Concurrent Diagnosis of Adenomyosis and Congenital Uterine Anomalies: A Review
Abstract
:1. Introduction
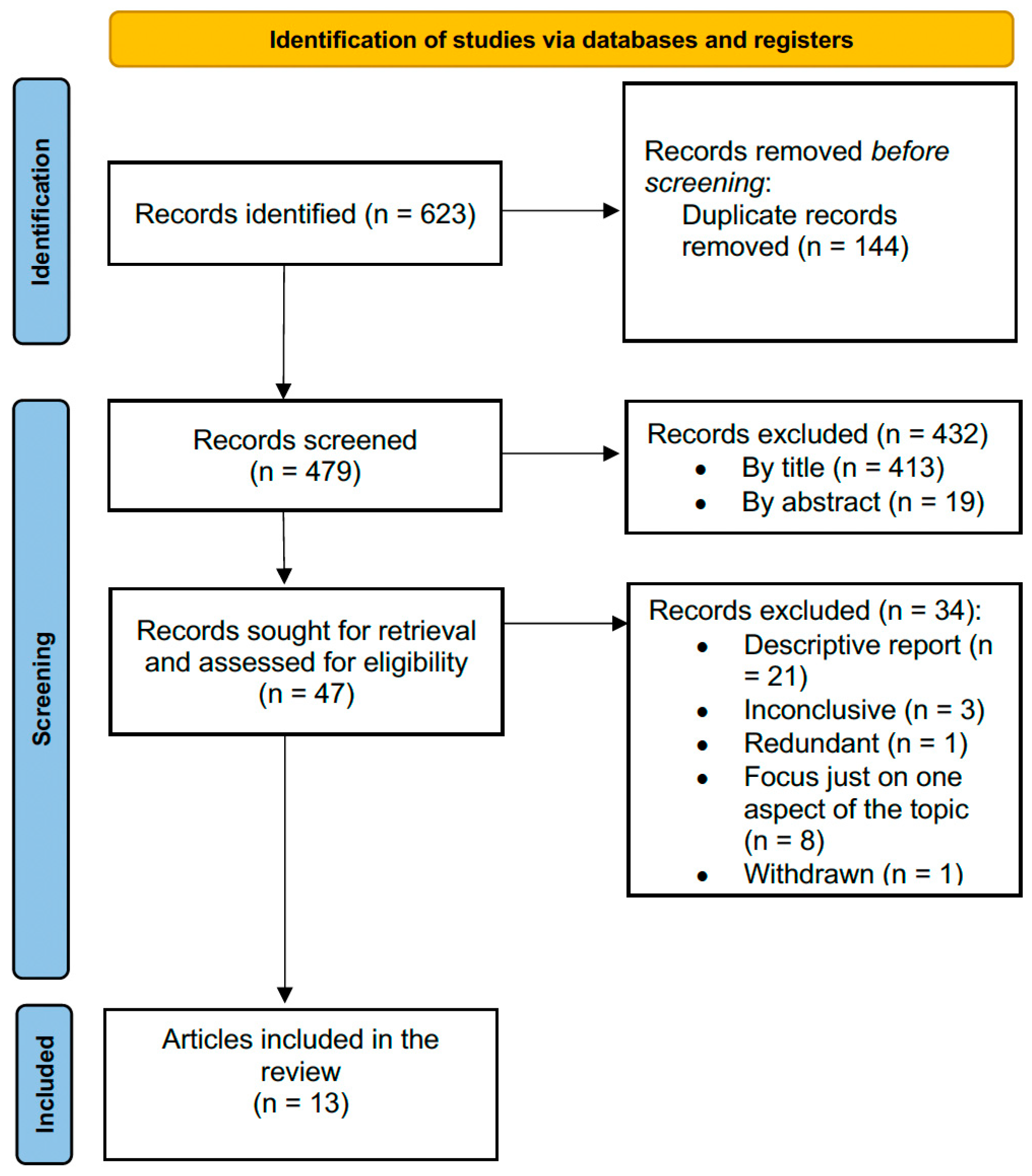
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garzon, S.; Laganà, A.S.; Di Spiezio Sardo, A.; Alonso Pacheco, L.; Haimovich, S.; Carugno, J.; Vitale, S.G.; Casarin, J.; Raffaelli, R.; Andrisani, A.; et al. Hysteroscopic Metroplasty for T-Shaped Uterus: A Systematic Review and Meta-Analysis of Reproductive Outcomes. Obstet. Gynecol. Surv. 2020, 75, 431–444. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Gordts, S.; Di Spiezio Sardo, A.; Brucker, S.; De Angelis, C.; Gergolet, M.; Li, T.C.; Tanos, V.; Brölmann, H.; Gianaroli, L.; et al. The ESHRE/ESGE Consensus on the Classification of Female Genital Tract Congenital Anomalies. Hum. Reprod. 2013, 28, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Muñoz, E.; Vitale, S.G.; Alvarado-Rosales, D.; Iyune-Cojab, E.; Vitagliano, A.; Lohmeyer, F.M.; Guevara-Gómez, Y.P.; Villarreal-Barranca, A.; Romo-Yañez, J.; Montoya-Estrada, A.; et al. Müllerian Anomalies Prevalence Diagnosed by Hysteroscopy and Laparoscopy in Mexican Infertile Women: Results from a Cohort Study. Diagnostics 2019, 9, 149. [Google Scholar] [CrossRef]
- Letterie, G.S. Management of Congenital Uterine Abnormalities. Reprod. Biomed. Online 2011, 23, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Freytag, D.; Mettler, L.; Maass, N.; Günther, V.; Alkatout, I. Uterine Anomalies and Endometriosis. Minerva Med. 2020, 111, 33–49. [Google Scholar] [CrossRef]
- Angioni, S.; Cela, V.; Sedda, F.; Stochino Loi, E.; Cofelice, V.; Pontis, A.; Melis, G.B. Focusing on Surgery Results in Infertile Patients with Deep Endometriosis. Gynecol. Endocrinol. 2015, 31, 595–598. [Google Scholar] [CrossRef]
- LaMonica, R.; Pinto, J.; Luciano, D.; Lyapis, A.; Luciano, A. Incidence of Septate Uterus in Reproductive-Aged Women With and Without Endometriosis. J. Minim. Invasive Gynecol. 2016, 23, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Vannuccini, S.; Petraglia, F. Recent Advances in Understanding and Managing Adenomyosis. F1000Res 2019, 8, 283. [Google Scholar] [CrossRef]
- Garcia, L.; Isaacson, K. Adenomyosis: Review of the Literature. J. Minim. Invasive Gynecol. 2011, 18, 428–437. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Ban Frangež, H.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro Omnibus, Omnes pro Uno: A Novel, Evidence-Based, Unifying Theory for the Pathogenesis of Endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef]
