Prospects of Novel and Repurposed Immunomodulatory Drugs against Acute Respiratory Distress Syndrome (ARDS) Associated with COVID-19 Disease
Abstract
:1. Introduction
2. Pathogenesis of COVID-19 Disease
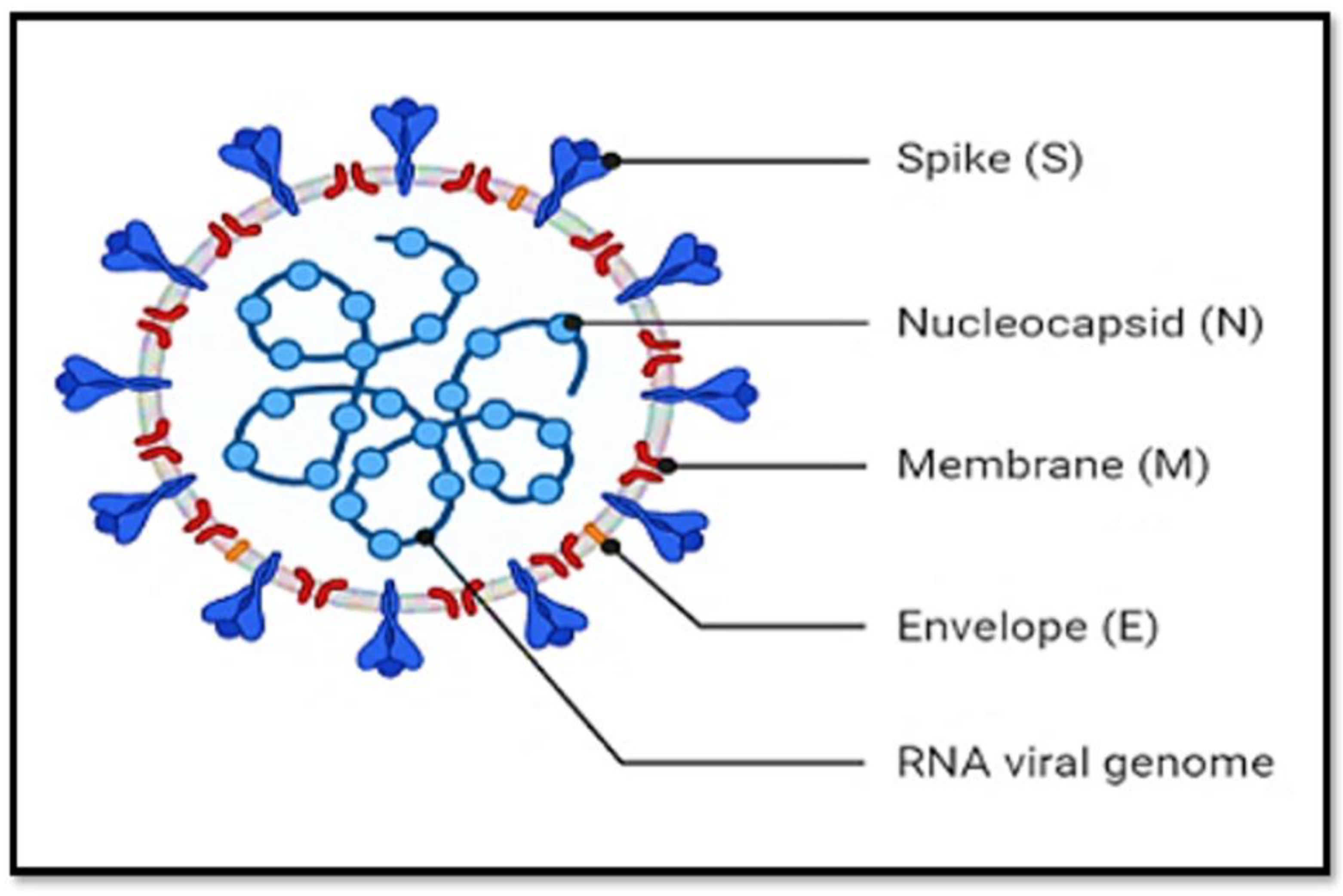
2.1. SARS-CoV-2 Structural Components
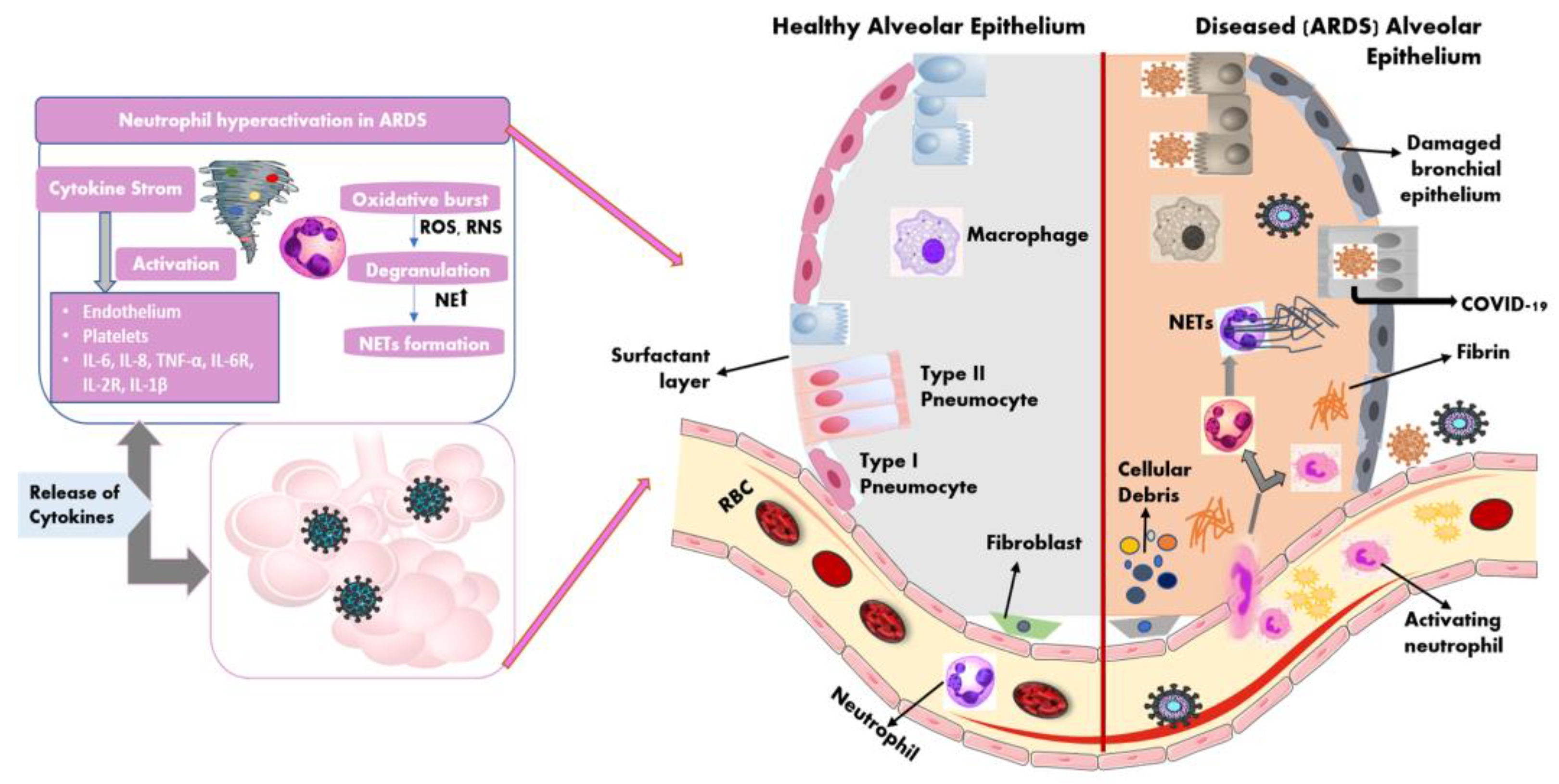
2.2. Immune Dysregulation during COVID-19—ARDS
2.3. COVID-19 in Patients with Autoimmune Diseases
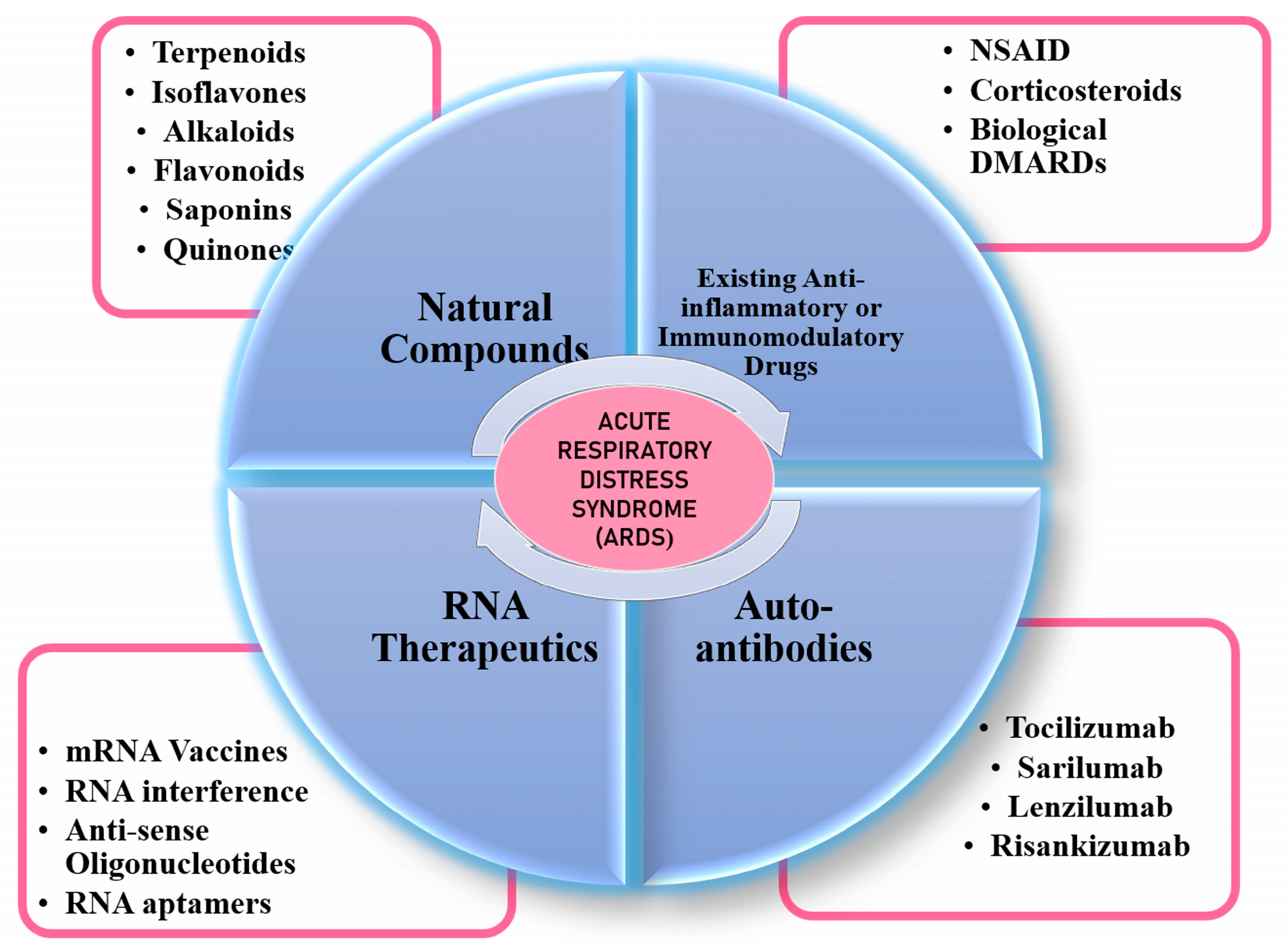
3. Management of ARDS
3.1. Anti-Rheumatic Drugs for COVID-19
3.1.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
3.1.2. Corticosteroids
