Relationship between Exercise Test Parameters, Device-Delivered Electric Shock and Adverse Clinical Events in Patients with an Implantable Cardioverter Defibrillator for Primary Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exercise Test
2.3. Electric Shocks Delivered by the Device and Clinical Events
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Exercise Test Results
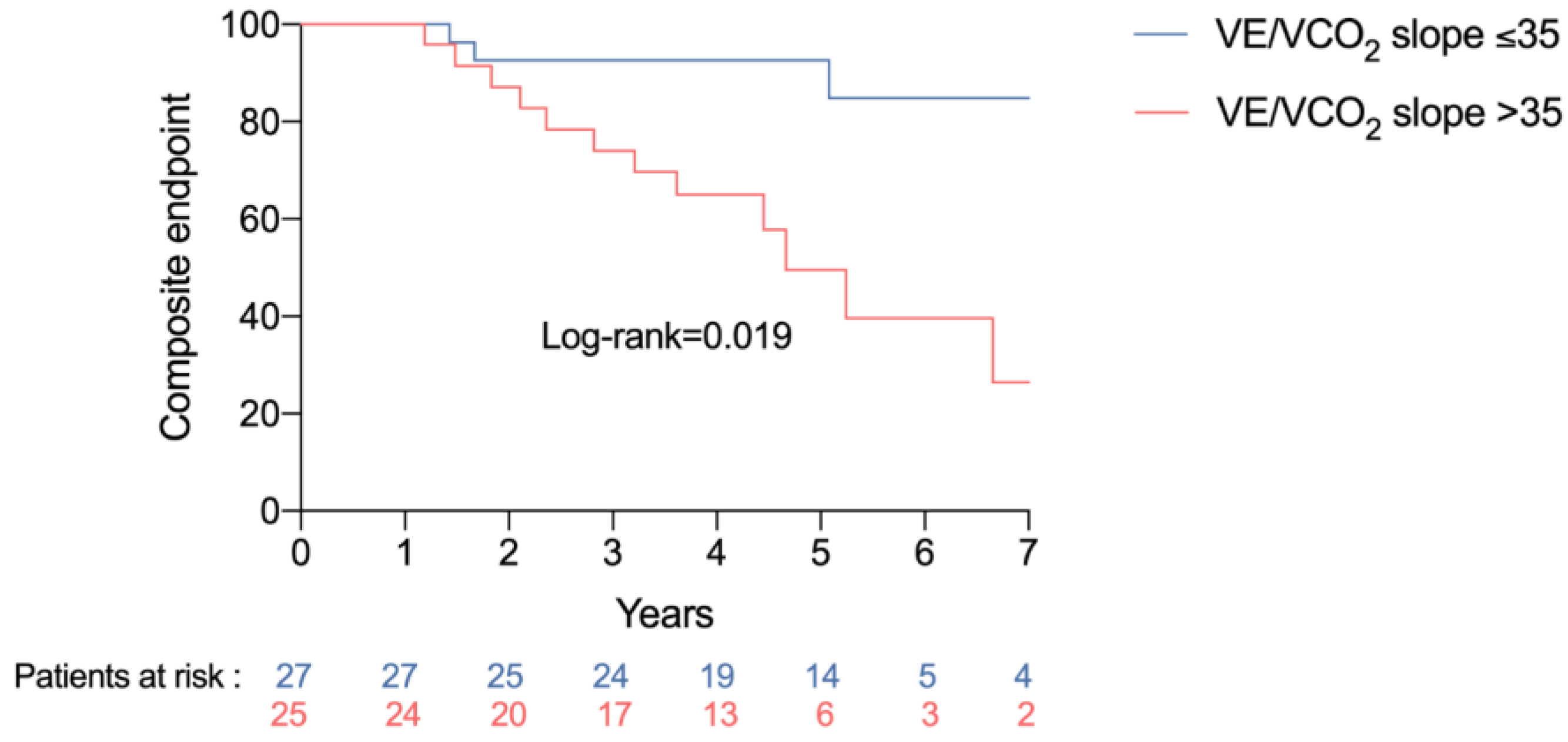
3.3. Association between Exercise Test Variables and Clinical Events
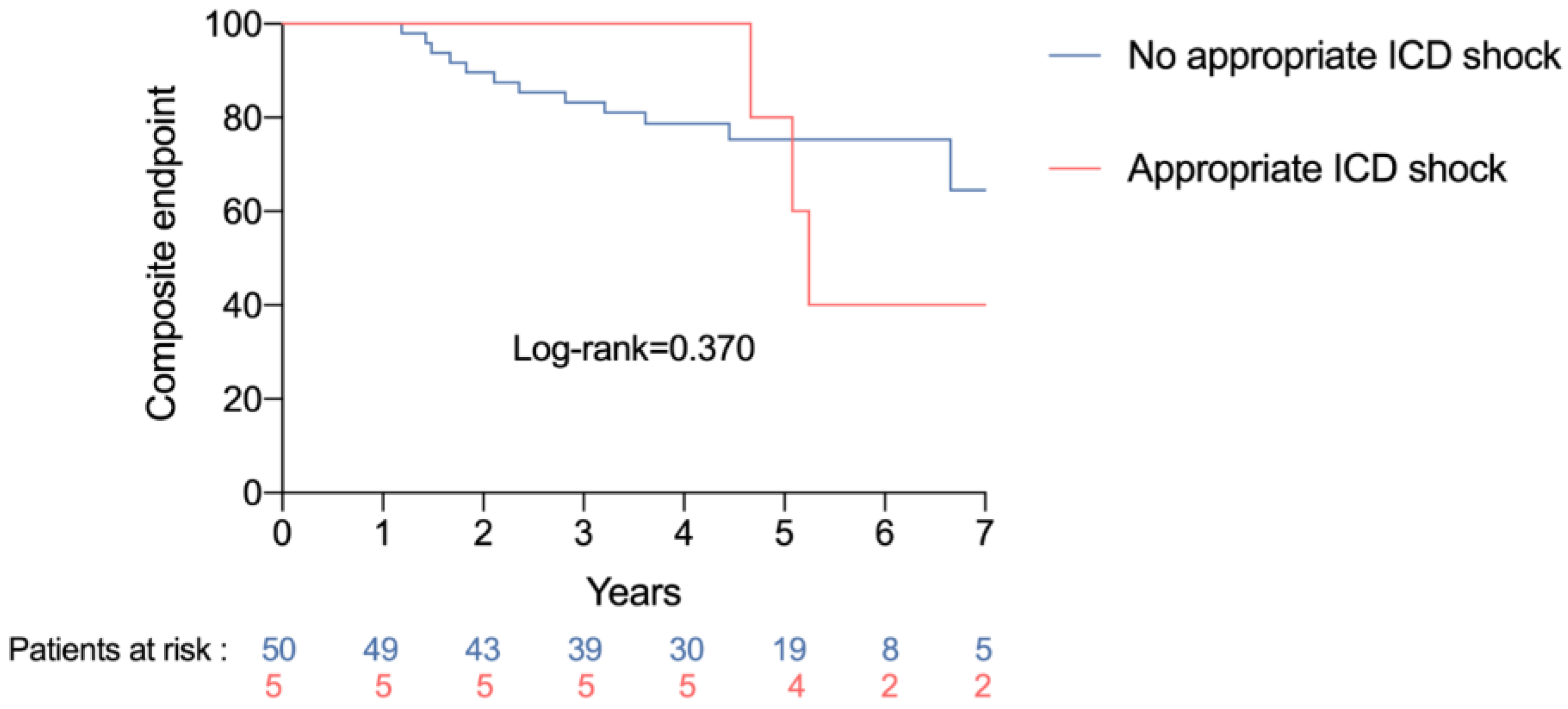
3.4. Association between Device-Delivered Electric Shock and Clinical Events
3.5. Association between Exercise Test Variables and Appropriate Electric Shock
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Celutkien, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Zipes, D.P.; Camm, A.J.; Borggrefe, M.; Buxton, A.E.; Chaitman, B.; Fromer, M.; Gregoratos, G.; Klein, G.; Myerburg, R.J.; Quinones, M.A.; et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J. Am. Coll. Cardiol. 2006, 48, e247–e346. [Google Scholar] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.A.M.; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008, 51, e1–e62. [Google Scholar]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic importance of defibrillator shocks in patients with heart failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef]
- Swank, A.M.; Horton, J.; Fleg, J.L.; Fonarow, G.C.; Keteyian, S.; Goldberg, L.; Wolfel, G.; Handberg, E.M.; Bensimhon, D.; Illiou, M.-C.; et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ. Heart Fail. 2012, 5, 579–585. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e272–e391. [Google Scholar] [PubMed]
- Tabet, J.-Y.; Beauvais, F.; Thabut, G.; Tartière, J.-M.; Logeart, D.; Cohen-Solal, A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. Eur. J. Cardiovasc. Prev. Rehabil. Off. J. Eur. Soc. Cardiol. Work Groups Epidemiol. Prev. Card. Rehabil. Exerc. Physiol. 2003, 10, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef] [PubMed]
