The Evaluation of Cartilage Regeneration Efficacy of Three-Dimensionally Biofabricated Human-Derived Biomaterials on Knee Osteoarthritis: A Single-Arm, Open Label Study in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment Criteria
2.2. Surgical Procedures and Biomaterial Ink Preparation
2.3. Postoperative Rehabilitation Programs and Follow-up Procedures
2.4. Histopathologic Examination
2.5. Statistical Analysis
3. Results
3.1. Clinical Evaluation
3.1.1. WOMAC Survey
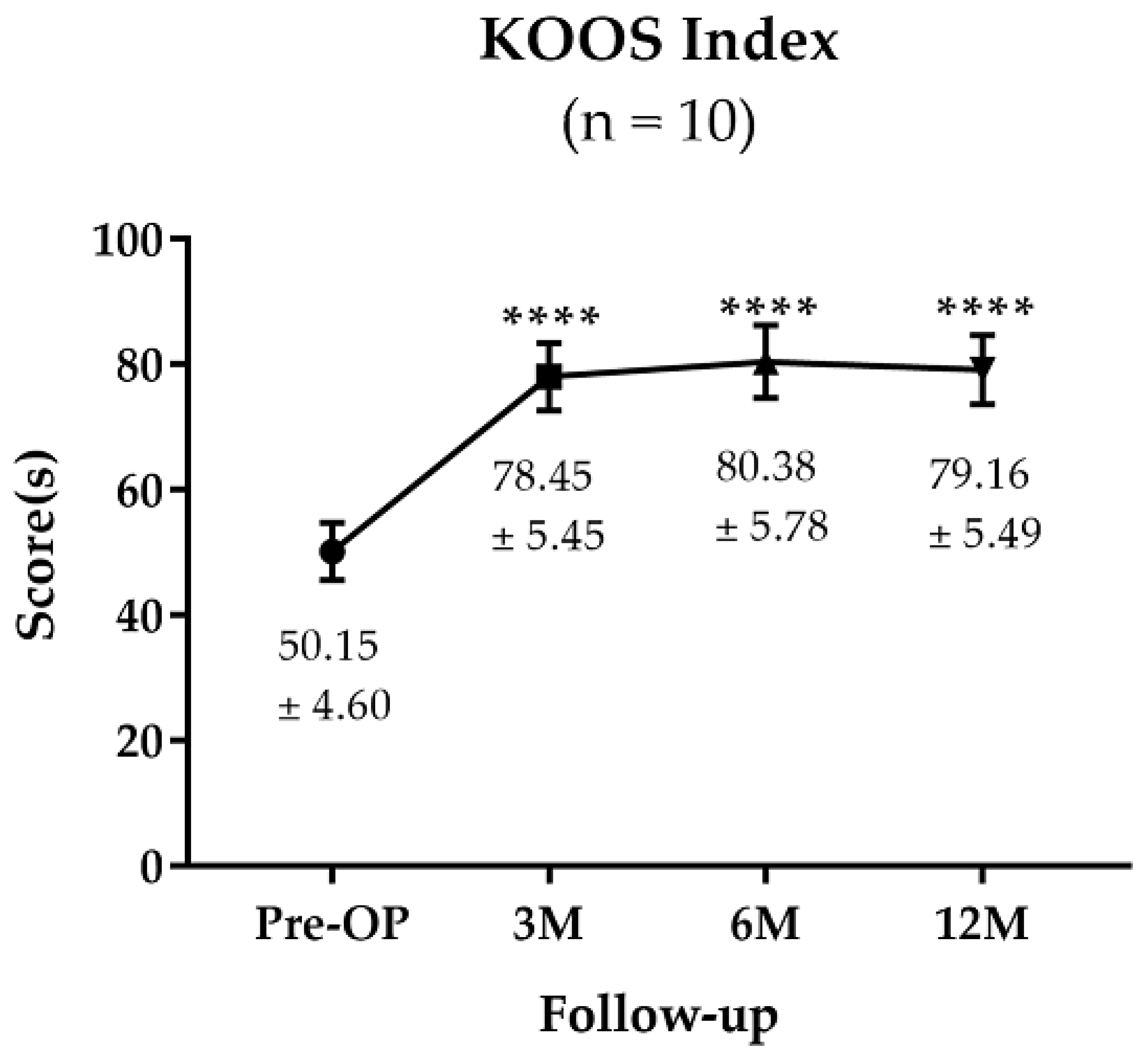
3.1.2. KOOS Survey
3.2. Medical Imaging Analysis
3.2.1. MRI Analysis and MOCART Score
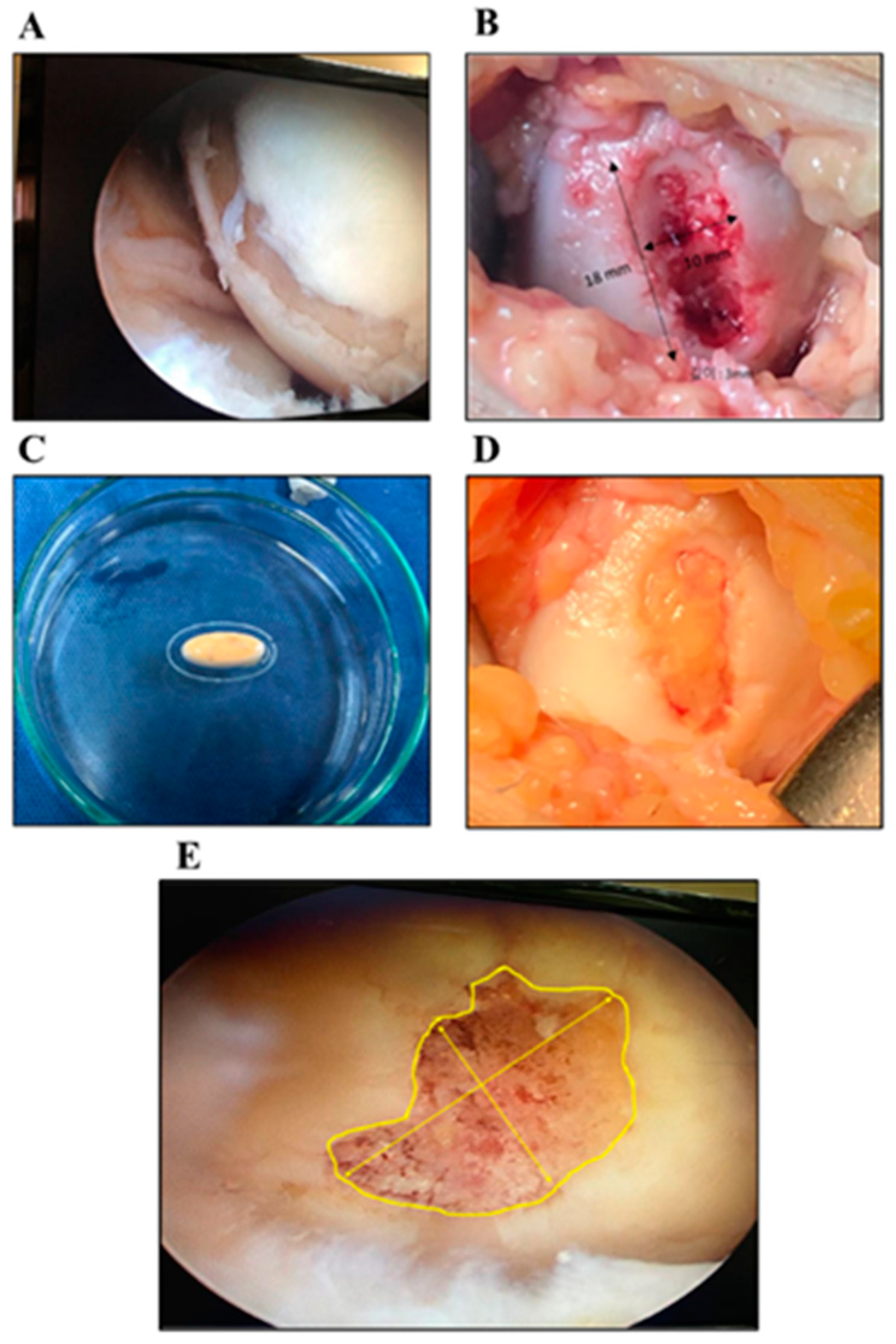
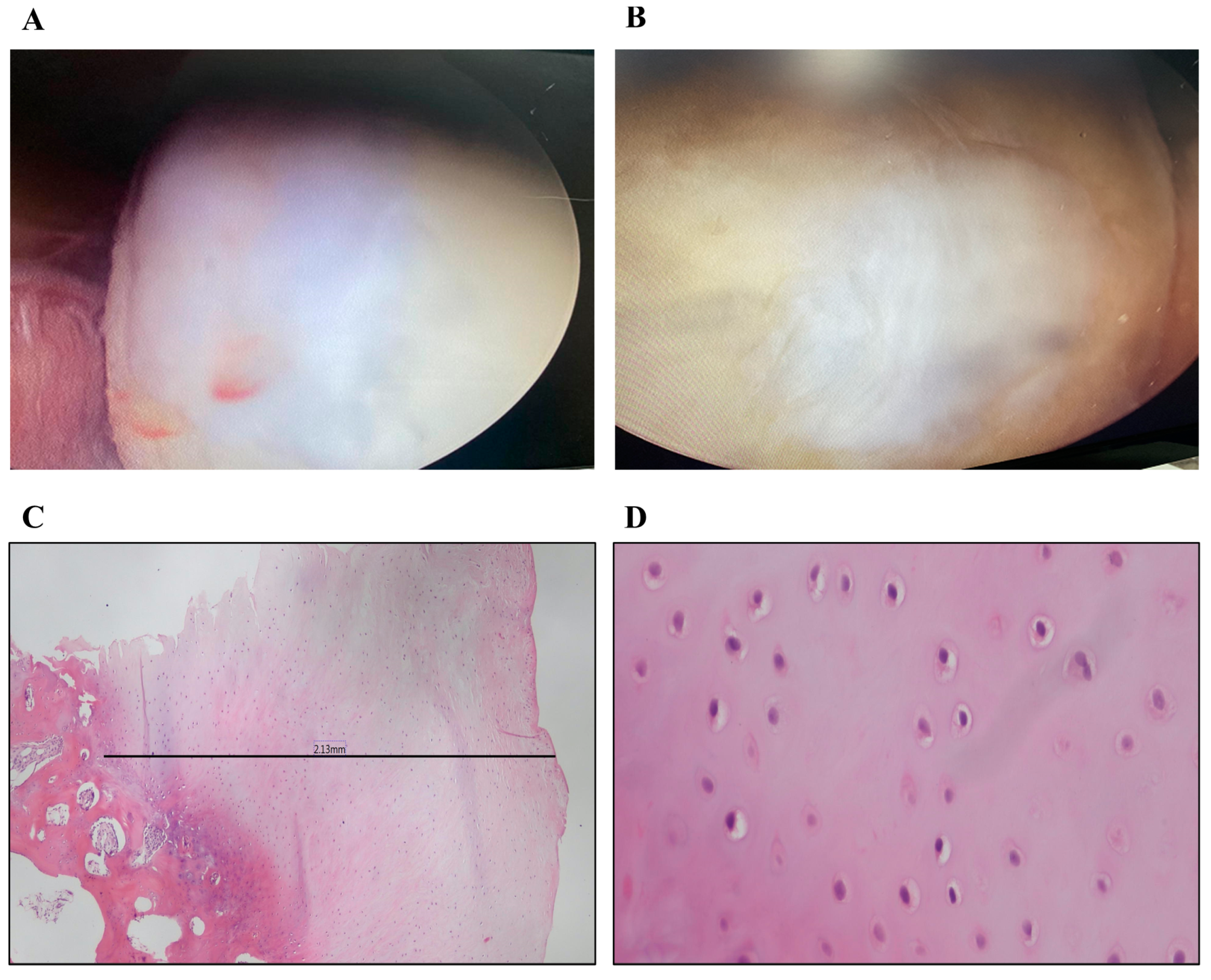
3.2.2. Arthroscopic Evaluation
3.3. Histopathologic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, X.; Li, C.; Yin, F.; Yang, G. Adipose-derived stem cells in articular cartilage regeneration: Current concepts and optimization strategies. Histol. Histopathol. 2018, 33, 639–653. [Google Scholar] [PubMed]
- Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar]
- Oldershaw, R.A. Cell sources for the regeneration of articular cartilage: The past, the horizon and the future. Int. J. Exp. Pathol. 2012, 93, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Alford, J.W.; Cole, B.J. Cartilage restoration, part 1: Basic science, historical perspective, patient evaluation, and treatment options. Am. J. Sports Med. 2005, 33, 295–306. [Google Scholar] [CrossRef]
- Kalamegam, G.; Memic, A.; Budd, E.; Abbas, M.; Mobasheri, A. A Comprehensive Review of Stem Cells for Cartilage Regeneration in Osteoarthritis. Adv. Exp. Med. Biol. 2018, 1089, 23–36. [Google Scholar]