- García-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.-M. Pathogenesis of Uterine Adenomyosis: Invagination or Metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Frontino, G.; Bianchi, S.; Ciappina, N.; Restelli, E.; Borruto, F.; Fedele, L. The Unicornuate Uterus with an Occult Adenomyotic Rudimentary Horn. J. Minim. Invasive Gynecol. 2009, 16, 622–625. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.D.; Cho, J.H.; Park, S.I.; Lee, M.S.; Lee, M.S. Uterine Artery Embolization for Symptomatic Adenomyosis in a Patient with Uterus Didelphys. J. Vasc. Interv. Radiol. 2011, 22, 1489–1491. [Google Scholar] [CrossRef]
- Takeuchi, K.; Tateiwa, H.; Hamana, S.; Yoshida, S.; Kitazawa, S.; Maruo, T. Invasive Adenocarcinoma Arising from Adenomyosis in a Septate Uterus. Acta Obstet. Gynecol. Scand. 2006, 85, 1146–1147. [Google Scholar] [CrossRef]
- Su, H.-Y.; Chen, C.-H.; Gao, H.-W.; Liu, J.-Y. A Bicornuate Uterus with a Unilateral Cornual Adenomyosis. Obstet. Gynecol. 2005, 105, 1191–1193. [Google Scholar] [CrossRef]
- Narayanan, R.; Mariappan, S.; Paulraj, S.; Shankar, B. Imaging of Leiomyomas Arising from Müllerian Remnants in a Case of Mayer-Rokitansky-Küster-Hauser Syndrome. BMJ Case Rep. 2015, 2015, bcr2015210737. [Google Scholar] [CrossRef]
- Ferrero, S.; Bentivoglio, G. Adenomyosis in a Patient with Mosaic Turner’s Syndrome. Arch. Gynecol. Obstet. 2005, 271, 249–250. [Google Scholar] [CrossRef]
- Yan, C.M.; Mok, K.M. Uterine Fibroids and Adenomyosis in a Woman with Rokitansky-Kuster-Hauser Syndrome. J. Obstet. Gynaecol. 2002, 22, 561–562. [Google Scholar] [CrossRef]
- Enatsu, A.; Harada, T.; Yoshida, S.; Iwabe, T.; Terakawa, N. Adenomyosis in a Patient with the Rokitansky-Kuster-Hauser Syndrome. Fertil. Steril. 2000, 73, 862–863. [Google Scholar] [CrossRef]
- Feghali, E.J.; Daccache, A.; Feghali, E.; Sleiman, Z. Rudimentary Horn Adenomyosis in A 20-Year-Old Patient With Unicornuate Uterus: A Case Report. Int. J. Clin. Res. 2022, 3, 31–36. [Google Scholar] [CrossRef]
- Morelli, M.; Venturella, R.; Mocciaro, R.; Lico, D.; Zullo, F. An Unusual Extremely Distant Noncommunicating Uterine Horn with Myoma and Adenomyosis Treated with Laparoscopic Hemihysterectomy. Case Rep. Obstet. Gynecol. 2013, 2013, 160291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, S.; Yang, L.; Songhong, Y. MRI Image Features and Differential Diagnoses of Herlyn-Werner-Wunderlich Syndrome. Gynecol. Endocrinol. 2020, 36, 484–488. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef] [PubMed]
