Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinicopathologic Data of EC Patients
2.2. Immunohistochemistry
2.3. DNA Extraction and Next-Generation Sequencing
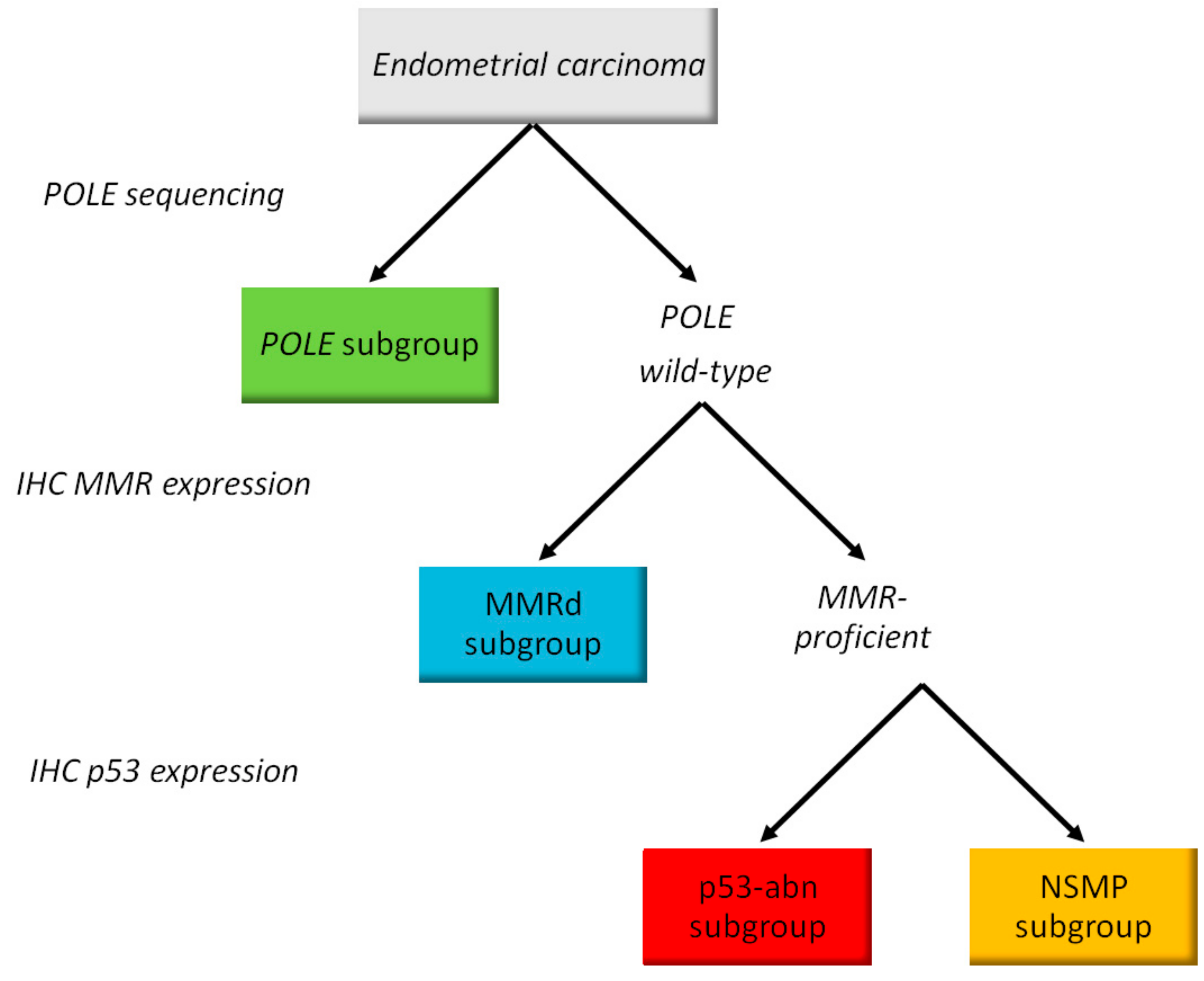
2.4. Molecular Classification
2.5. Statistics
3. Results
3.1. Characteristics of the Endometrial Carcinoma Cohort
3.2. Characteristics of Molecular Subgroups
3.3. Histopathologic Parameters in Molecular Subgroups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Odicino, F.; Maisonneuve, P.; Quinn, M.A.; Beller, U.; Benedet, J.L.; Heintz, A.P.; Ngan, H.Y.; Pecorelli, S. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006, 95 (Suppl. 1), S105–S143. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Briet, J.M.; Hollema, H.; Reesink, N.; Aalders, J.G.; Mourits, M.J.; ten Hoor, K.A.; Pras, E.; Boezen, H.M.; van der Zee, A.G.; Nijman, H.W. Lymphvascular space involvement: An independent prognostic factor in endometrial cancer. Gynecol. Oncol. 2005, 96, 799–804. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef]
- Keys, H.M.; Roberts, J.A.; Brunetto, V.L.; Zaino, R.J.; Spirtos, N.M.; Bloss, J.D.; Pearlman, A.; Maiman, M.A.; Bell, J.G.; Gynecologic Oncology, G. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2004, 92, 744–751. [Google Scholar] [CrossRef]
- Morrow, C.P.; Bundy, B.N.; Kurman, R.J.; Creasman, W.T.; Heller, P.; Homesley, H.D.; Graham, J.E. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecol. Oncol. 1991, 40, 55–65. [Google Scholar] [CrossRef]
- Stalberg, K.; Bjurberg, M.; Borgfeldt, C.; Carlson, J.; Dahm-Kahler, P.; Floter-Radestad, A.; Hellman, K.; Hjerpe, E.; Holmberg, E.; Kjolhede, P.; et al. Lymphovascular space invasion as a predictive factor for lymph node metastases and survival in endometrioid endometrial cancer—A Swedish Gynecologic Cancer Group (SweGCG) study. Acta Oncol. 2019, 58, 1628–1633. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Osse, E.M.; Jurgenliemk-Schulz, I.J.