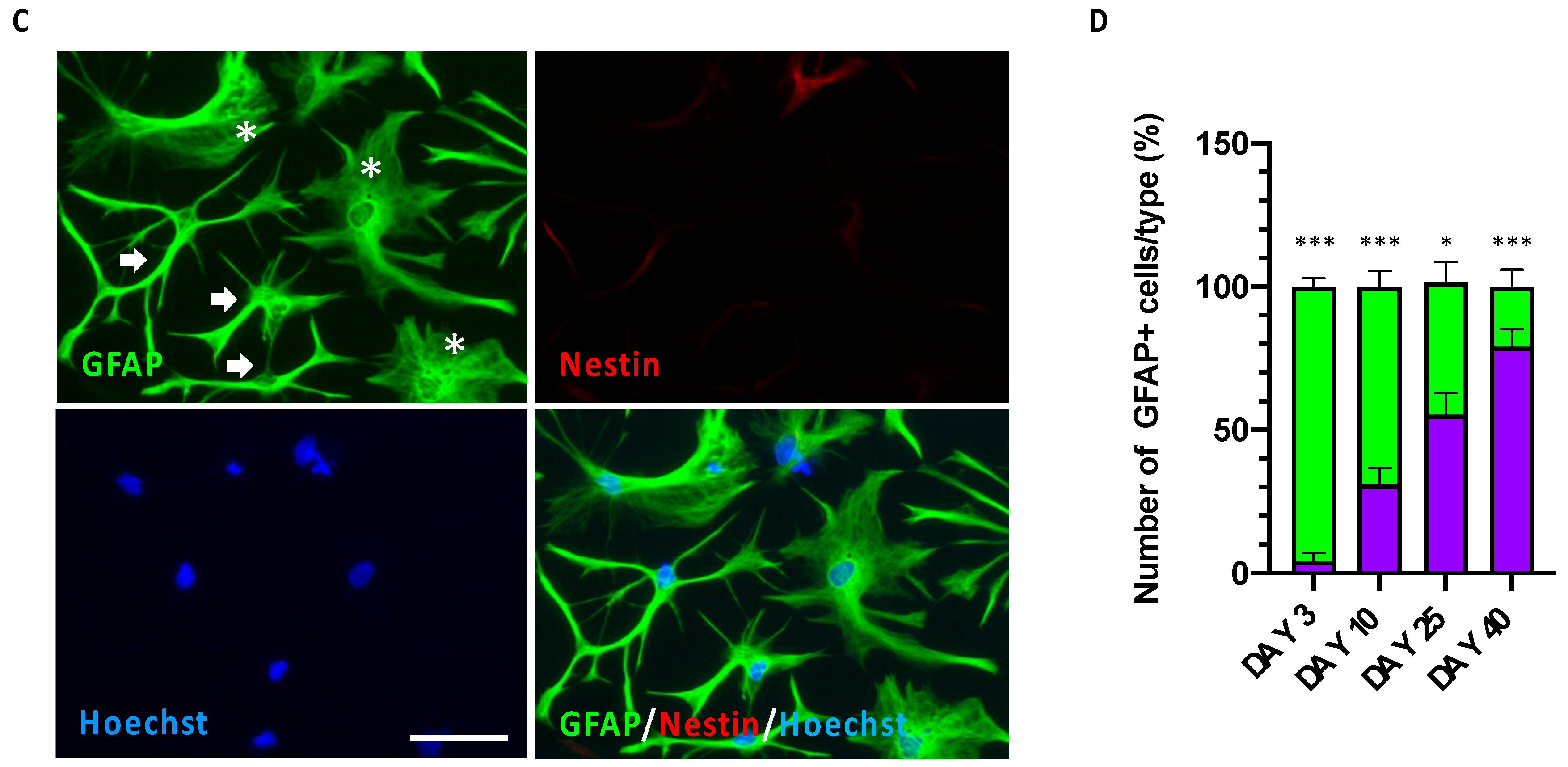
3.3. Time-Dependent Increased Maturation of hiPSC-Derived CX Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jäkel, S.; Dimou, L. Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, M.R. Specification and morphogenesis of astrocytes. Science 2010, 330, 774–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, D.; Volterra, A. Astrocytic dysfunction: Insights on the role in neurodegeneration. Brain Res. Bull. 2009, 80, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Tian, G.F.; Peng, W.; Lou, N.; Libionka, W.; Han, X.; Nedergaard, M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006, 9, 260–267. [Google Scholar] [CrossRef]
- Eroglu, C.; Barres, B.A. Regulation of synaptic connectivity by glia. Nature 2010, 468, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef] [Green Version]
- De Pittà, M.; Brunel, N.; Volterra, A. Astrocytes: Orchestrating synaptic plasticity? Neuroscience 2016, 323, 43–61. [Google Scholar] [CrossRef] [Green Version]
- Windrem, M.S.; Osipovitch, M.; Liu, Z.; Bates, J.; Chandler-Militello, D.; Zou, L.; Munir, J.; Schanz, S.; McCoy, K.; Miller, R.H.; et al. Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell Stem Cell 2017, 21, 195–208.e6. [Google Scholar] [CrossRef]
- Kim, R.; Healey, K.L.; Sepulveda-Orengo, M.T.; Reissner, K.J. Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 126–146. [Google Scholar] [CrossRef]
- Kindler, J.; Lim, C.K.; Weickert, C.S.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.R.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 2860–2872. [Google Scholar] [CrossRef] [Green Version]
- Rajkowska, G.; Miguel-Hidalgo, J.J.; Makkos, Z.; Meltzer, H.; Overholser, J.; Stockmeier, C. Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr. Res. 2002, 57, 127–138. [Google Scholar] [CrossRef]
- Williams, M.R.; Hampton, T.; Pearce, R.K.B.; Hirsch, S.R.; Ansorge, O.; Thom, M.; Maier, M. Astrocyte decrease in the subgenual cingulate and callosal genu in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 41–52. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Chana, G.; Beasley, C.; Landau, S.; Everall, I.P. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb. Cortex 2002, 12, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Torres-Platas, S.G.; Hercher, C.; Davoli, M.A.; Maussion, G.; Labonté, B.; Turecki, G.; Mechawar, N. Astrocytic hypertrophy in anterior cingulate white matter of depressed suicides. Neuropsychopharmacology 2011, 36, 2650–2658. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Haidet-Phillips, A.M.; Roybon, L.; Gross, S.K.; Tuteja, A.; Donnelly, C.J.; Richard, J.P.; Ko, M.; Sherman, A.; Eggan, K.; Henderson, C.E.; et al. Gene profiling of human induced pluripotent stem cell-derived astrocyte progenitors following spinal cord engraftment. Stem Cells Transl. Med. 2014, 3, 575–585. [Google Scholar] [CrossRef]