- Hall-Craggs, M.A.; Williams, C.E.; Pattison, S.H.; Kirkham, A.P.; Creighton, S.M. Mayer-Rokitansky-Kuster-Hauser Syndrome: Diagnosis with MR Imaging. Radiology 2013, 269, 787–792. [Google Scholar] [CrossRef]
- Miao, Y.; Wen, J.; Huang, L.; Wu, J.; Zhao, Z. Diagnosis and Management of Ovarian Tumor in Mayer-Rokitansky-Küster-Hauser (MRKH) Syndrome. Biomed. Res. Int. 2018, 2018, 2369430. [Google Scholar] [CrossRef]
- Zanatta, A.; Rocha, A.M.; Carvalho, F.M.; Pereira, R.M.A.; Taylor, H.S.; Motta, E.L.A.; Baracat, E.C.; Serafini, P.C. The Role of the Hoxa10/HOXA10 Gene in the Etiology of Endometriosis and Its Related Infertility: A Review. J. Assist. Reprod. Genet. 2010, 27, 701–710. [Google Scholar] [CrossRef]
- Maniglio, P.; Ricciardi, E.; Laganà, A.S.; Triolo, O.; Caserta, D. Epigenetic Modifications of Primordial Reproductive Tract: A Common Etiologic Pathway for Mayer-Rokitansky-Kuster-Hauser Syndrome and Endometriosis? Med. Hypotheses 2016, 90, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Zajec, V.; Mikuš, M.; Vitale, S.G.; D’alterio, M.N.; Gregov, M.; Šarić, M.J.; Carugno, J.; Angioni, S.; Ćorić, M. Current Status and Challenges of Drug Development for Hormonal Treatment of Endometriosis: A Systematic Review of Randomized Control Trials. Gynecol. Endocrinol. 2022, 38, 713–720. [Google Scholar] [CrossRef]
- Mikuš, M.; Vitale, S.G.; Ćorić, M.; Zajec, V.; Ciebiera, M.; Carugno, J.; D’alterio, M.N.; Herman, M.; Puževski, T.; Angioni, S. State of the Art, New Treatment Strategies, and Emerging Drugs for Non-Hormonal Treatment of Endometriosis: A Systematic Review of Randomized Control Trials. Gynecol. Endocrinol. 2022, 38, 911–917. [Google Scholar] [CrossRef]
- Kitawaki, J.; Obayashi, H.; Ishihara, H.; Koshiba, H.; Kusuki, I.; Kado, N.; Tsukamoto, K.; Hasegawa, G.; Nakamura, N.; Honjo, H. Oestrogen Receptor-Alpha Gene Polymorphism Is Associated with Endometriosis, Adenomyosis and Leiomyomata. Hum. Reprod. 2001, 16, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Salastekar, N.; Coelho, M.; Majmudar, A.; Gupta, S. Herlyn-Werner-Wunderlich Syndrome: A Rare Cause of Abdominal Pain and Dyspareunia. Radiol. Case Rep. 2019, 14, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Type | Congenital Abnormality | Country | Patient (n) | Age (Mean) |
|---|---|---|---|---|---|---|