- Chua, T.P.; Ponikowski, P.; Harrington, D.; Anker, S.D.; Webb-Peploe, K.; Clark, A.L.; Poole-Wilson, P.A.; Coats, A.J. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J. Am. Coll. Cardiol. 1997, 29, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Poggio, R.; Arazi, H.C.; Giorgi, M.; Miriuka, S.G. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: A meta-analysis of the published literature. Am Heart J. 2010, 160, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Methvin, A.B.; Owens, A.T.; Emmi, A.G.; Allen, M.; Wiegers, S.E.; Dries, D.L.; Margulies, K.B.; Forfia, P.R. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest 2011, 139, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, A.; Brawner, C.A.; Aldred, H.A.; Lewis, B.; Williams, C.T.; Tita, C.; Schairer, J.R.; Ehrman, J.K.; Velez, M.; Selektor, Y.; et al. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT-CPX) project. Am. Heart J. 2016, 174, 167–172. [Google Scholar] [CrossRef]
- Moss, A.J.; Greenberg, H.; Case, R.B.; Zareba, W.; Hall, W.J.; Brown, M.W.; Daubert, J.P.; McNitt, S.; Andrews, M.L.; Elkin, A.D. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation 2004, 110, 3760–3765. [Google Scholar] [CrossRef]
- Xie, J.; Weil, M.H.; Sun, S.; Tang, W.; Sato, Y.; Jin, X.; Bisera, J. High-energy defibrillation increases the severity of postresuscitation myocardial dysfunction. Circulation 1997, 96, 683–688. [Google Scholar] [CrossRef]
- Osswald, S.; Trouton, T.G.; O’Nunain, S.S.; Holden, H.B.; Ruskin, J.N.; Garan, H. Relation between shock-related myocardial injury and defibrillation efficacy of monophasic and biphasic shocks in a canine model. Circulation 1994, 90, 2501–2509. [Google Scholar] [CrossRef]
- Welch, P.J.; Joglar, J.A.; Hamdan, M.H.; Nelson, L.; Page, R.L. The Effect of Biphasic Defibrillation on the Immediate Pacing Threshold of a Dedicated Bipolar, Steroid-Eluting Lead. Pacing. Clin. Electrophysiol. 1999, 22, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Singer, I.; Hutchins, G.M.; Mirowski, M.; Mower, M.M.; Veltri, E.P.; Guarnieri, T.; Juanteguy, J.; Fisher, S.; Reid, P.R.; Weisfeldt, M.L. Pathologic findings related to the lead system and repeated defibrillations in patients with the automatic implantable cardioverter-defibrillator. J. Am. Coll. Cardiol. 