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Nijman, H.W.; Putter, H.; Bosse, T.; et al. Improved Risk Assessment by Integrating Molecular and Clinicopathological Factors in Early-stage Endometrial Cancer-Combined Analysis of the PORTEC Cohorts. Clin. Cancer Res. 2016, 22, 4215–4224. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grillini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S.; et al. ARID1A and CTNNB1/beta-Catenin Molecular Status Affects the Clinicopathologic Features and Prognosis of Endometrial Carcinoma: Implications for an Improved Surrogate Molecular Classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef]
- WHO. WHO Classification of Tumours. Female Genital Tumours, 5th ed.; IARC: Lyon, France, 2020; Volume 4. [Google Scholar]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol 2019, 38 (Suppl. 1), S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer--A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Murray, S.K.; Young, R.H.; Scully, R.E. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: A study of their frequency, associated diagnostic problems, and prognostic significance. Int. J. Gynecol. Pathol. 2003, 22, 324–333. [Google Scholar] [CrossRef]
- Euscher, E.; Fox, P.; Bassett, R.; Al-Ghawi, H.; Ali-Fehmi, R.; Barbuto, D.; Djordjevic, B.; Frauenhoffer, E.; Kim, I.; Hong, S.R.; et al. The pattern of myometrial invasion as a predictor of lymph node metastasis or extrauterine disease in low-grade endometrial carcinoma. Am. J. Surg. Pathol. 2013, 37, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Piskorz, A.M.; Bosse, T.; Jimenez-Linan, M.; Rous, B.; Brenton, J.D.; Gilks, C.B.; Kobel, M. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J. Pathol. 2020, 250, 336–345. [Google Scholar] [CrossRef]
- Garg, K.; Broaddus, R.R.; Soslow, R.A.; Urbauer, D.L.; Levine, D.A.; Djordjevic, B. Pathologic scoring of PTEN immunohistochemistry in endometrial carcinoma is highly reproducible. Int. J. Gynecol. Pathol. 2012, 31, 48–56. [Google Scholar] [CrossRef]