3.2. Natural Compounds as Immunomodulatory Agents
3.3. Monoclonal Antibodies
3.4. RNA Therapeutics
4. Advanced Computational Tools for Informed Drug Screening
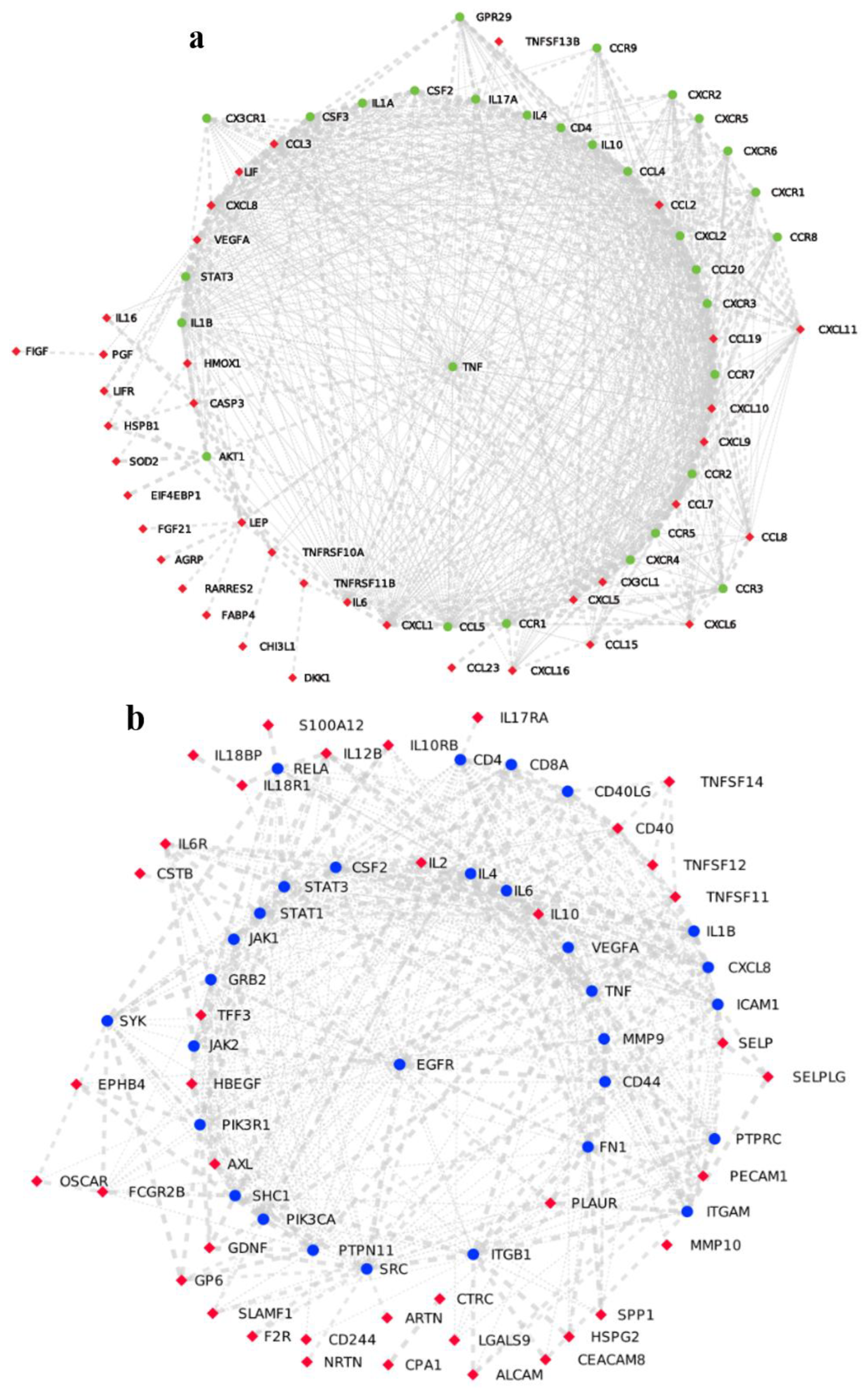
4.1. Application of Network Medicine to Screen Immunomodulatory Drugs
4.2. Applications of Machine Learning Algorithms to Screen Immune-Modulatory Drugs
Applications of Deep Learning
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, L. Lymphopenia Predicts Disease Severity of COVID-19: A Descriptive and Predictive Study. Signal Transduct. Target Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 4 February 2023).