- De L’escalopier, N.; Anract, P.; Biau, D. Surgical treatments for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef]
- Bedi, A.; Feeley, B.T.; Williams, R.J. Management of articular cartilage defects of the knee. J. Bone Jt. Surg. Am. 2010, 92, 994–1009. [Google Scholar] [CrossRef]
- Murawski, C.D.; Kennedy, J.G. Operative treatment of osteochondral lesions of the talus. J. Bone Jt. Surg. Am. 2013, 95, 1045–1054. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths & misconceptions—Progress & prospects. Osteoarthr. Cartil. 2015, 23, 334–350. [Google Scholar]
- Im, G.I.; Kim, T.K. Regenerative Therapy for Osteoarthritis: A Perspective. Int. J. Stem. Cells 2020, 13, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.C.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef] [PubMed]
- Minas, T.; Gomoll, A.H.; Solhpour, S.; Rosenberger, R.; Probst, C.; Bryant, T. Autologous chondrocyte implantation for joint preservation in patients with early osteoarthritis. Clin. Orthop. Relat. Res. 2010, 468, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Seon, J.-K.; Cho, I.-S.; Ko, J.-W. Current Update of Cartilage Regeneration Using Stem Cells in Osteoarthritis. J. Korean Orthop. Assoc. 2019, 54, 478–489. [Google Scholar] [CrossRef]
- Freitag, J.; Kenihan, M.A. Mesenchymal Stem Cell Therapy in Osteoarthritis and Regenerative Medicine. Curr. Sports Med. Rep. 2018, 17, 441–443. [Google Scholar] [CrossRef]
- Di Matteo, B.; El Araby, M.M.; D’angelo, A.; Iacono, F.; Nannini, A.; Vitale, N.D.; Marcacci, M.; Respizzi, S.; Kon, E. Adipose-Derived Stem Cell Treatments and Formulations. Clin. Sports Med. 2019, 38, 61–78. [Google Scholar] [CrossRef]