1987, 10, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Moss, A.J.; Hall, W.J.; McNitt, S.; Zareba, W.; Andrews, M.L.; Cannom, D.S. Causes and Consequences of Heart Failure after Prophylactic Implantation of a Defibrillator in the Multicenter Automatic Defibrillator Implantation Trial II. Circulation 2006, 113, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Semmler, V.; Biermann, J.; Haller, B.; Jilek, C.; Sarafoff, N.; Lennerz, C.; Vrazic, H.; Zrenner, B.; Asbach, S.; Kolb, C. ICD Shock, Not Ventricular Fibrillation, Causes Elevation of High Sensitive Troponin T after Defibrillation Threshold Testing—The Prospective, Randomized, Multicentre TropShock-Trial. PLoS ONE 2015, 10, e0131570. [Google Scholar] [CrossRef]
- Gasparini, M.; Proclemer, A.; Klersy, C.; Kloppe, A.; Lunati, M.; Ferrer, J.B.M.; Hersi, A.; Gulaj, M.; Wijfels, M.C.E.F.; Santi, E.; et al. Effect of long-detection interval vs. standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: The ADVANCE III randomized clinical trial. JAMA 2013, 309, 1903–1911. [Google Scholar] [CrossRef]
- Powell, B.D.; Saxon, L.A.; Boehmer, J.P.; Day, J.D.; Gilliam, F.R.; Heidenreich, P.A.; Jones, P.W.; Rousseau, M.J.; Hayes, D.L. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J. Am. Coll. Cardiol. 2013, 62, 1674–1679. [Google Scholar] [CrossRef]
- Calé, R.; Mendes, M.; Brito, J.; Sousa, P.; Carmo, P.; Almeida, S.; Gomes, R.; Ferreira, A.; Santos, K.R.; Cavaco, D.; et al. Resting heart rate is a powerful predictor of arrhythmic events in patients with dilated cardiomyopathy and implantable cardioverter-defibrillator. Rev. Port. Cardiol. Orgao. Soc. Port. Cardiol. Port. J. Cardiol. Off. J. Port. Soc. Cardiol. 2011, 30, 199–212. [Google Scholar]
- Bergau, L.; Willems, R.; Sprenkeler, D.J.; Fischer, T.H.; Flevari, P.; Hasenfuß, G.; Katsaras, D.; Kirova, A.; Lehnart, S.E.; Lüthje, L.; et al. Differential multivariable risk prediction of appropriate shock versus competing mortality—A prospective cohort study to estimate benefits from ICD therapy. Int. J. Cardiol. 2018, 272, 102–107. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.H.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Porcari, A.; De Luca, A.; Grigoratos, C.; Biondi, F.; Faganello, G.; Vitrella, G.; Nucifora, G.; Aquaro, G.D.; Merlo, M.; Sinagra, G. Arrhythmic risk stratification by cardiac magnetic resonance tissue characterization: Disclosing the arrhythmic substrate within the heart muscle. Heart Fail Rev. 2022, 27, 49–69. [Google Scholar] [CrossRef]