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors Associated with COVID-19-Related Death in People with Rheumatic Diseases: Results from the COVID-19 Global Rheumatology Alliance Physician-Reported Registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Marotto, D.; Sarzi-Puttini, P. What Is the Role of Rheumatologists in the Era of COVID-19? Autoimmun. Rev. 2020, 19, 102539. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Manger, B.; Simon, D.; Caporali, R. COVID-19 Revisiting Inflammatory Pathways of Arthritis. Nat. Rev. Rheumatol. 2020, 16, 465–470. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation Abnormalities and Thrombosis in Patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and Tissue Factor-Enriched Neutrophil Extracellular Traps Are Key Drivers in COVID-19 Immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent Progress in Angiotensin II Type 2 Receptor Research in the Cardiovascular System. Hypertension 1999, 33, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 Cytokine Storm: Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Available online: https://www.hindawi.com/journals/mi/2020/8829674/ (accessed on 8 August 2021).
- Domon, H.; Nagai, K.; Maekawa, T.; Oda, M.; Yonezawa, D.; Takeda, W.; Hiyoshi, T.; Tamura, H.; Yamaguchi, M.; Kawabata, S.; et al. Neutrophil Elastase Subverts the Immune Response by Cleaving Toll-like Receptors and Cytokines in Pneumococcal Pneumonia. Front. Immunol. 2018, 9, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenne, C.N.; Wong, C.H.Y.; Zemp, F.J.; McDonald, B.; Rahman, M.M.; Forsyth, P.A.; McFadden, G.; Kubes, P. Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host Microbe 2013, 13, 169–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, R.; Uni, R.; Akchurin, O.M.; Alvarez-Mulett, S.; Gómez-Escobar, L.G.; Patino, E.; Hoffman, K.L.; Simmons, W.; Whalen, W.; Chetnik, K.; et al. Urine-Based Multi-Omic Comparative Analysis of COVID-19 and Bacterial Sepsis-Induced ARDS. Mol. Med. 2023, 29, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Tsai, Y.F.; Pan, Y.L.; Hwang, T.L. Understanding the Role of Neutrophils in Acute Respiratory Distress Syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef]
- Marks, M.; Marks, J.L. Viral Arthritis. Clin. Med. 2016, 16, 129–134. [Google Scholar] [CrossRef]
- Shin, Y.H.; Shin, J.I.; Moon, S.Y.; Jin, H.Y.; Kim, S.Y.; Yang, J.M.; Cho, S.H.; Kim, S.; Lee, M.; Park, Y.; et al. Autoimmune Inflammatory Rheumatic Diseases and COVID-19 Outcomes in South Korea: A Nationwide Cohort Study. Lancet Rheumatol. 2021, 3, 698–706. [Google Scholar] [CrossRef]
- Tan, E.H.; Sena, A.G.; Prats-Uribe, A.; You, S.C.; Ahmed, W.U.R.; Kostka, K.; Reich, C.; Duvall, S.L.; Lynch, K.E.; Matheny, M.E.; et al. COVID-19 in Patients with Autoimmune Diseases: Characteristics and Outcomes in a Multinational Network of Cohorts across Three Countries. Rheumatology 2021, 60, SI37–SI50. [Google Scholar] [CrossRef]
- Galeotti, C.; Bayry, J. Autoimmune and Inflammatory Diseases Following COVID-19. Nat. Rev. Rheumatol. 2020, 16, 413–414. [Google Scholar] [CrossRef]
- Gracia-Ramos, A.E.; Martin-Nares, E.; Hernández-Molina, G. New Onset of Autoimmune Diseases Following COVID-19 Diagnosis. Cells 2021, 10, 3592. [Google Scholar] [CrossRef]
- Saad, M.A.; Alfishawy, M.; Nassar, M.; Mohamed, M.; Esene, I.N.; Elbendary, A. COVID-19 and Autoimmune Diseases: A Systematic Review of Reported Cases. Curr. Rheumatol. Rev. 2020, 17, 193–204. [Google Scholar] [CrossRef]
- Know Your Treatment Options for COVID-19|FDA. Available online: https://www.fda.gov/consumers/consumer-updates/know-your-treatment-options-covid-19 (accessed on 3 February 2023).
- Vu, C.A.; DeRonde, K.J.; Vega, A.D.; Maxam, M.; Holt, G.; Natori, Y.; Zamora, J.G.; Salazar, V.; Boatwright, R.; Morris, S.R.; et al. Effects of Tocilizumab in COVID-19 Patients: A Cohort Study. BMC Infect. Dis. 2020, 20, 964. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Arribas, J.R.; Assoumou, L.; Holten, A.R.; Poissy, J.; Terzić, V.; Mazzaferri, F.; Baño, J.R.; Eustace, J.; Hites, M.; et al. Efficacy and Safety of Baricitinib in Hospitalized Adults with Severe or Critical COVID-19 (Bari-SolidAct): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Crit. Care 2023, 27, 9. [Google Scholar] [CrossRef]
- Kaliamurthi, S.; Selvaraj, G.; Selvaraj, C.; Singh, S.K.; Wei, D.Q.; Peslherbe, G.H. Structure-Based Virtual Screening Reveals Ibrutinib and Zanubrutinib as Potential Repurposed Drugs against COVID-19. Int. J. Mol. Sci. 2021, 22, 7071. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Tang, P.C.H.; Mahalingam, S.; Liu, X. Repurposing of Drugs Targeting the Cytokine Storm Induced by SARS-CoV-2. Br. J. Pharmacol. 2022, 180, 133–143. [Google Scholar] [CrossRef]
- Furci, F.; Murdaca, G.; Allegra, A.; Gammeri, L.; Senna, G.; Gangemi, S. IL-33 and the Cytokine Storm in COVID-19: From a Potential Immunological Relationship towards Precision Medicine. Int. J. Mol. Sci. 2022, 23, 14532. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial Intelligence in COVID-19 Drug Repurposing. Lancet Digit. Health 2020, 2, e667–e676. [Google Scholar] [CrossRef]
- Barati, S.; Hashemian, S.M.; Tabarsi, P.; Abedini, A.; Ashrafzadeh, M.; Haseli, S.; Abtahian, Z.; Yousefian, S.; Dastan, A.; Sobhanian, A.; et al. Combined Therapy of Ciclosporin Plus Favipiravir in the Management of Patients with Severe COVID-19, Not Responding to Dexamethasone: A Non-Controlled Prospective Trial. Int. Immunopharmacol. 2021, 99, 108043. [Google Scholar] [CrossRef]
- Dastan, F.; Nadji, S.A.; Saffaei, A.; Marjani, M.; Moniri, A.; Jamaati, H.; Hashemian, S.M.R.; Baghaei, P.; Abedini, A.; Varahram, M.; et al. Subcutaneous Administration of Interferon Beta-1a for COVID-19: A Non-Controlled Prospective Trial. Int. Immunopharmacol. 2020, 85, 106688. [Google Scholar] [CrossRef]
- Maldonado, V.; Hernandez-Ramírez, C.; Oliva-Pérez, E.A.; Sánchez-Martínez, C.O.; Pimentel-González, J.F.; Molina-Sánchez, J.R.; Jiménez-Villalba, Y.Z.; Chávez-Alderete, J.; Loza-Mejía, M.A. Pentoxifylline Decreases Serum LDH Levels and Increases Lymphocyte Count in COVID-19 Patients: Results from an External Pilot Study. Int. Immunopharmacol. 2021, 90, 107209. [Google Scholar] [CrossRef]
- Malekzadeh, R.; Abedini, A.; Mohsenpour, B.; Sharifipour, E.; Ghasemian, R.; Javad-Mousavi, S.A.; Khodashahi, R.; Darban, M.; Kalantari, S.; Abdollahi, N.; et al. Subcutaneous Tocilizumab in Adults with Severe and Critical COVID-19: A Prospective Open-Label Uncontrolled Multicenter Trial. Int. Immunopharmacol. 2020, 89, 107102. [Google Scholar] [CrossRef]