- Dondi, G.; Coluccelli, S.; De Leo, A.; Ferrari, S.; Gruppioni, E.; Bovicelli, A.; Godino, L.; Coada, C.A.; Morganti, A.G.; Giordano, A.; et al. An Analysis of Clinical, Surgical, Pathological and Molecular Characteristics of Endometrial Cancer According to Mismatch Repair Status. A Multidisciplinary Approach. Int. J. Mol. Sci. 2020, 21, 7188. [Google Scholar] [CrossRef]
- De Biase, D.; Acquaviva, G.; Visani, M.; Sanza, V.; Argento, C.M.; De Leo, A.; Maloberti, T.; Pession, A.; Tallini, G. Molecular Diagnostic of Solid Tumor Using a Next Generation Sequencing Custom-Designed Multi-Gene Panel. Diagnostics 2020, 10, 250. [Google Scholar] [CrossRef]
- De Leo, A.; Ravegnini, G.; Musiani, F.; Maloberti, T.; Visani, M.; Sanza, V.; Angelini, S.; Perrone, A.M.; De Iaco, P.; Corradini, A.G.; et al. Relevance of ARID1A Mutations in Endometrial Carcinomas. Diagnostics 2022, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef]
- de Biase, D.; Maloberti, T.; Corradini, A.G.; Rosini, F.; Grillini, M.; Ruscelli, M.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Sanza, V.; et al. Integrated clinicopathologic and molecular analysis of endometrial carcinoma: Prog-nostic impact of the new ESGO-ESTRO-ESP endometrial cancer risk classification and proposal of histopathologic algorithm for its implementation in clinical practice. Front. Med. 2023, 10. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; Stelloo, E.; Dreef, E.; Nijman, H.W.; Jurgenliemk-Schulz, I.M.; Jobsen, J.J.; Creutzberg, C.L.; Smit, V.T. L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: Pooled PORTEC trial results. Eur. J. Cancer 2014, 50, 2602–2610. [Google Scholar] [CrossRef] [PubMed]