| Baseline characteristics: | n = 55 |
| Age, years | 60 (47–73) |
| Male sex | 41 (75) |
| NYHA class ≥ 3 | 47 (85) |
| LVEF, % | 30 (24–36) |
| Dyslipidemia | 27 (49) |
| Diabetes | 19 (35) |
| Active smoking | 36 (65) |
| Hypertension | 17 (31) |
| Obesity | 14 (25) |
| Atrial fibrillation | 10 (18) |
| GFR < 60 mL/min | 20 (36) |
| Stroke | 2 (4) |
| COPD | 4 (8) |
| Hyperthyroidism | 4 (8) |
| Anemia | 11 (20) |
| Obstructive sleep apnea | 6 (11) |
| Underlying cardiomyopathy: | |
| Ischemic | 31 (56) |
| Dilated | 24 (44) |
| Treatment: | |
| ACEI/ARB | 31 (56) |
| Sacubitril/Valsartan | 23 (42) |
| Beta-blocker | 54 (98) |
| Anti-aldosterone agent | 41 (75) |
| Amiodarone | 11 (20) |
| Loop diuretic | 33 (60) |
| Ivabradine | 15 (27) |
| Type of device implanted: | |
| Single chamber | 24 (43) |
| Dual chamber | 12 (22) |
| Cardiac resynchronization device | 19 (35) |
| Negative outcomes on exercise test: | |
| Peak VO2 < 12 mL/min/kg | 7 (13) |
| Maximum theoretical VO2 < 50% | 12 (22) |
| VE/VCO2 slope > 35 | 25 (48) |
| Oscillatory ventilation | 3 (7) |
| Circulatory power < 2000 | 15 (33) |
| Drop in pulse oximetry during exercise | 23 (45) |
| Drop in blood pressure during exercise | 25 (46) |
| Clinical events: | |
| Appropriate DDES | 5 (9) |
| Appropriate shock or ATP | 14 (25) |
| Death or heart transplant | 8 (15) |
| Composite endpoint * | 17 (31) |
| Median follow-up duration, months | 59 (47–68) |
| Median time between ICD implantation and first DDES, months | 37 (13–61) |
| Death or Heart Transplant | Composite Endpoint * | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Peak VO2 < 12 mL/min/kg | 0.60 (0.07–5.14) | 0.640 | 1.73 (0.55–5.43) | 0.350 |
| Maximum theoretical VO2 < 50% | 0.89 (0.17–4.55) | 0.893 | 2.04 (0.75–5.56) | 0.163 |
| VE/VCO2 slope > 35 | 3.36 (0.67–16.80) | 0.140 | 3.28 (1.15–9.41) | 0.027 |