| Frontino et al. [16] | 2009 | Case report | Unicornuate uterus | Italy | 2 | 13 |
| Kim et al. [17] | 2011 | Case report | Uterus didelphys | Korea | 1 | 38 |
| Takeuchi et al. [18] | 2003 | Case report | Septate uterus | Japan | 1 | 56 |
| Zhang et al. [26] | 2019 | Retrospective analysis | HWWS syndrome | China | 1 | 17.67 |
| Du et al. [27] | 2015 | Theoretical article | - | US | - | - |
| Hall-Craggs et al. [28] | 2013 | Retrospective review | Rudimentary uteri in MRKH syndrome | UK | 2 | 19 |
| Su et al. [19] | 2005 | Case report | Unicornuate uterus | Taiwan | 1 | 41 |
| Narayanan et al. [20] | 2015 | Case report | Müllerian remnants in MRKH syndrome | India | 1 | 43 |
| Ferrero and Bentivoglio [21] | 2004 | Case report | Mosaic Turner syndrome | Italy | 1 | 31 |
| Yan and Mok [22] | 2002 | Case report | Rudimentary uteri, cervical agenesis, and vaginal hypoplasia in MRKH syndrome | Hong Kong | 1 | 52 |
| Enatsu et al. [23] | 2000 | Case report | Rudimentary uteri in MRKH syndrome | Japan | 1 | 27 |
| Morelli et al. [25] | 2013 | Case report | Noncommunicating uterine horn | Italy | 1 | 41 |
| Feghali et al. [24] | 2022 | Case report | Unicornuate uterus | Lebanon | 1 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feghali, E.; Etrusco, A.; Haydamous, J.; Ayed, A.; Laganà, A.S.; Chiantera, V.; Vitale, S.G.; Angioni, S.; Stabile, G.; Sleiman, Z. Concurrent Diagnosis of Adenomyosis and Congenital Uterine Anomalies: A Review. J. Pers. Med. 2023, 13, 716. https://doi.org/10.3390/jpm13050716
Feghali E, Etrusco A, Haydamous J, Ayed A, Laganà AS, Chiantera V, Vitale SG, Angioni S, Stabile G, Sleiman Z. Concurrent Diagnosis of Adenomyosis and Congenital Uterine Anomalies: A Review. Journal of Personalized Medicine. 2023; 13(5):716. https://doi.org/10.3390/jpm13050716
Chicago/Turabian StyleFeghali, Edwin, Andrea Etrusco, Joe Haydamous, Amal Ayed, Antonio Simone Laganà, Vito Chiantera, Salvatore Giovanni Vitale, Stefano Angioni, Guglielmo Stabile, and Zaki Sleiman. 2023. "Concurrent Diagnosis of Adenomyosis and Congenital Uterine Anomalies: A Review" Journal of Personalized Medicine 13, no. 5: 716. https://doi.org/10.3390/jpm13050716
APA StyleFeghali, E., Etrusco, A., Haydamous, J., Ayed, A., Laganà, A. S., Chiantera, V., Vitale, S. G., Angioni, S., Stabile, G., & Sleiman, Z. (2023). Concurrent Diagnosis of Adenomyosis and Congenital Uterine Anomalies: A Review. Journal of Personalized Medicine, 13(5), 716. https://doi.org/10.3390/jpm13050716