- Krencik, R.; Weick, J.P.; Liu, Y.; Zhang, Z.; Zhang, S.C. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 528–534. [Google Scholar] [CrossRef] [Green Version]
- McGivern, J.V.; Patitucci, T.N.; Nord, J.A.; Barabas, M.A.; Stucky, C.L.; Ebert, A.D. Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production. Glia 2013, 61, 1418–1428. [Google Scholar] [CrossRef] [Green Version]
- Serio, A.; Bilican, B.; Barmada, S.J.; Ando, D.M.; Zhao, C.; Siller, R.; Burr, K.; Haghi, G.; Story, D.; Nishimura, A.L.; et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc. Natl. Acad. Sci. USA 2013, 110, 4697–4702. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Qian, K.; Chen, W.; Hu, B.; Blackbourn, L.W.; Du, Z.; Ma, L.; Liu, H.; Knobel, K.M.; Ayala, M.; et al. Human-derived neural progenitors functionally replace astrocytes in adult mice. J. Clin. Investig. 2015, 125, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Chen, C.; Wang, R.; Chechneva, O.V.; Chung, S.H.; Rao, M.S.; Pleasure, D.E.; Liu, Y.; Zhang, Q.; Deng, W. hESC-derived Olig2+ progenitors generate a subtype of astroglia with protective effects against ischaemic brain injury. Nat. Commun. 2013, 4, 2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaltouki, A.; Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells 2013, 31, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Chaboub, L.S.; Deneen, B. Astrocyte form and function in the developing central nervous system. Semin. Pediatr. Neurol. 2013, 20, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voulgaris, D.; Nikolakopoulou, P.; Herland, A. Generation of Human iPSC-Derived Astrocytes with a mature star-shaped phenotype for CNS modeling. Stem Cell Rev. Rep. 2022, 18, 2494–2512. [Google Scholar] [CrossRef]
- Soubannier, V.; Maussion, G.; Chaineau, M.; Sigutova, V.; Rouleau, G.; Durcan, T.M.; Stifani, S. Characterization of human iPSC-derived astrocytes with potential for disease modeling and drug discovery. Neurosci. Lett. 2020, 731, 135028. [Google Scholar] [CrossRef]
- Tcw, J.; Wang, M.; Pimenova, A.A.; Bowles, K.R.; Hartley, B.J.; Lacin, E.; Machlovi, S.I.; Abdelaal, R.; Karch, C.M.; Phatnani, H.; et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2017, 9, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Cutarelli, A.; Martínez-Rojas, V.A.; Tata, A.; Battistella, I.; Rossi, D.; Arosio, D.; Musio, C.; Conti, L. A Monolayer System for the Efficient Generation of Motor Neuron Progenitors and Functional Motor Neurons from Human Pluripotent Stem Cells. Cells 2021, 10, 1127. [Google Scholar] [CrossRef]
- Marsoner, F.; Marcatili, M.; Karnavas, T.; Bottai, D.; D’Agostino, A.; Scarone, S.; Conti, L. Generation and characterization of an induced pluripotent stem cell (iPSC) line from a patient with clozapine-resistant Schizophrenia. Stem Cell Res. 2016, 17, 661–664. [Google Scholar] [CrossRef] [Green Version]