| Oscillatory ventilation ** | - | - | - | - |
| Circulatory power < 2000 | 1.25 (0.28–5.52) | 0.765 | 1.65 (0.59–4.65) | 0.341 |
| Drop in pulse oximetry during exercise | 0.92 (0.23–3.71) | 0.904 | 0.70 (0.27–1.86) | 0.480 |
| Drop in blood pressure during exercise | 1.80 (0.43–7.61) | 0.423 | 1.40 (0.54–3.66) | 0.488 |
| Any two or more criteria | 5.56 (0.68–46) | 0.108 | 2.27 (0.78–6.54) | 0.130 |
| Death or Heart Transplant | Composite Endpoint * | |||
|---|---|---|---|---|
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Electric shock | 3.25 (0.69–15.21) | 0.135 | 2.58 (0.78–8.53) | 0.121 |
| Shock or antitachycardia pacing | 2.43 (0.52–11.36) | 0.259 | 1.97 (0.61–6.41) | 0.260 |
| Appropriate shock | 10.11 (1.66–61.52) | 0.012 | 5.39 (1.29–22.50) | 0.021 |
| Appropriate shock or antitachycardia pacing | 2.43 (0.52–11.36) | 0.259 | 1.97 (0.61–6.41) | 0.260 |
| Shock (n = 5) | No Shock (n = 50) | p-Value | |
|---|---|---|---|
| Baseline characteristics: | |||
| Age, years | 59 (55–60) | 61 (47–68) | 0.703 |
| Men | 5 (100) | 36 (72) | 0.314 |
| NYHA Class ≥ 3 | 0 | 8 (16) | 1.000 |
| LVEF, % | 30 (30–33) | 30 (25–30) | 0.276 |
| Dyslipidemia | 3 (60) | 24 (48) | 0.670 |
| Diabetes | 0 | 19 (38) | 0.152 |
| Active smoking | 4 (80) | 32 (64) | 0.650 |
| Hypertension | 0 | 17 (34) | 0.310 |
| Obesity | 0 | 14 (28) | 0.314 |
| Atrial fibrillation | 1 (20) | 9 (18) | 1.000 |
| GFR < 60 mL/min | 2 (40) | 18 (36) | 1.000 |
| Stroke | 0 | 2 (4) | 1.000 |
| COPD | 0 | 4 (8) | 1.000 |
| Hyperthyroidism | 0 | 4 (8) | 1.000 |
| Anemia | 0 | 11 (22) | 0.571 |
| Obstructive sleep apnea | 0 | 6 (12) | 1.000 |
| Underlying cardiomyopathy: | |||
| Ischemic | 2 (40) | 29 (58) | 0.643 |
| Dilated | 3 (60) | 21 (42) | |
| Treatment: | |||
| ACE/ARB | 2 (40) | 29 (58) | 0.643 |
| Sacubitril/Valsartan | 3 (60) | 20 (40) | 0.639 |
| Beta-blocker | 5 (100) | 49 (98) | 1.000 |
| Anti-aldosterone agent | 4 (80) | 37 (74) | 1.000 |
| Amiodarone | 4 (80) | 7 (14) | 0.004 |
| Loop diuretic | 4 (80) | 29 (58) | 0.638 |
| Ivabradine | 5 (100) | 35 (70) | 0.308 |
| Type of device implanted: | |||
| Single chamber | 4 (80) | 20 (40) | - |
| Dual chamber | 0 | 12 (24) | - |