- Soin, A.S.; Kumar, K.; Choudhary, N.S.; Sharma, P.; Mehta, Y.; Kataria, S.; Govil, D.; Deswal, V.; Chaudhry, D.; Singh, P.K.; et al. Tocilizumab plus Standard Care versus Standard Care in Patients in India with Moderate to Severe COVID-19-Associated Cytokine Release Syndrome (COVINTOC): An Open-Label, Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Respir. Med. 2021, 9, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Broman, N.; Feuth, T.; Vuorinen, T.; Valtonen, M.; Hohenthal, U.; Löyttyniemi, E.; Hirvioja, T.; Jalava-Karvinen, P.; Marttila, H.; Nordberg, M.; et al. Early Administration of Tocilizumab in Hospitalized COVID-19 Patients with Elevated Inflammatory Markers; COVIDSTORM—A Prospective, Randomized, Single-Centre, Open-Label Study. Clin. Microbiol. Infect. 2022, 28, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour-Aghdam, S.; Hazrati, A.; Abdolmohammadi-Vahid, S.; Tahmasebi, S.; Mohseni, J.; Valizadeh, H.; Nadiri, M.; Mikaeili, H.; Sadeghi, A.; Yousefi, M.; et al. Immunomodulatory Role of Nanocurcumin in COVID-19 Patients with Dropped Natural Killer Cells Frequency and Function. Eur. J. Pharmacol. 2022, 933, 175267. [Google Scholar] [CrossRef] [PubMed]
- Plaze, M.; Attali, D.; Petit, A.C.; Blatzer, M.; Simon-Loriere, E.; Vinckier, F.; Cachia, A.; Chrétien, F.; Gaillard, R. Repurposing Chlorpromazine to Treat COVID-19: The ReCoVery Study. Encephale 2020, 46, 169–172. [Google Scholar] [CrossRef]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Jannot, A.S.; Neuraz, A.; Blanco, C.; Lemogne, C.; Airagnes, G.; Paris, N.; Daniel, C.; et al. Observational Study of Chlorpromazine in Hospitalized Patients with COVID-19. Clin. Drug Investig. 2021, 41, 221–233. [Google Scholar] [CrossRef]
- Sharma, A. Randomized Trial Drug Controlled Compendious Transcriptome Analysis Supporting Broad and Phase Specific Therapeutic Potential of Multiple Candidates in COVID-19. Cytokine 2021, 148, 155719. [Google Scholar] [CrossRef]
- Umemura, Y.; Mitsuyama, Y.; Minami, K.; Nishida, T.; Watanabe, A.; Okada, N.; Yamakawa, K.; Nochioka, K.; Fujimi, S. Efficacy and Safety of Nintedanib for Pulmonary Fibrosis in Severe Pneumonia Induced by COVID-19: An Interventional Study. Int. J. Infect. Dis. 2021, 108, 454. [Google Scholar] [CrossRef]
- Hamed, D.M.; Belhoul, K.M.; Al Maazmi, N.A.; Ghayoor, F.; Moin, M.; Al Suwaidi, M.; Narainen, M.; Makki, M.; AbdulRahman, M. Intravenous Methylprednisolone with or without Tocilizumab in Patients with Severe COVID-19 Pneumonia Requiring Oxygen Support: A Prospective Comparison. J. Infect. Public Health 2021, 14, 985–989. [Google Scholar] [CrossRef]
- Lescure, F.X.; Honda, H.; Fowler, R.A.; Lazar, J.S.; Shi, G.; Wung, P.; Patel, N.; Hagino, O.; Bazzalo, I.J.; Casas, M.M.; et al. Sarilumab in Patients Admitted to Hospital with Severe or Critical COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Respir. Med. 2021, 9, 522–532. [Google Scholar] [CrossRef]
- Kulkarni, S.; Fisk, M.; Kostapanos, M.; Banham-Hall, E.; Bond, S.; Hernan-Sancho, E.; Norton, S.; Cheriyan, J.; Cope, A.; Galloway, J.; et al. Repurposed Immunomodulatory Drugs for COVID-19 in Pre-ICu Patients-MulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted with COVID-19—Repurposed Drugs (TACTIC-R): A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2020, 21, 626. [Google Scholar] [CrossRef] [PubMed]
- Lu, I.N.; Kulkarni, S.; Fisk, M.; Kostapanos, M.; Banham-Hall, E.; Kadyan, S.; Bond, S.; Norton, S.; Cope, A.; Galloway, J.; et al. MuLTi-Arm Therapeutic Study in Pre-ICu Patients Admitted with COVID-19-Experimental Drugs and Mechanisms (TACTIC-E): A Structured Summary of a Study Protocol for a Randomized Controlled Trial. Trials 2020, 21, 690. [Google Scholar] [CrossRef] [PubMed]
- Favalli, E.G.; Bugatti, S.; Klersy, C.; Biggioggero, M.; Rossi, S.; de Lucia, O.; Bobbio-Pallavicini, F.; Murgo, A.; Balduzzi, S.; Caporali, R.; et al. Impact of Corticosteroids and Immunosuppressive Therapies on Symptomatic SARS-CoV-2 Infection in a Large Cohort of Patients with Chronic Inflammatory Arthritis. Arthritis Res. Ther. 2020, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Roostaei Firozabad, A.; Meybodi, Z.A.; Mousavinasab, S.R.; Sahebnasagh, A.; Jelodar, M.G.; Karimzadeh, I.; Habtemariam, S.; Saghafi, F. Efficacy and Safety of Levamisole Treatment in Clinical Presentations of Non-Hospitalized Patients with COVID-19: A Double-Blind, Randomized, Controlled Trial. BMC Infect. Dis. 2021, 21, 297. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-Dose versus Standard-Dose Vitamin D Supplementation in Older Adults with COVID-19 (COVIT-TRIAL): A Multicenter, Open-Label, Randomized Controlled Superiority Trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Mohamed, H.S.; Abd-ellatief, R.B.; Gomaa, M.A.; Hammam, D.S. Advancing Combination Treatment with Glycyrrhizin and Boswellic Acids for Hospitalized Patients with Moderate COVID-19 Infection: A Randomized Clinical Trial. Inflammopharmacology 2022, 30, 477–486. [Google Scholar] [CrossRef]
- Hu, K.; Guan, W.-J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 2021, 85, 153242. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa for the Treatment of COVID-19: An Open-Label Randomized Controlled Clinical Trial. Complement. Ther. Med. 2021, 61, 102769. [Google Scholar] [CrossRef]
- Diáz, Y.; Ramos-Suzarte, M.; Martín, Y.; Calderón, N.A.; Santiago, W.; Viñet, O.; La, O.Y.; Oyarzábal, J.P.A.; Pérez, Y.; Lorenzo, G.; et al. Use of a Humanized Anti-CD6 Monoclonal Antibody (Itolizumab) in Elderly Patients with Moderate COVID-19. Gerontology 2020, 66, 553–561. [Google Scholar] [CrossRef]
- Kumar, S.; de Souza, R.; Nadkar, M.; Guleria, R.; Trikha, A.; Joshi, S.R.; Loganathan, S.; Vaidyanathan, S.; Marwah, A.; Athalye, S.N. A Two-Arm, Randomized, Controlled, Multi-Centric, Open-Label Phase-2 Study to Evaluate the Efficacy and Safety of Itolizumab in Moderate to Severe ARDS Patients Due to COVID-19. Expert Opin. Biol. Ther. 2021, 21, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, J.; Arai, H.; Iida, H.; Mukai, J.; Furukawa, K.; Ohtsu, S.; Nakade, S.; Hikima, T.; Haranaka, M.; Uemura, N. A Phase I Study of High Dose Camostat Mesylate in Healthy Adults Provides a Rationale to Repurpose the TMPRSS2 Inhibitor for the Treatment of COVID-19. Clin. Transl. Sci. 2021, 14, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef] [PubMed]
- Kragholm, K.; Torp-Pedersen, C.; Fosbol, E. Non-Steroidal Anti-Inflammatory Drug Use in COVID-19. Lancet Rheumatol. 2021, 3, e465–e466. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.H.; You, S.C.; Kim, J.; Yang, K. Non-Steroidal Anti-Inflammatory Agent Use May Not Be Associated with Mortality of Coronavirus Disease 19. Sci. Rep. 2021, 11, 5087. [Google Scholar] [CrossRef]
- Drake, T.M.; Fairfield, C.J.; Pius, R.; Knight, S.R.; Norman, L.; Girvan, M.; Hardwick, H.E.; Docherty, A.B.; Thwaites, R.S.; Openshaw, P.J.M.; et al. Non-Steroidal Anti-Inflammatory Drug Use and Outcomes of COVID-19 in the ISARIC Clinical Characterisation Protocol UK Cohort: A Matched, Prospective Cohort Study. Lancet Rheumatol. 2021, 3, e498–e506. [Google Scholar] [CrossRef]
- Chen, J.S.; Alfajaro, M.M.; Chow, R.D.; Wei, J.; Filler, R.B.; Eisenbarth, S.C.; Wilen, C.B. Nonsteroidal Anti-Inflammatory Drugs Dampen the Cytokine and Antibody Response to SARS-CoV-2 Infection. J. Virol. 2021, 95, e00014-21. [Google Scholar] [CrossRef]
- FDA Advises Patients on Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) for COVID-19|FDA. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-patients-use-non-steroidal-anti-inflammatory-drugs-nsaids-covid-19 (accessed on 4 February 2023).
