The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report
Abstract
:1. Introduction
2. Case Report
3. Materials and Methods
3.1. Oral Dietary Supplementation
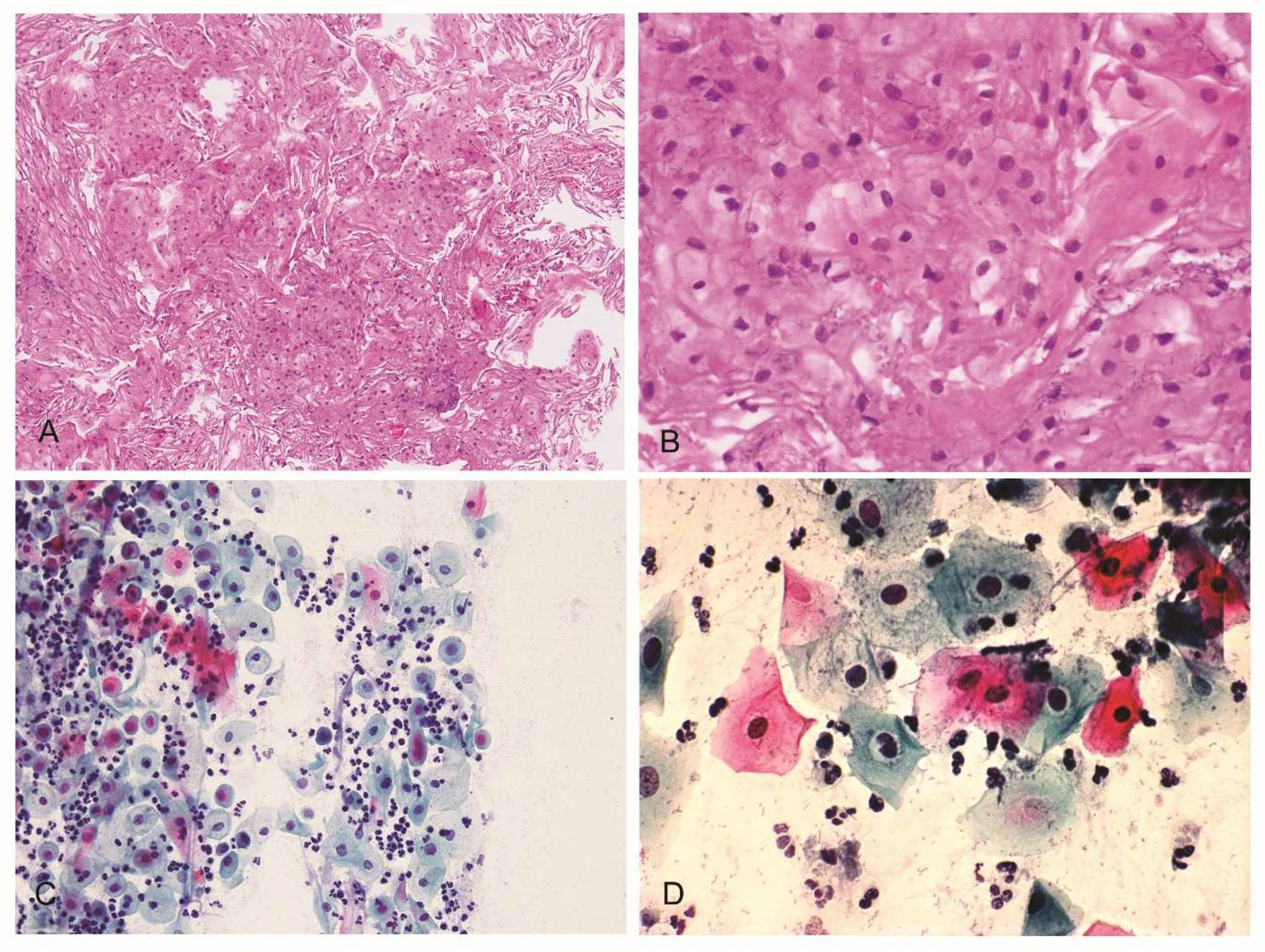
3.2. Cervical–Vaginal Cytology (Pap-Test)
3.3. Histological Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Garutti, S.M.; Bazzan, E.; Tarabbia, C. Gender differences in the epidemiology and prevention of human papillomavirus (HPV) and HPV-related diseases. Ital. J. Gend.-Specif. Med. 2018, 4, 152–161. [Google Scholar]
- Cogliano, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F. WHO International Agency for Research on Cancer. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005, 6, 204. [Google Scholar] [CrossRef]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Kundu, R. Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 2019, 10, 3116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016, 2, 16086. [Google Scholar] [CrossRef]
- Peralta-Zaragoza, O.; Deas, J.; Gómez-Cerón, C.; García-Suastegui, W.A.; Fierros-Zárate, G.D.S.; Jacobo-Herrera, N.J. HPV-Based Screening, Triage, Treatment, and Followup Strategies in the Management of Cervical Intraepithelial Neoplasia. Obstet. Gynecol. Int. 2013, 2013, 912780. [Google Scholar] [CrossRef]
- Nuovo, J.; Melnikow, J.; Willan, A.; Chan, B. Treatment outcomes for squamous intraepithelial lesions. Int. J. Gynecol. Obstet. 2000, 68, 25–33. [Google Scholar] [CrossRef]
- Liverani, C.A. The four steps in the prevention of human papillomavirus-associated neoplasia. Arch. Gynecol. Obstet. 2013, 288, 979–988. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.-H.S.; Garcia, F. Green Tea Compound in Chemoprevention of Cervical Cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The Major Constituent of Green Tea, Epigallocatechin-3-Gallate (EGCG), Inhibits the Growth of HPV18-Infected Keratinocytes by Stimulating Proteasomal Turnover of the E6 and E7 Oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea Polyphenols Induce Apoptosis Through Mitochondrial Pathway and by Inhibiting Nuclear Factor-κB and Akt Activation in Human Cervical Cancer Cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef]