| Cardiac resynchronization device | 1 (20) | 18 (36) | 0.649 |
| Negative outcomes on exercise test: | |||
| Peak VO2 < 12 mL/min/kg | 0 | 7 (14) | 1.000 |
| Maximum theoretical VO2 < 50% | 1 (20) | 11 (22) | 1.000 |
| VE/VCO2 slope > 35 | 2 (40) | 23 (46) | 1.000 |
| Oscillatory ventilation | 0 | 3 (6) | 1.000 |
| Circulatory power < 2000 | 1 (20) | 14 (28) | 1.000 |
| Drop in pulse oximetry during exercise | 3 (60) | 20 (40) | 0.647 |
| Drop in blood pressure during exercise | 2 (40) | 23 (46) | 1.000 |
| Any two negative outcomes | 3 (60) | 26 (52) | 1.000 |
| Any three negative outcomes | 2 (40) | 16 (32) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Théry, G.; Faroux, L.; Boyer, F.; Nazeyrollas, P.; Chabert, J.-P.; Metz, D.; Lesaffre, F. Relationship between Exercise Test Parameters, Device-Delivered Electric Shock and Adverse Clinical Events in Patients with an Implantable Cardioverter Defibrillator for Primary Prevention. J. Pers. Med. 2023, 13, 589. https://doi.org/10.3390/jpm13040589
Théry G, Faroux L, Boyer F, Nazeyrollas P, Chabert J-P, Metz D, Lesaffre F. Relationship between Exercise Test Parameters, Device-Delivered Electric Shock and Adverse Clinical Events in Patients with an Implantable Cardioverter Defibrillator for Primary Prevention. Journal of Personalized Medicine. 2023; 13(4):589. https://doi.org/10.3390/jpm13040589
Chicago/Turabian StyleThéry, Guillaume, Laurent Faroux, Fanny Boyer, Pierre Nazeyrollas, Jean-Pierre Chabert, Damien Metz, and François Lesaffre. 2023. "Relationship between Exercise Test Parameters, Device-Delivered Electric Shock and Adverse Clinical Events in Patients with an Implantable Cardioverter Defibrillator for Primary Prevention" Journal of Personalized Medicine 13, no. 4: 589. https://doi.org/10.3390/jpm13040589
APA StyleThéry, G., Faroux, L., Boyer, F., Nazeyrollas, P., Chabert, J.-P., Metz, D., & Lesaffre, F. (2023). Relationship between Exercise Test Parameters, Device-Delivered Electric Shock and Adverse Clinical Events in Patients with an Implantable Cardioverter Defibrillator for Primary Prevention. Journal of Personalized Medicine, 13(4), 589. https://doi.org/10.3390/jpm13040589