- Chaplin, S. Summary of the New EULAR Rheumatoid Arthritis Guideline. Prescriber 2020, 31, 15–19. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. The Rheumatoid Arthritis Patient in the Clinic: Comparing More than 1300 Consecutive DMARD Courses. Rheumatology 2002, 41, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Lou, M.; Yuan, D.; Liao, S.; Tong, L.; Li, J. Potential Mechanisms of Cerebrovascular Diseases in COVID-19 Patients. J. Neurovirol. 2021, 27, 35–51. [Google Scholar] [CrossRef]
- Lo, M.W.; Kemper, C.; Woodruff, T.M. COVID-19: Complement, Coagulation, and Collateral Damage. J. Immunol. 2020, 205, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, K.M.; Wallace, Z.S. COVID-19 and Disease-Modifying Anti-Rheumatic Drugs. Curr. Rheumatol. Rep. 2021, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Liu, J.; Shi, D.; Chen, W.; Li, J.; Yan, R.; Bi, Y.; Hu, W.; Zhu, Z.; Yu, Y.; et al. Glucocorticoids Improve Severe or Critical COVID-19 by Activating Ace2 and Reducing Il-6 Levels. Int. J. Biol. Sci. 2020, 16, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, Y. Glucocorticoids Are Double-Edged Sword in the Treatment of COVID-19 and Cancers. Int. J. Biol. Sci. 2021, 17, 1530–1537. [Google Scholar] [CrossRef]
- Sieniawska, E.; Michel, P.; Mroczek, T.; Granica, S.; Skalicka-Woźniak, K. Nigella damascena L. Essential Oil and Its Main Constituents, Damascenine and β-Elemene Modulate Inflammatory Response of Human Neutrophils Ex Vivo. Food Chem. Toxicol. 2019, 125, 161–169. [Google Scholar] [CrossRef]
- Shi, J.; Weng, J.H.; Mitchison, T.J. Immunomodulatory Drug Discovery from Herbal Medicines: Insights from Organ-Specific Activity and Xenobiotic Defenses. eLife 2021, 10, e73673. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on Its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 28. [Google Scholar] [CrossRef]
- Nayak, S.S.; Sundararajan, V. Robust Anti-Inflammatory Activity of Genistein against Neutrophil Elastase: A Microsecond Molecular Dynamics Simulation Study. J. Biomol. Struct. Dyn. 2023; 1–17, online ahead of print. [Google Scholar] [CrossRef]
- Nadi, A.; Shiravi, A.A.; Mohammadi, Z.; Aslani, A.; Zeinalian, M. Thymus Vulgaris, a Natural Pharmacy against COVID-19: A Molecular Review. J. Herb. Med. 2023, 38, 100635. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and Immunomodulatory Activity of Curcumin: A Case for Prophylactic Therapy for COVID-19. Heliyon 2021, 7, E063520. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef] [PubMed]
- Cron, R.Q.; Caricchio, R.; Chatham, W.W. Calming the Cytokine Storm in COVID-19. Nat Med 2021, 27, 1674–1675. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Nintedanib: A Review in Fibrotic Interstitial Lung Diseases. Drugs 2021, 81, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Naidu, A.; Lulu, S.S. Mucosal and Systemic Immune Responses to Vibrio Cholerae Infection and Oral Cholera Vaccines (OCVs) in Humans: A Systematic Review. Expert Rev. Clin. Immunol. 2022, 18, 1307–1318. [Google Scholar] [CrossRef]
- Le Jan, S.; Muller, C.; Plee, J.; Durlach, A.; Bernard, P.; Antonicelli, F. IL-23/IL-17 Axis Activates IL-1β-Associated Inflammasome in Macrophages and Generates an Auto-Inflammatory Response in a Subgroup of Patients with Bullous Pemphigoid. Front. Immunol. 2019, 10, 1972. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.H.; Hua, K.F.; Lin, Y.C.; Chu, C.L.; Hsieh, C.Y.; Hsu, Y.J.; Ka, S.M.; Tsai, Y.L.; Liu, F.C.; Chen, A. IL-36 Signaling Facilitates Activation of the NLRP3 Inflammasome and IL-23/IL-17 Axis in Renal Inflammation and Fibrosis. J. Am. Soc. Nephrol. 2017, 28, 2022–2037. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.; Molla, M.M.A.; Saif-Ur-Rahman, K.M. An Update to Monoclonal Antibody as Therapeutic Option against COVID-19. Biosaf. Health 2021, 3, 87–91. [Google Scholar] [CrossRef]
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131–132. [Google Scholar] [CrossRef]
- Selvaraj, G.; Kaliamurthi, S.; Peslherbe, G.H.; Wei, D.-Q. Are the Allergic Reactions of COVID-19 Vaccines Caused by MRNA Constructs or Nanocarriers? Immunological Insights. Interdiscip. Sci. 2021, 13, 344–347. [Google Scholar] [CrossRef]
- Kaushik, A.C.; Mehmood, A.; Selvaraj, G.; Dai, X.; Pan, Y.; Wei, D.Q. CoronaPep: An Anti-Coronavirus Peptide Generation Tool. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021, 18, 1299–1304. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-Amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 2020, 28, 117–129. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-Based Therapeutics: An Overview and Prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef]
- Uludağ, H.; Parent, K.; Aliabadi, H.M.; Haddadi, A. Prospects for RNAi Therapy of COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 916. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.I.; Moazzam, M.; Cho, Y.; Kato, S.; Xu, A.; Way, J.J.; Lohan, S.; Tiwari, R.K. SiRNA Therapeutics for the Therapy of COVID-19 and Other Coronaviruses. Mol. Pharm. 2021, 18, 2105–2121. [Google Scholar] [CrossRef]
- Zogg, H.; Singh, R.; Ro, S. Current Advances in RNA Therapeutics for Human Diseases. Int. J. Mol. Sci. 2022, 23, 2736. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.; Ambite, I.; Wan, M.L.Y.; Tran, T.H.; Wullt, B.; Svanborg, C. Immunomodulation Therapy Offers New Molecular Strategies to Treat UTI. Nat. Rev. Urol. 2022, 19, 419–437. [Google Scholar] [CrossRef]
- Jagrosse, M.L.; Dean, D.A.; Rahman, A.; Nilsson, B.L. RNAi Therapeutic Strategies for Acute Respiratory Distress Syndrome. Transl. Res. 2019, 214, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.E. MicroRNA Immunomodulating Therapeutics. Blood 2020, 135, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Fei, Y.; Chaulagain, A.; Wang, T.; Chen, Y.; Liu, J.; Yi, M.; Wang, Y.; Huang, Y.; Lin, L.; Chen, S.; et al. MiR-146a down-Regulates Inflammatory Response by Targeting TLR3 and TRAF6 in Coxsackievirus B Infection. RNA 2020, 26, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Sagulkoo, P.; Chuntakaruk, H.; Rungrotmongkol, T.; Suratanee, A.; Plaimas, K. Multi-Level Biological Network Analysis and Drug Repurposing Based on Leukocyte Transcriptomics in Severe COVID-19: In Silico Systems Biology to Precision Medicine. J. Pers. Med. 2022, 12, 1030. [Google Scholar] [CrossRef]
- MotieGhader, H.; Safavi, E.; Rezapour, A.; Amoodizaj, F.F.; Iranifam, R.A. Drug Repurposing for Coronavirus (SARS-CoV-2) Based on Gene Co-Expression Network Analysis. Sci. Rep. 2021, 11, 21872. [Google Scholar] [CrossRef] [PubMed]
- Cartesian Therapeutics Initiates Clinical Trial of First RNA-Engineered Cell Therapy for Acute Respiratory Distress Syndrome and COVID-19. Available online: https://www.prnewswire.com/news-releases/cartesian-therapeutics-initiates-clinical-trial-of-first-rna-engineered-cell-therapy-for-acute-respiratory-distress-syndrome-and-covid-19-301121921.html (accessed on 1 February 2023).