- Bekkers, J.E.; Tsuchida, A.I.; Van Rijen, M.H.; Vonk, L.A.; Dhert, W.; Creemers, L.; Saris, D.B. Single-stage cell-based cartilage regeneration using a combination of chondrons and mesenchymal stromal cells: Comparison with microfracture. Am. J. Sports Med. 2013, 41, 2158–2166. [Google Scholar] [CrossRef]
- Ryu, J.; Brittberg, M.; Nam, B.; Chae, J.; Kim, M.; Iban, Y.C.; Magneli, M.; Takahashi, E.; Khurana, B.; Bragdon, C.R. Evaluation of Three-Dimensional Bioprinted Human Cartilage Powder Combined with Micronized Subcutaneous Adipose Tissues for the Repair of Osteochondral Defects in Beagle Dogs. Int. J. Mol. Sci. 2022, 23, 2743. [Google Scholar] [CrossRef]
- Salzmann, G.M.; Ossendorff, R.; Gilat, R.; Cole, B.J. Autologous Minced Cartilage Implantation for Treatment of Chondral and Osteochondral Lesions in the Knee Joint: An Overview. Cartilage 2020, 13, 1124S–1136S. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting Technology: A Current State-of-the-Art Review. J. Manuf. Sci. Eng. 2014, 136, 061016. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Moncal, K.K.; Dey, M.; Ozbolat, I.T. Extrusion-Based Biofabrication in Tissue Engineering and Regenerative Medicine, in 3D Printing and Biofabrication; Ovsianikov, A., Yoo, J., Mironov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 255–281. [Google Scholar]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Bauge, C.; Boumediene, K. Use of Adult Stem Cells for Cartilage Tissue Engineering: Current Status and Future Developments. Stem. Cells Int. 2015, 2015, 438026. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef]