- Depreeuw, J.; Stelloo, E.; Osse, E.M.; Creutzberg, C.L.; Nout, R.A.; Moisse, M.; Garcia-Dios, D.A.; Dewaele, M.; Willekens, K.; Marine, J.C.; et al. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin. Cancer Res. 2017, 23, 7232–7241. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Nguyen, B.; Vanderbilt, C.M.; Ladanyi, M.; Abu-Rustum, N.R.; Aghajanian, C.; Ellenson, L.H.; Weigelt, B.; Soslow, R.A. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod. Pathol. 2022, 35, 1269–1278. [Google Scholar] [CrossRef]
- Ravegnini, G.; De Leo, A.; Coada, C.; Gorini, F.; de Biase, D.; Ceccarelli, C.; Dondi, G.; Tesei, M.; De Crescenzo, E.; Santini, D.; et al. Identification of miR-499a-5p as a Potential Novel Biomarker for Risk Stratification in Endometrial Cancer. Front. Oncol. 2021, 11, 757678. [Google Scholar] [CrossRef]
- Vermij, L.; Jobsen, J.J.; Leon-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef]
- de Boer, S.M.; Wortman, B.G.; Bosse, T.; Powell, M.E.; Singh, N.; Hollema, H.; Wilson, G.; Chowdhury, M.N.; Mileshkin, L.; Pyman, J.; et al. Clinical consequences of upfront pathology review in the randomised PORTEC-3 trial for high-risk endometrial cancer. Ann. Oncol. 2018, 29, 424–430. [Google Scholar] [CrossRef]
- Ali, A.; Black, D.; Soslow, R.A. Difficulties in assessing the depth of myometrial invasion in endometrial carcinoma. Int. J. Gynecol. Pathol. 2007, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Manion, E.; Cohen, M.B.; Weydert, J. Mandatory second opinion in surgical pathology referral material: Clinical consequences of major disagreements. Am. J. Surg. Pathol. 2008, 32, 732–737. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Hirschowitz, L.; Wilson, G.E.; Oliva, E.; Soslow, R.A.; Zaino, R.J. Significant variation in the assessment of cervical involvement in endometrial carcinoma: An interobserver variation study. Am. J. Surg. Pathol. 2011, 35, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Hirschowitz, L.; Zaino, R.; Alvarado-Cabrero, I.; Duggan, M.A.; Ali-Fehmi, R.; Euscher, E.; Hecht, J.L.; Horn, L.C.; Ioffe, O.; et al. Pathologic Prognostic Factors in Endometrial Carcinoma (Other Than Tumor Type and Grade). Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S93–S113. [Google Scholar] [CrossRef]