- Al-Hazzani, A.A.; Alshatwi, A.A. Catechin hydrate inhibits proliferation and mediates apoptosis of SiHa human cervical cancer cells. Food Chem. Toxicol. 2011, 49, 3281–3286. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Han, J.H.; Song, Y.; Lee, J.H.; Choi, S.Y.; Park, Y.M. Epigallocatechin-3-gallate Can Prevent Type 2 Human Papillomavirus E7 from Suppressing Interferon-Stimulated Genes. Int. J. Mol. Sci. 2021, 22, 2418. [Google Scholar] [CrossRef]
- Ahn, W.-S.; Yoo, J.; Huh, S.-W.; Kim, C.-K.; Lee, J.-M.; Namkoong, S.-E.; Bae, S.-M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Wu, W.; Guo, Y.; Cui, W. Elevated Homocysteine Level and Folate Deficiency Associated with Increased Overall Risk of Carcinogenesis: Meta-Analysis of 83 Case-Control Studies Involving 35,758 Individuals. PLoS ONE 2015, 10, e0123423. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, Y.-S.; Kusumanchi, P.; Stabler, S.P.; Zhang, Y.; Antony, A.C. Folate Deficiency Facilitates Genomic Integration of Human Papillomavirus Type 16 DNA In Vivo in a Novel Mouse Model for Rapid Oncogenic Transformation of Human Keratinocytes. J. Nutr. 2018, 148, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Piyathilake, C.J.; Macaluso, M.; Chambers, M.M.; Badiga, S.; Siddiqui, N.R.; Bell, W.C.; Edberg, J.C.; Partridge, E.E.; Alvarez, R.D.; Johanning, G.L. Folate and Vitamin B12 May Play a Critical Role in Lowering the HPV 16 Methylation–Associated Risk of Developing Higher Grades of CIN. Cancer Prev. Res. 2014, 7, 1128–1137. [Google Scholar] [CrossRef] [Green Version]
- Piyathilake, C.J.; Henao, O.L.; Macaluso, M.; Cornwell, P.E.; Meleth, S.; Heimburger, D.C.; Partridge, E.E. Folate Is Associated with the Natural History of High-Risk Human Papillomaviruses. Cancer Res 2004, 64, 8788–8793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin. Investig. Med. 2008, 31, E106–E116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemma, G.; Schettino, M.T.; Munno, G.M.; Fasulo, D.D.; Sandullo, L.; Amabile, E.; La Verde, M.; Torella, M. Echinacea angustifolia and Echinacea purpurea Supplementation Combined with Vaginal Hyaluronic Acid to Boost the Remission of Cervical Low-Grade Squamous Intraepithelial Lesions (L-SILs): A Randomized Controlled Trial. Medicina 2022, 58, 646. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Cui, L.; Bian, C.; Zhao, X.; Wang, X. Clearance of human papillomavirus infection in patients with cervical intraepithelial neoplasia: A systemic review and meta-analysis. Medicine 2020, 99, e23155. [Google Scholar] [CrossRef]