- Marcatili, M.; Marsoner, F.; D’Agostino, A.; Karnavas, T.; Bottai, D.; Scarone, S.; Conti, L. Establishment of an induced pluripotent stem cell (iPSC) line from a patient with Clozapine-responder Schizophrenia. Stem Cell Res. 2016, 17, 630–633. [Google Scholar] [CrossRef] [Green Version]
- Cutarelli, A.; Ghio, S.; Zasso, J.; Speccher, A.; Scarduelli, G.; Roccuzzo, M.; Crivellari, M.; Pugno, N.M.; Casarosa, S.; Boscardin, M.; et al. Vertically-Aligned Functionalized Silicon Micropillars for 3D Culture of Human Pluripotent Stem Cell-Derived Cortical Progenitors. Cells 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, C.; Sahlgren, C.; Berthold, C.H.; Stakeberg, J.; Celis, J.E.; Betsholtz, C.; Eriksson, J.E.; Pekny, M. Intermediate Filament Protein Partnership in Astrocytes. J. Biol. Chem. 1999, 274, 23996–24006. [Google Scholar] [CrossRef] [Green Version]
- Potokar, M.; Morita, M.; Wiche, G.; Jorgačevski, J. The Diversity of Intermediate Filaments in Astrocytes. Cells 2020, 9, 1604. [Google Scholar] [CrossRef]
- Roybon, L.; Lamas, N.J.; Garcia, A.D.; Yang, E.J.; Sattler, R.; Lewis, V.J.; Kim, Y.A.; Kachel, C.A.; Rothstein, J.D.; Przedborski, S.; et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013, 4, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci. Ther. 2019, 25, 665–673. [Google Scholar] [CrossRef]
- Akkouh, I.A.; Hribkova, H.; Grabiec, M.; Budinska, E.; Szabo, A.; Kasparek, T.; Andreassen, O.A.; Sun, Y.; Djurovic, S. Derivation and Molecular Characterization of a Morphological Subpopulation of Human iPSC Astrocytes Reveal a Potential Role in Schizophrenia and Clozapine Response. Schizophr. Bull. 2022, 48, 190–198. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Jill, G.; Chaineau, M.; Brennand, K.J. The Importance of Non-neuronal Cell Types in hiPSC-Based Disease Modeling and Drug Screening. Front. Cell Dev. Biol. 2017, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Mormone, E.; D’Sousa, S.; Alexeeva, V.; Bederson, M.M.; Germano, I.M. “Footprint-free” human induced pluripotent stem cell-derived astrocytes for in vivo cell-based therapy. Stem Cells Dev. 2014, 23, 2626–2636. [Google Scholar] [CrossRef] [Green Version]
- Lafaille, F.G.; Pessach, I.M.; Zhang, S.Y.; Ciancanelli, M.J.; Herman, M.; Abhyankar, A.; Ying, S.-W.; Keros, S.; Goldstein, P.A.; Mostoslavsky, G.; et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 2012, 491, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Leventoux, N.; Morimoto, S.; Imaizumi, K.; Sato, Y.; Takahashi, S.; Mashima, K.; Ishikawa, M.; Sonn, I.; Kondo, T.; Watanabe, H.; et al. Human Astrocytes Model Derived from Induced Pluripotent Stem Cells. Cells 2020, 9, 2680. [Google Scholar] [CrossRef]