- Peters, E.E.M.; Leon-Castillo, A.; Hogdall, E.; Boennelycke, M.; Smit, V.; Hogdall, C.; Creutzberg, C.L.; Bosse, T.; Nout, R.A.; Ortoft, G. Substantial Lymphovascular Space Invasion Is an Adverse Prognostic Factor in High-Risk Endometrial Cancer. Int. J. Gynecol. Pathol. 2022, 41, 227–234. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Neola, D.; Maletta, M.; Santoro, A.; Insabato, L.; Casadio, P.; Fanfani, F.; Zannoni, G.F.; et al. Lymphovascular space invasion in endometrial carcinoma: A prognostic factor independent from molecular signature. Gynecol. Oncol. 2022, 165, 192–197. [Google Scholar] [CrossRef]
- Barnes, E.A.; Martell, K.; Parra-Herran, C.; Taggar, A.S.; Donovan, E.; Leung, E. Substantial lymphovascular space invasion predicts worse outcomes in early-stage endometrioid endometrial cancer. Brachytherapy 2021, 20, 527–535. [Google Scholar] [CrossRef]
- Son, J.; Chambers, L.M.; Carr, C.; Michener, C.M.; Yao, M.; Beavis, A.; Yen, T.T.; Stone, R.L.; Wethington, S.L.; Fader, A.N.; et al. Adjuvant treatment improves overall survival in women with high-intermediate risk early-stage endometrial cancer with lymphovascular space invasion. Int. J. Gynecol. Cancer 2020, 30, 1738–1747. [Google Scholar] [CrossRef]
- Peters, E.E.M.; Bartosch, C.; McCluggage, W.G.; Genestie, C.; Lax, S.F.; Nout, R.; Oosting, J.; Singh, N.; Smit, H.; Smit, V.; et al. Reproducibility of lymphovascular space invasion (LVSI) assessment in endometrial cancer. Histopathology 2019, 75, 128–136. [Google Scholar] [CrossRef]





| Characteristics of EC Cases | n = 219 (%) | |
|---|---|---|
| Age, years | 62.5 ± 10.4 | |
| (34–86) | ||
| Body mass index, kg/m2 | 28.11 ± 7.2 | |
| (18.2–55.3) | ||
| Histotype | ||
| Endometrioid | 167 (76.3) | |
| Dedifferentiated/ Undifferentiated | 25 (11.4) | |
| Serous | 20 (9.1) | |
| Clear cell | 3 (1.4) | |
| Carcinosarcoma | 4 (1.8) | |
| Grade | ||
| Low | 127 (58) | |