- Jani, M.S.; Veetil, A.T.; Krishnan, Y. Precision Immunomodulation with Synthetic Nucleic Acid Technologies. Nat. Rev. Mater. 2019, 4, 451–458. [Google Scholar] [CrossRef]
- IEEE Xplore Full-Text PDF. Available online: https://ieeexplore.ieee.org/stamp/stamp.jsp?arnumber=9508641 (accessed on 7 February 2023).
- Selvaraj, G.; Kaliamurthi, S.; Peslherbe, G.H.; Wei, D.-Q. Identifying Potential Drug Targets and Candidate Drugs for COVID-19: Biological Networks and Structural Modeling Approaches. F1000Research 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Fava, V.M.; Bourgey, M.; Nawarathna, P.M.; Orlova, M.; Cassart, P.; Vinh, D.C.; Pellan Cheng, M.; Bourque, G.; Schurr, E.; Langlais, D. A Systems Biology Approach Identifies Candidate Drugs to Reduce Mortality in Severely Ill Patients with COVID-19. Sci. Adv. 2022, 8, eabm2510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-Based Drug Repurposing for Novel Coronavirus 2019-NCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Siminea, N.; Popescu, V.; Martin, J.A.S.; Florea, D.; Gavril, G.; Gheorghe, A.M.; Iţcuş, C.; Kanhaiya, K.; Pacioglu, O.; Popa, L.I.; et al. Network Analytics for Drug Repurposing in COVID-19. Brief. Bioinform. 2022, 23, bbab490. [Google Scholar] [CrossRef]
- Fiscon, G.; Conte, F.; Farina, L.; Paci, P. SAveRUNNER: A Network-Based Algorithm for Drug Repurposing and Its Application to COVID-19. PLoS Comput. Biol. 2021, 17, e1008686. [Google Scholar] [CrossRef]
- Aghdam, R.; Habibi, M.; Taheri, G. Using Informative Features in Machine Learning Based Method for COVID-19 Drug Repurposing. J. Cheminform. 2021, 13, 70. [Google Scholar] [CrossRef]
- Richman, S.; Lyman, C.; Nesterova, A.; Yuryev, A.; Morris, M.; Cao, H.; Cheadle, C.; Skuse, G.; Broderick, G. Old Drugs, New Tricks: Leveraging Known Compounds to Disrupt Coronavirus-Induced Cytokine Storm. npj Syst. Biol. Appl. 2022, 8, 38. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of Machine Learning in Drug Discovery and Development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Alves, V.M.; Lima, M.N.N.; Cassiano, G.C.; Muratov, E.N.; Costa, F.T.M.; Andrade, C.H. Deep Learning-Driven Research for Drug Discovery: Tackling Malaria. PLoS Comput. Biol. 2020, 16, e1007025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.K.; Meena, A.; Srivastava, A.; Chanda, D.; Khan, F.F.; Chattopadhyay, S. Development of QsAr Model for Immunomodulatory Activity of Natural Coumarinolignoids. Drug Des. Dev. Ther. 2010, 6, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Nagai, J.; Imamura, M.; Sakagami, H.; Uesawa, Y. QSAR Prediction Model to Search for Compounds with Selective Cytotoxicity Against Oral Cell Cancer. Medicines 2019, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Bae, H.; Jo, J.; Yoon, S. Comprehensive Ensemble in QSAR Prediction for Drug Discovery. BMC Bioinform. 2019, 20, 521. [Google Scholar] [CrossRef] [Green Version]
- Zanni, R.; Galvez-Llompart, M.; Galvez, J.; García-Domenech, R. QSAR Multi-Target in Drug Discovery: A Review. Curr. Comput. Aided Drug Des. 2014, 10, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gantla, M.R.; Tsigelny, I.F.; Kouznetsova, V.L. Repurposing of Drugs for Combined Treatment of COVID-19 Cytokine Storm Using Machine Learning. Med. Drug Discov. 2023, 17, 100148. [Google Scholar] [CrossRef]
- Han, X.; Shih, J.; Lin, Y.; Chai, Q.; Cramer, S.M. Development of QSAR Models for in Silico Screening of Antibody Solubility. mAbs 2022, 14, 2062807. [Google Scholar] [CrossRef]
- Stebbing, J.; Krishnan, V.; de Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Mechanism of Baricitinib Supports Artificial Intelligence-Predicted Testing in COVID-19 Patients. EMBO Mol. Med. 2020, 12, e12697. [Google Scholar] [CrossRef]
- Zeng, X.; Song, X.; Ma, T.; Pan, X.; Zhou, Y.; Hou, Y.; Zhang, Z.; Li, K.; Karypis, G.; Cheng, F. Repurpose Open Data to Discover Therapeutics for COVID-19 Using Deep Learning. J. Proteome Res. 2020, 19, 4624–4636. [Google Scholar] [CrossRef]
- Saha, S.; Chatterjee, P.; Halder, A.K.; Nasipuri, M.; Basu, S.; Plewczynski, D. ML-DTD: Machine Learning-Based Drug Target Discovery for the Potential Treatment of COVID-19. Vaccines 2022, 10, 1643. [Google Scholar] [CrossRef]
- Ozdemir, E.S.; Ranganathan, S.V.; Nussinov, R. How Has Artificial Intelligence Impacted COVID-19 Drug Repurposing and What Lessons Have We Learned? Expert Opin. Drug Discov. 2022, 17, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, K.; Wang, Y.; Chen, L.; Zhao, Z.; Savitz, S.; Jiang, X.; Tang, J.; Kim, Y. Drug Repurposing for COVID-19 Using Graph Neural Network and Harmonizing Multiple Evidence. Sci. Rep. 2021, 11, 23179. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Qiu, Y.; Zeng, J.; Xie, L.; Zhang, P. A Deep Learning Framework for High-Throughput Mechanism-Driven Phenotype Compound Screening and Its Application to COVID-19 Drug Repurposing. Nat. Mach. Intell. 2021, 3, 247–257. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, C.; Zhang, Z.; Liu, S.; Xu, M.; Yuan, X.; Zhang, Y.; Chen, J.; Cai, H.; Lu, J.; et al. TorchDrug: A Powerful and Flexible Machine Learning Platform for Drug Discovery. arXiv 2022, arXiv:2202.08320. [Google Scholar]
- Aittokallio, T. What Are the Current Challenges for Machine Learning in Drug Discovery and Repurposing? Expert Opin. Drug Discov. 2022, 17, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Mirsadeghi, S.; Larijani, B. Personalized Medicine: Pharmacogenomics and Drug Development. Acta Med. Iran. 2017, 55, 150–165. [Google Scholar] [PubMed]
- Morselli Gysi, D.; da Valle, I.; Zitnik, M.; Ameli, A.; Gan, X.; Varol, O.; Ghiassian, S.D.; Patten, J.J.; Davey, R.A.; Loscalzo, J.; et al. Network Medicine Framework for Identifying Drug-Repurposing Opportunities for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, 1205472. [Google Scholar] [CrossRef] [PubMed]
- Ozsert Yigit, G.; Baransel, C. A Novel Autoencoder-Based Feature Selection Method for Drug-Target Interaction Prediction with Human-Interpretable Feature Weights. Symmetry 2023, 15, 192. [Google Scholar] [CrossRef]
- Yue, R.; Dutta, A. Computational Systems Biology in Disease Modeling and Control, Review and Perspectives. npj Syst. Biol. Appl. 2022, 8, 37. [Google Scholar] [CrossRef]