- Yuan, S.H.; Martin, J.; Elia, J.; Flippin, J.; Paramban, R.I.; Hefferan, M.P.; Vidal, J.G.; Mu, Y.; Killian, R.L.; Israel, M.A.; et al. Cell surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS ONE 2011, 6, e17540. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, A.; Avci, H.X.; Leist, M.; Kobolák, J.; Dinnyés, A. Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research. Front. Cell. Neurosci. 2016, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, R.A.; Liu, J.; Miller, J.W.; Rothstein, J.D.; Farrell, K.; Stein, B.A.; Longuemare, M.C. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J. Neurosci. 1997, 17, 932–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, F.C.; Paulin, D.; Moura Neto, V. Glial fibrillary acidic protein (GFAP): Modulation by growth factors and its implication in astrocyte differentiation. Braz. J. Med. Biol. Res. 1999, 32, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.; Gomes, A.; Garcia-Abreu, J.; Galou, M.; Paulin, D.; Moura Neto, V. Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. Glia 1999, 26, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human astrocytes: Structure and functions in the healthy brain. Brain Struct. Funct. 2017, 222, 2017–2029. [Google Scholar] [CrossRef] [Green Version]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [Green Version]
- Bradley, R.A.; Shireman, J.; McFalls, C.; Choi, J.; Canfield, S.C.; Dong, Y.; Liu, K.; Lisota, B.; Jones, J.R.; Petersen, J.; et al. Regionally specified human pluripotent stem cell-derived astrocytes exhibit different molecular signatures and functional properties. Development 2019, 146, dev170910. [Google Scholar] [CrossRef] [Green Version]
| Gene | Fw Primer | Rev Primer |
|---|---|---|
| hNESTIN | GGAGAAGGACCAAGAACTG | ACCTCCTCTGTGGCATTC |
| hGFAP | CTGGAGAGGAAGATTGAGTCGC | ACGTCAAGCTCCACATGGACCT |
| hALDOLASE C | CATTCTGGCTGCGGATGAGTCT | CACACGGTCATCAGCACTGAAC |
| hEAAT1 | GGTTGCTGCAAGCACTCATCAC | CACGCCATTGTTCTCTTCCAGG |
| hEAAT2 | TGCCAACAGAGGACATCAGCCT | CAGCTCAGACTTGGAGAGGTGA |
| hVIMENTIN | TGGACCAGCTAACCAACGAC | GCCAGAGACGCATTGTCAAC |
| hS100B | TGTAGACCCTAACCCGGAGG | TGCATGGATGAGGAACGCAT |
| hGAPDH | CCACTCCTCCACCTTTGAC | ACCCTGTTGCTGTAGCCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistella, I.; Cutarelli, A.; Zasso, J.; Clerici, M.; Sala, C.; Marcatili, M.; Conti, L. Cortical Astrocyte Progenitors and Astrocytes from Human Pluripotent Stem Cells. J. Pers. Med. 2023, 13, 538. https://doi.org/10.3390/jpm13030538
Battistella I, Cutarelli A, Zasso J, Clerici M, Sala C, Marcatili M, Conti L. Cortical Astrocyte Progenitors and Astrocytes from Human Pluripotent Stem Cells. Journal of Personalized Medicine. 2023; 13(3):538. https://doi.org/10.3390/jpm13030538
Chicago/Turabian StyleBattistella, Ingrid, Alessandro Cutarelli, Jacopo Zasso, Massimo Clerici, Carlo Sala, Matteo Marcatili, and Luciano Conti. 2023. "Cortical Astrocyte Progenitors and Astrocytes from Human Pluripotent Stem Cells" Journal of Personalized Medicine 13, no. 3: 538. https://doi.org/10.3390/jpm13030538
APA StyleBattistella, I., Cutarelli, A., Zasso, J., Clerici, M., Sala, C., Marcatili, M., & Conti, L. (2023). Cortical Astrocyte Progenitors and Astrocytes from Human Pluripotent Stem Cells. Journal of Personalized Medicine, 13(3), 538. https://doi.org/10.3390/jpm13030538