| High | 92 (42) | |
| Depth of invasion | ||
| <50% | 154 (70.3) | |
| ≥50% | 65 (29.7) | |
| Lymphovascular space invasion (LVSI) | ||
| Absent | 72 (32.9) | |
| Focal | 68 (31.1) | |
| Substantial | 79 (36.1) | |
| Extensive necrosis * | ||
| Absent | 105 (47.9) | |
| Present | 113 (51.6) | |
| Unknown/Not tested | 1 (0.5) | |
| MELF * | ||
| Absent | 147 (67.1) | |
| Present | 71 (32.4) | |
| Unknown/Not tested | 1 (0.5) | |
| Tumor budding * | ||
| Absent | 128 (58.4) | |
| Present | 91 (41.6) | |
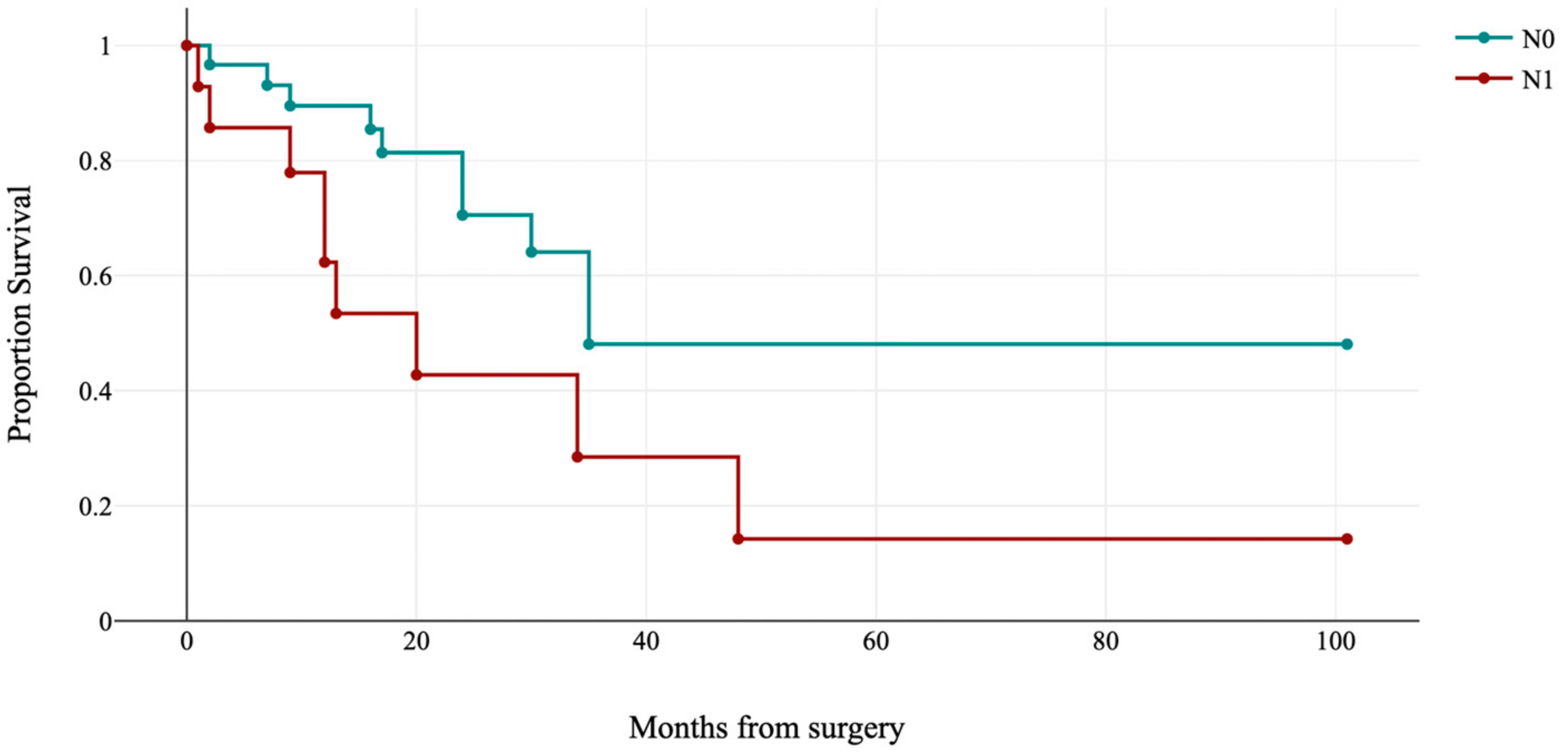
| Lymph node status | ||
| Negative | 180 (82.2) | |
| Positive | 33 (15.1) | |
| Unknown/Not tested | 6 (2.7) | |
| FIGO stage | ||
| IA | 124 (56.6) | |
| IB/II | 44 (20.1) | |
| III | 42 (19.2) | |
| IV | 9 (4.1) | |
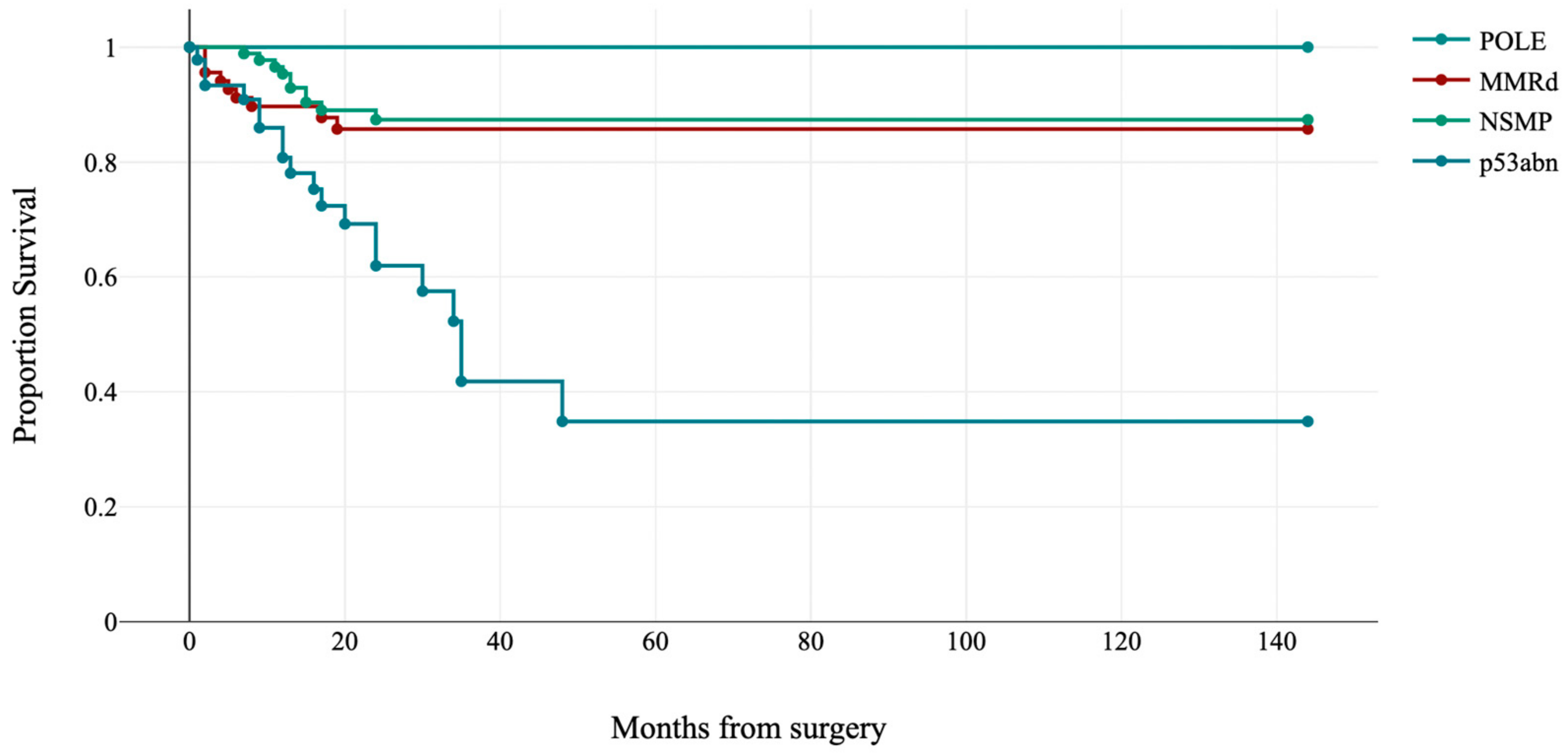
| Molecular subgroups | ||
| POLE | 17 (7.8) | |
| MMRd | 68 (31.0) | |
| NSMP | 88 (40.2) | |
| p53abn | 46 (21.0) | |
| ESGO/ESTRO/ESP risk group | ||
| Low | 91 (41.6) | |
| Intermediate | 19 (8.7) | |
| High–intermediate | 23 (10.5) | |
| High | 77 (35.2) | |
| Advanced/metastatic | 9 (4.1) | |
| Surgical approach | ||
| Minimally-invasive | 168 (76.7) | |
| Laparotomy | 51 (23.3) | |
| Disease recurrence | ||
| Absent | 181 (82.6) | |
| Present | 38 (17.4) | |
| Characteristics | POLE | MMRd | p53abn | NSMP | p-Value |
|---|---|---|---|---|---|
| (n = 17; 7.8%) | (n = 68; 31.0%) | (n = 46; 21.0%) | (n = 88; 40.2%) | ||
| Age, years | 57.8 ± 11.8 | 63.0 ± 9.4 | 66.8 ± 10.1 | 60.7 ± 10.3 | 0.002 |
| Body mass index, kg/m2 | 26.4 ± 8.4 | 28.0 ± 7.1 | 26.0 ± 4.8 | 29.6 ± 7.9 | 0.036 |
| Histotype | <0.001 | ||||
| Endometrioid | 14 (82.4) | 54 (79.4) | 17 (37.0) | 82 (93.2) | |
| Dedifferentiated/Undifferentiated | 3 (17.6) | 14 (20.6) | 2 (4.3) | 6 (6.8) | |
| Serous | 0 (0.0) | 0 (0.0) | 20 (43.5) | 0 (0.0) | |
| Clear cell | 0 (0.0) | 0 (0.0) | 3 (6.5) | 0 (0.0) | |
| Carcinosarcoma | 0 (0.0) | 0 (0.0) | 4 (8.7) | 0 (0.0) | |
| Grade | <0.001 | ||||