- Bianchi, F.; Maioli, M.; Leonardi, E.; Olivi, E.; Pasquinelli, G.; Valente, S.; Mendez, A.J.; Ricordi, C.; Raffaini, M.; Tremolada, C.; et al. A New Nonenzymatic Method and Device to Obtain a Fat Tissue Derivative Highly Enriched in Pericyte-Like Elements by Mild Mechanical Forces from Human Lipoaspirates. Cell Transplant. 2013, 22, 2063–2077. [Google Scholar] [CrossRef]
- Schreiner, M.M.; Raudner, M.; Marlovits, S.; Bohndorf, K.; Weber, M.; Zalaudek, M.; Röhrich, S.; Szomolanyi, P.; Filardo, G.; Windhager, R.; et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage 2021, 13, 571S–587S. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Kuroda, R.; Ishida, K.; Matsumoto, T.; Akisue, T.; Fujioka, H.; Mizuno, K.; Ohgushi, H.; Wakitani, S.; Kurosaka, M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthr. Cartil. 2007, 15, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Saris, T.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.; Saris, D.B. Five-year outcome of 1-stage cell-based cartilage repair using recycled autologous chondrons and allogenic mesenchymal stromal cells: A first-in-human clinical trial. Am. J. Sports Med. 2021, 49, 941–947. [Google Scholar] [CrossRef] [PubMed]



| Age | Number of Patients |
|---|---|
| >18–20 years old | 1 |
| 40–50 years old | 8 |
| >50 years old | 1 |
| Gender | Number of Patients |
| Male | 5 |
| Female | 5 |
| Body Mass Index | Number of Patients |
| >18.5–24.9 kg/m2 | 4 |
| 25–29.9 kg/m2 | 5 |
| 30–34.9 kg/m2 | 1 |
| Patient Number | De novo Cartilage (mm) | Fibrous Tissue (mm) |
|---|---|---|
| 3 | 2.20–2.60 | - 1 |
| 4 | 2.12 | 0.4 |
| 7 | 2.13–2.30 | 0.3 |
| 8 | 2.0–2.60 | - |