- Shim, J.V.; Chun, B.; van Hasselt, J.G.C.; Birtwistle, M.R.; Saucerman, J.J.; Sobie, E.A. Mechanistic Systems Modeling to Improve Understanding and Prediction of Cardiotoxicity Caused by Targeted Cancer Therapeutics. Front. Physiol. 2017, 8, 651. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, L.; Xenarios, I. A Method for the Generation of Standardized Qualitative Dynamical Systems of Regulatory Networks. Theor. Biol. Med. Model. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Hajek, B.; Hanzawa, Y. From Graph Topology to ODE Models for Gene Regulatory Networks. PLoS ONE 2020, 15, e0235070. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.V.; Arastoo, R.; Bhat, A.; Subramanian, K.; Kothare, M.V.; Riedel, M.C. Gene Regulatory Network Modeling Using Literature Curated and High Throughput Data. Syst. Synth. Biol. 2012, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Khan, A.A.; Dinner, A.R. Gene Regulatory Networks in the Immune System. Trends Immunol. 2014, 35, 211–218. [Google Scholar] [CrossRef]
- Van Ijzendoorn, D.G.P.; Glass, K.; Quackenbush, J.; Kuijjer, M.L. PyPanda: A Python Package for Gene Regulatory Network Reconstruction. Bioinformatics 2016, 32, 3363–3365. [Google Scholar] [CrossRef] [Green Version]

| S. No. | Drug Used | Current Pharmacological Indication | Drug Class/Function | Study Design | No. of Patients | Outcome | Ref |
|---|---|---|---|---|---|---|---|
| 1 | Ciclosporin and Favipiravir | Ciclosporin is used for the treatment of RA and Favipiravir used against influenza | Immuno-suppressant | Prospective, Non-controlled | 20 | No favorable effect detected | [31] |
| 2 | IFN-beta-1a along with Hydroxy-chloroquine and lopinavir/ritonavir | Pulmonary infections | Immuno-modulating | Prospective, Non-controlled | 20 | Significant improvement observed in patients | [32] |
| 3 | Pentoxifylline | Chronic occlusive arterial disease (COAD) | Rheologic modifier | Prospective, Controlled | 38 (26T + 12C) | No statistically significant effect, but trend toward improvement in outcome parameters | [33] |
| 4 | Tocilizumab (subcutaneous) | Severely ill patients with COVID-19 | mAb against IL6RA | Multi-center, prospective, open-label, uncontrolled study | 126 | Reduction in the risk of death when treatment begins in early stages of respiratory failure | [34] |
| 5 | Tocilizumab (subcutaneous) | Severely ill patients with COVID-19 | ,, | Open-label, multi-center, randomized, controlled, phase 3 trial (COVINTOC) | 180 (90T, 90C) | No statistically significant reduction in disease progression, future studies advocated in severe patients | [35] |
| 6 | Tocilizumab | Severely ill patients with COVID-19 | ,, | Randomized prospective trial (COVIDSTORM) | 84 (56T, 28C) | The intervention arm showed statistically significant clinical recovery and shortened stay in the hospital | [36] |
| 7 | Nanocurcumin | Liver cancer, colon cancer, and cancer of the central nervous system | Natural compound—Immunomodulatory | Prospective, controlled | 120 (60T + 60C) | Statistically significant increase in the frequency and function of NK cells | [37] |
| 8 | Chlorpromazine | Schizophrenia | Typical antipsychotics | Pilot, multi-center, randomized, single blind, controlled, phase III therapeutic trial (standard arm vs. CPZ arm) | 55T (in a cohort of 14,340 in-patients) | No statistical association between chlorpromazine administration and reduced mortality | [38,39] |
| 9 | Etanercept | Severely active RA and psoriatic arthritis | Tumor necrosis factor alpha inhibitor | Compendium of randomized controlled trials, transcriptional studies | 5 | Based on descriptive study showed therapeutic potential | [40] |
| 10 | Tofacitinib | RA and psoriatic arthritis | Janus kinase inhibitors | ‘’ | 5 | ‘’ | [40] |
| 11 | Adalimumab | Crohn’s disease and ankylosing spondylitis | DMARDs, tumor necrosis factor alpha inhibitor | ‘’ | 3 | ‘’ | [4] |
| 12 | Canakinumab | Autoinflammatory syndromes and systemic juvenile idiopathic arthritis (SJIA) | anti-IL-1beta monoclonal antibody | ‘’ | 4 | Potential effectiveness during high oxygen supplementation phase | [4] |
| 13 | Nintedanib | Pulmonary fibrosis and systemic sclerosis-associated lung disease | Tyrosine kinase inhibitors | Prospective, controlled study | 30T, 30C | Statistically significant reduction in lung damage based on CT volumetry data (in patients with severe pneumonia induced by COVID-19) | [41] |
| 14 | Methylprednisolone | Neoplastic diseases and endocrine conditions | Glucocorticoids | Randomized, controlled study | 76 (23T, 27C) | Statistically significant reduction in mortality and ICU admission in COVID-19 patients with severe pneumonia | [42] |
| 15 | Sarilumab | Moderate to severely affected patients with RA | mAB against IL-6 receptor | Randomized, double-blind, placebo-controlled, multinational phase 3 trial | 416 (159T, 84C) | No statistically significant efficacy observed | [43] |
| 16 | RavulizumabBaricitinib | mAB against C5 and JAK, respectively | Randomized, parallel 3-arm (1:1:1 ratio), open-label, Phase IV—mulTi-Arm Therapeutic study in pre-ICU patients admitted with COVID-19 (TACTIC-R) | [44,45] | |||
| 17 | Targeted-synthetic/biological (ts/b) disease-modifying drugs (DMARDs) | Rheumatoid arthritis conditions | Anti-RA medication | Observational study on RA parients | 2050 | DMARDs administration do not put patients at increased risk | [46] |
| 18 | Corticosteroids | Used for treating chronic inflammations | Anti-inflammatory | ,, | ,, | Association with increased risk of COVID-19 | [46] |
| 19 | Umbilical cord mesenchymal stem cell (UC) | Type 1 diabetes mellitus (T1DM) and T2DM, also gynecologic conditions | Stem cell supplement | Double-blind, phase 1/2a, randomized, controlled trial on subjects with COVID-19 induced ARDs | 24 (12T, 12C) | Significant improvement in patient survival | [47] |
| 20 | Levamisole | Parasitic infections | Antiprotozoal agents, immunomodulatory effect | Prospective, double-blind, randomized controlled clinical trial | 50 (50T, 50C) | Statistically improved cough status and dyspnea | [48] |
| 21 | High-dose Vitamin D | Hypoparathyroidism | Nutritional supplement | Multi-center, randomized, controlled, open-label, superiority trial | 254 (127T, 127C) | Early administration of high-dose Vit. D improved overall mortality by day 14 | [49] |
| 22 | Glycyrrhizin + Boswellic acids | Hyperglycemia and premenstrual syndromes | Natural compounds with anti-inflammatory and immunomodulatory properties | Single-center, randomized, double-blind, placebo-controlled, clinical trial | 50, 25T, 25C | Statistically significant decrease in CRP and increase in lymphocyte level in intervention arm compared to control arm | [50] |