| Low | 8 (47.1) | 43 (63.2) | 1 (2.2) | 75 (85.2) | |
| High | 9 (52.9) | 25 (36.8) | 45 (97.8) | 13 (14.8) | |
| Depth of invasion ≥50% | 3 (17.6) | 24 (35.3) | 19 (41.3) | 19 (21.6) | 0.047 |
| LVSI | <0.001 | ||||
| Absent | 5 (29.4) | 15 (22.1) | 11 (23.9) | 41 (46.6) | |
| Focal | 7 (41.2) | 26 (38.2) | 6 (13.0) | 29 (33.0) | |
| Substantial | 5 (29.4) | 27 (39.7) | 29 (63.1) | 18 (20.4) | |
| Lymph node status | 0.002 | ||||
| Negative | 16 (94.1) | 54 (81.8) | 31 (68.9) | 79 (92.9) | |
| Positive | 1 (5.9) | 12 (18.2) | 14 (31.1) | 6 (7.1) | |
| FIGO stage | <0.001 | ||||
| I | 10 (58.8) | 36 (53.0) | 15 (32.6) | 63 (71.6) | |
| IB/II | 5 (29.4) | 16 (23.5) | 7 (15.2) | 16 (18.2) | |
| III | 2 (11.8) | 13 (19.1) | 19 (41.3) | 8 (9.1) | |
| IV | 0 (0.0) | 3 (4.4) | 5 (10.9) | 1 (1.1) | |
| Extensive tumor necrosis | <0.001 | ||||
| Absent | 4 (23.5) | 27 (39.7) | 17 (37.8) | 57 (64.8) | |
| Present | 13 (76.5) | 41 (60.3) | 28 (62.2) | 31 (35.2) | |
| MELF | <0.001 | ||||
| Absent | 11 (64.7) | 33 (48.5) | 40 (88.9) | 63 (71.6) | |
| Present | 6 (35.3) | 35 (51.5) | 5 (11.1) | 25 (28.4) | |
| Tumor budding | 0.017 | ||||
| Absent | 9 (52.9) | 31 (45.6) | 25 (55.6) | 62 (70.5) | |
| Present | 8 (47.1) | 37 (54.4) | 20 (44.4) | 26 (29.5) | |
| Mitoses/10 HPF | 76.6 ± 35.9 | 55.5 ± 24.4 | 86.8 ± 43.9 | 32.2 ± 26.2 | <0.001 |
| Characteristics | Coefficients | Lower 95% CI | Upper 95% CI | Std. Error | z | p-Value |
|---|---|---|---|---|---|---|
| Endometrioid histotype | −2.73 | −9.98 | 1.52 | 2.17 | 1.26 | 0.208 |
| Dedifferentiated histotype | −4.31 | −8.17 | −0.46 | 1.97 | 2.19 | 0.028 |
| Stage I | −2.51 | −4.78 | −0.23 | 1.16 | 2.16 | 0.031 |
| Stage IB/II | −2.5 | −4.69 | −0.31 | 1.12 | 2.24 | 0.025 |
| High grade | 0.04 | −3.21 | 3.29 | 1.66 | 0.02 | 0.982 |
| Mitoses/10 HPF | 0.00 | −0.03 | 0.03 | 0.02 | 0.09 | 0.927 |
| Extensive tumor necrosis | 2.71 | 0.22 | 5.21 | 1.27 | 2.13 | 0.033 |