| 10 | 0.35 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, M.M.; Eid, G.; Othman, M.H.M.; Ibrahim, H.; Elsers, D.; Elyounsy, M.; Kwon, S.Y.; Kim, M.; Kim, D.; Kim, J.-W.; et al. The Evaluation of Cartilage Regeneration Efficacy of Three-Dimensionally Biofabricated Human-Derived Biomaterials on Knee Osteoarthritis: A Single-Arm, Open Label Study in Egypt. J. Pers. Med. 2023, 13, 748. https://doi.org/10.3390/jpm13050748
Abdelhamid MM, Eid G, Othman MHM, Ibrahim H, Elsers D, Elyounsy M, Kwon SY, Kim M, Kim D, Kim J-W, et al. The Evaluation of Cartilage Regeneration Efficacy of Three-Dimensionally Biofabricated Human-Derived Biomaterials on Knee Osteoarthritis: A Single-Arm, Open Label Study in Egypt. Journal of Personalized Medicine. 2023; 13(5):748. https://doi.org/10.3390/jpm13050748
Chicago/Turabian StyleAbdelhamid, Mohamed M., Gaber Eid, Moustafa H. M. Othman, Hamdy Ibrahim, Dalia Elsers, Mohamed Elyounsy, Soon Yong Kwon, Minju Kim, Doheui Kim, Jin-Wook Kim, and et al. 2023. "The Evaluation of Cartilage Regeneration Efficacy of Three-Dimensionally Biofabricated Human-Derived Biomaterials on Knee Osteoarthritis: A Single-Arm, Open Label Study in Egypt" Journal of Personalized Medicine 13, no. 5: 748. https://doi.org/10.3390/jpm13050748
APA StyleAbdelhamid, M. M., Eid, G., Othman, M. H. M., Ibrahim, H., Elsers, D., Elyounsy, M., Kwon, S. Y., Kim, M., Kim, D., Kim, J.-W., Ryu, J., El-Radi, M. A., & Fetih, T. N. (2023). The Evaluation of Cartilage Regeneration Efficacy of Three-Dimensionally Biofabricated Human-Derived Biomaterials on Knee Osteoarthritis: A Single-Arm, Open Label Study in Egypt. Journal of Personalized Medicine, 13(5), 748. https://doi.org/10.3390/jpm13050748