- Boardman, L.A.; Kennedy, C.M. Management of atypical squamous cells, low-grade squamous intraepithelial lesions, and cervical intraepithelial neoplasia 1. Obstet. Gynecol. Clin. N. Am. 2008, 35, 599–614. [Google Scholar] [CrossRef]
- Jancar, N.; Rakar, S.; Poljak, M.; Fujs, K.; Kocjan, B.J.; Vrtacnik-Bokal, E. Efficiency of three surgical procedures in eliminating high-risk human papillomavirus infection in women with precancerous cervical lesions. Eur. J. Gynaecol. Oncol. 2006, 27, 239–242. [Google Scholar]
- Bogani, G.; DI Donato, V.; Sopracordevole, F.; Ciavattini, A.; Ghelardi, A.; Lopez, S.; Simoncini, T.; Plotti, F.; Casarin, J.; Serati, M.; et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser Conization: A 5-year follow-up study. Gynecol. Oncol. 2020, 159, 636–641. [Google Scholar] [CrossRef]
- Ouh, Y.-T.; Cho, H.W.; Kim, S.M.; Min, K.-J.; Lee, S.-H.; Song, J.-Y.; Lee, J.-K.; Lee, N.W.; Hong, J.H. Risk factors for type-specific persistence of high-risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment. Obstet. Gynecol. Sci. 2020, 63, 631–642. [Google Scholar] [CrossRef]
- Aragona, C.; Bezerra Espinola, M.S.; Bilotta, G.; Porcaro, G.; Calcagno, M. Evaluating the Efficacy of Pervistop®, a New Combination Based on EGCG, Folic Acid, Vitamin B12 and Hyaluronic Acid on Patients with Human Papilloma Virus (HPV) Persistent Infections and Cervical Lesions: A Pilot Study. J. Clin. Med. 2023, 12, 2171. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandi, G.; Botticelli, L.; Fraia, P.D.; Babalini, C.; Masini, M.; Unfer, V. The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report. J. Pers. Med. 2023, 13, 567. https://doi.org/10.3390/jpm13030567
Grandi G, Botticelli L, Fraia PD, Babalini C, Masini M, Unfer V. The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report. Journal of Personalized Medicine. 2023; 13(3):567. https://doi.org/10.3390/jpm13030567
Chicago/Turabian StyleGrandi, Giovanni, Laura Botticelli, Pietro Di Fraia, Carla Babalini, Meris Masini, and Vittorio Unfer. 2023. "The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report" Journal of Personalized Medicine 13, no. 3: 567. https://doi.org/10.3390/jpm13030567
APA StyleGrandi, G., Botticelli, L., Fraia, P. D., Babalini, C., Masini, M., & Unfer, V. (2023). The Association of Four Natural Molecules—EGCG, Folic Acid, Vitamin B12, and HA—To Counteract HPV Cervical Lesions: A Case Report. Journal of Personalized Medicine, 13(3), 567. https://doi.org/10.3390/jpm13030567