| Substantial LVSI | −2.05 | −4.14 | 0.03 | 1.06 | 1.93 | 0.054 |
| Characteristics | Coefficients | Lower 95% CI | Upper 95% CI | Std. Error | z | p-Value |
|---|---|---|---|---|---|---|
| Endometrioid histotype | −15.53 | −385.57 | 354.51 | 188.80 | 0.08 | 0.93 |
| Dedifferentiated histotype | −2.66 | −6.54 | 1.22 | 1.98 | 1.34 | 0.18 |
| High grade | 11.98 | −358.05 | 382.01 | 188.79 | 0.06 | 0.95 |
| Mitoses/10 HPF | 0.01 | −0.04 | 0.06 | 0.03 | 0.33 | 0.74 |
| Extensive tumor necrosis | 1.28 | −1.23 | 3.78 | 1.28 | 1.00 | 0.32 |
| Substantial LVSI | −2.99 | −5.33 | −0.64 | 1.20 | 2.50 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruscelli, M.; Maloberti, T.; Corradini, A.G.; Rosini, F.; Querzoli, G.; Grillini, M.; Altimari, A.; Gruppioni, E.; Sanza, V.; Costantino, A.; et al. Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma. J. Pers. Med. 2023, 13, 723. https://doi.org/10.3390/jpm13050723
Ruscelli M, Maloberti T, Corradini AG, Rosini F, Querzoli G, Grillini M, Altimari A, Gruppioni E, Sanza V, Costantino A, et al. Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma. Journal of Personalized Medicine. 2023; 13(5):723. https://doi.org/10.3390/jpm13050723
Chicago/Turabian StyleRuscelli, Martina, Thais Maloberti, Angelo Gianluca Corradini, Francesca Rosini, Giulia Querzoli, Marco Grillini, Annalisa Altimari, Elisa Gruppioni, Viviana Sanza, Alessia Costantino, and et al. 2023. "Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma" Journal of Personalized Medicine 13, no. 5: 723. https://doi.org/10.3390/jpm13050723
APA StyleRuscelli, M., Maloberti, T., Corradini, A. G., Rosini, F., Querzoli, G., Grillini, M., Altimari, A., Gruppioni, E., Sanza, V., Costantino, A., Ciudino, R., Errani, M., Papapietro, A., Coluccelli, S., Turchetti, D., Ferioli, M., Giunchi, S., Dondi, G., Tesei, M., ... De Leo, A. (2023). Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma. Journal of Personalized Medicine, 13(5), 723. https://doi.org/10.3390/jpm13050723