| 23 | Lianhuaqingwen | Influenza | Natural compounds with anti-inflammatory property | Prospective multi-center, open-label, randomized, controlled trial | 284 (142T, 142C) | Significantly improved rate of recovery of symptoms and radiological reports | [51] |
| 24 | Nigella sativa | COVID-19 | Natural compound with immunomodulatory properties | Open-label, randomized, controlled trial | 183 (91T, 92C) | Significantly faster recovery of symptoms for mild COVID-19 infection | [52] |
| 25 | Itolizumab | Psoriasis | mAb against anti-CD46 | Open, multi-center trial in elderly infected patients | 19 | Significant (up to 10 times) reduction of the risk of death | [53] |
| 26 | Itolizumab | Psoriasis | ,, | ARDS paients | 36 | Significantly greater number of people had improved SpO2 level | [54] |
| 27 | Camostat mesylate | Psoriasis | Protease inhibitors | An open-label, phase I study to assess the safety, tolerability, and PK | 14 | The drug was well tolerated and the range of dosage was determined | [55] |
| Sl. No. | Compound Name | Molecular Formula | Molecular Weight |
|---|---|---|---|
| 1 | 8-METHOXY-PSORALEN | C12H7ClO4 | 250.63 |
| 2 | 9-HYDROXYELLIPTICINE | C17H14N2O | 262.30 |
| 3 | ACEMANNAN | C66H100NO49 | 1691.5 |
| 4 | ANTHRAGALLOL | C14H8O5 | 256.21 |
| 5 | ASIMICIN | C37H66O7 | 622.9 |
| 6 | BAOHUOSIDE-1 | C27H30O11 | 530.5 |
| 7 | CHRYSAZIN | C14H8O4 | 240.21 |
| 8 | EMODIN | C15H10O5 | 270.24 |
| 9 | GALLIC-ACID | C7H6O5 | 170.12 |
| 10 | GRAPHINONE | C16H24O5 | 296.36 |
| 11 | HARMINE | C13H12N2O | 212.25 |
| 12 | LAPACHOL | C15H14O3 | 242.27 |
| 13 | MATRINE | C15H24N2O | 248.36 |
| 14 | OSTHOL | C15H16O3 | 244.28 |
| 15 | P-HYDROXY-BENZOIC-ACID | C7H6O3 | 138.12 |
| 16 | PHORBOL | C20H28O6 | 364.4 |
| 17 | POLYPHENOLS | C20H22O9 | 406.4 |
| 18 | QUINIDINE | C20H24N2O2 | 324.4 |
| 19 | SCOPARONE | C11H10O4 | 206.19 |
| 20 | SESAMIN | C20H18O6 | 354.4 |
| 21 | TANNIN | C42H32O26 | 952.7 |
| 22 | MARINOL | C21H30O2 | 314.5 |
| 23 | TETRANDRINE | C38H42N2O6 | 622.7 |
| 24 | TYLOPHORINE | C24H27NO4 | 393.5 |
| 25 | VANILLIC-ACID | C8H8O4 | 168.15 |
| 26 | VANILLIN | C8H8O3 | 152.15 |
| 27 | VERBASCOSIDE | C29H36O15 | 624.6 |
| 28 | VINBLASTINE | C46H58N4O9 | 811.0 |
| 29 | VINCRISTINE | C46H56N4O10 | 825.0 |
| 30 | WITHAFERIN-A | C28H38O6 | 470.6 |
| 31 | WITHANOLIDE-D | C28H38O6 | 470.6 |
| 32 | (+)−EPIPINORESINOL | C20H22O6 | 358.4 |
| 33 | 3-ACETYLACONITINE | C36H49NO12 | 687.8 |
| 34 | ACONITINE | C34H47NO11 | 645.7 |
| 35 | ADENOSINE | C10H13N5O4 | 267.24 |
| 36 | ALKANNIN | C16H16O5 | 288.29 |
| 37 | ALPHA-TOCOPHEROL | C29H50O2 | 430.7 |
| 38 | ARCTIGENIN | C21H24O6 | 372.4 |
| 39 | ARTEMISININ | C15H22O5 | 282.33 |
| 40 | ASCORBIC-ACID | C6H8O6 | 176.12 |
| 41 | BOLDINE | C19H21NO4 | 327.4 |
| 42 | CHIMAPHYLIN | C12H10O2 | 186.21 |
| 43 | EUCOMMIN-A | C27H34O12 | 550.6 |
| 44 | GAMMA-LINOLENIC-ACID | C18H30O2 | 278.4 |
| 45 | GINKGOLIDE | C20H24O10 | 424.4 |
| 46 | INOSINE | C10H12N4O5 | 268.23 |
| 47 | IRILONE | C16H10O6 | 298.25 |
| 48 | LIMONENE | C10H16 | 136.23 |
| 49 | LINOLEIC-ACID | C18H32O2 | 280.4 |
| 50 | OLEANOLIC-ACID | C30H48O3 | 456.7 |
| 51 | PAEONOL | C9H10O3 | 166.17 |
| 52 | ROSMARINIC-ACID | C18H16O8 | 360.3 |
| 53 | RUTIN | C27H30O16 | 610.5 |
| 54 | SAIKOSAPONIN | C42H68O13 | 781.0 |
| 55 | SAPONINS | C55H86O24 | 1131.3 |
| 56 | SWAINSONINE | C8H15NO3 | 173.21 |
| 57 | SYRINGIN | C17H24O9 | 372.4 |
| 58 | TOCOPHEROL | C29H50O2 | 430.7 |
| 59 | URSOLIC-ACID | C30H48O3 | 456.7 |
| 60 | WITHANOLIDE | C28H38O6 | 470.6 |
| Sl. No. | Inflammatory Mediator | Classification | Anti-Sense RNAs |
|---|---|---|---|
| 1 | IL-1β | Pro-inflammatory | NA |
| 2 | IL-6 | Pro-inflammatory | IL6-AS1 |
| 3 | IL-18 | Pro-inflammatory | NA |
| 4 | IL-1 | Pro-inflammatory | NA |
| 5 | TNFα | Pro-inflammatory | HOTAIR, KCNK15-AS1 |
| 6 | IL-10 | Anti-inflammatory | GNAS-AS1 |
| 7 | TGF-β | Anti-inflammatory | CDKN2B-AS1, KCNQ1OT1, AFAP1-AS1, AFAP1-AS1, NNT-AS1, WT1-AS, HAS2-AS1, HAS2-AS1, MBNL1-AS1, PRR34-AS1, TGFB2-AS1 |
| 8 | ELANE | Innate immunity | NA |
| 9 | CXCR2 | Chemokines | NA |
| 10 | MMP9 | Chemokines | SLC12A5-AS1, TP73-AS1, HAGLR |
| 11 | CXCL2 | Chemokines | NA |
| 12 | IFN-γ | Pro-inflammatory | IFNG-AS1 |
| 13 | PAF | Signaling pathway (intercellular mediator) | NA |
| 14 | GM-CSF | Adaptive immune system | NA |
| 15 | C5a | Pro-inflammatory | NA |
| 16 | ICAM-1 | Neutrophil adhesion | LIMASI, ICAM4-AS1 |
| 17 | VEGF | Endothelial cytokine | HOTAIR |
| 18 | IGF-I | Alveolar macrophage | HOXA-AS2 |
| 19 | ROS | Regulation of vascular tone | NA |
| 20 | NLRP3 | Extracellular histone | HOTAIR, CDKN2B-AS1, HAGLR, DLX6-AS1, ADAMTS9-AS2, RGMB-AS1, ZNF561-AS1 |
| 21 | IL-2 | Adaptive immunity | NA |
| 22 | IL-37(IL-1F7) | Anti-inflammatory | NA |
| 23 | TLR4 | Extracellular histones | PAPPA-AS1, MGAT3-AS1 |
| 24 | CXCL10 | Cytokines | NA |
| 25 | M-CSF | Pro-inflammatory | NA |
| 26 | NF-κΒ | Pro-inflammatory | SLC26A4-AS1 |
| 27 | MIP-1α | Chemokine | NA |
| 28 | MIP-1β | Chemokine | NA |
| 29 | IL-6R | Pro-inflammatory | IL6R-AS1 |
| 30 | CXCL9 | Monokine | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, S.S.; Naidu, A.; Sudhakaran, S.L.; Vino, S.; Selvaraj, G. Prospects of Novel and Repurposed Immunomodulatory Drugs against Acute Respiratory Distress Syndrome (ARDS) Associated with COVID-19 Disease. J. Pers. Med. 2023, 13, 664. https://doi.org/10.3390/jpm13040664
Nayak SS, Naidu A, Sudhakaran SL, Vino S, Selvaraj G. Prospects of Novel and Repurposed Immunomodulatory Drugs against Acute Respiratory Distress Syndrome (ARDS) Associated with COVID-19 Disease. Journal of Personalized Medicine. 2023; 13(4):664. https://doi.org/10.3390/jpm13040664
Chicago/Turabian StyleNayak, Smruti Sudha, Akshayata Naidu, Sajitha Lulu Sudhakaran, Sundararajan Vino, and Gurudeeban Selvaraj. 2023. "Prospects of Novel and Repurposed Immunomodulatory Drugs against Acute Respiratory Distress Syndrome (ARDS) Associated with COVID-19 Disease" Journal of Personalized Medicine 13, no. 4: 664. https://doi.org/10.3390/jpm13040664
APA StyleNayak, S. S., Naidu, A., Sudhakaran, S. L., Vino, S., & Selvaraj, G. (2023). Prospects of Novel and Repurposed Immunomodulatory Drugs against Acute Respiratory Distress Syndrome (ARDS) Associated with COVID-19 Disease. Journal of Personalized Medicine, 13(4), 664. https://doi.org/10.3390/jpm13040664








