Male Clinical Parameters (Age, Stature, Weight, Body Mass Index, Smoking History, Alcohol Consumption) Bear Minimal Relationship to the Level of Sperm DNA Fragmentation
Abstract
:1. Introduction
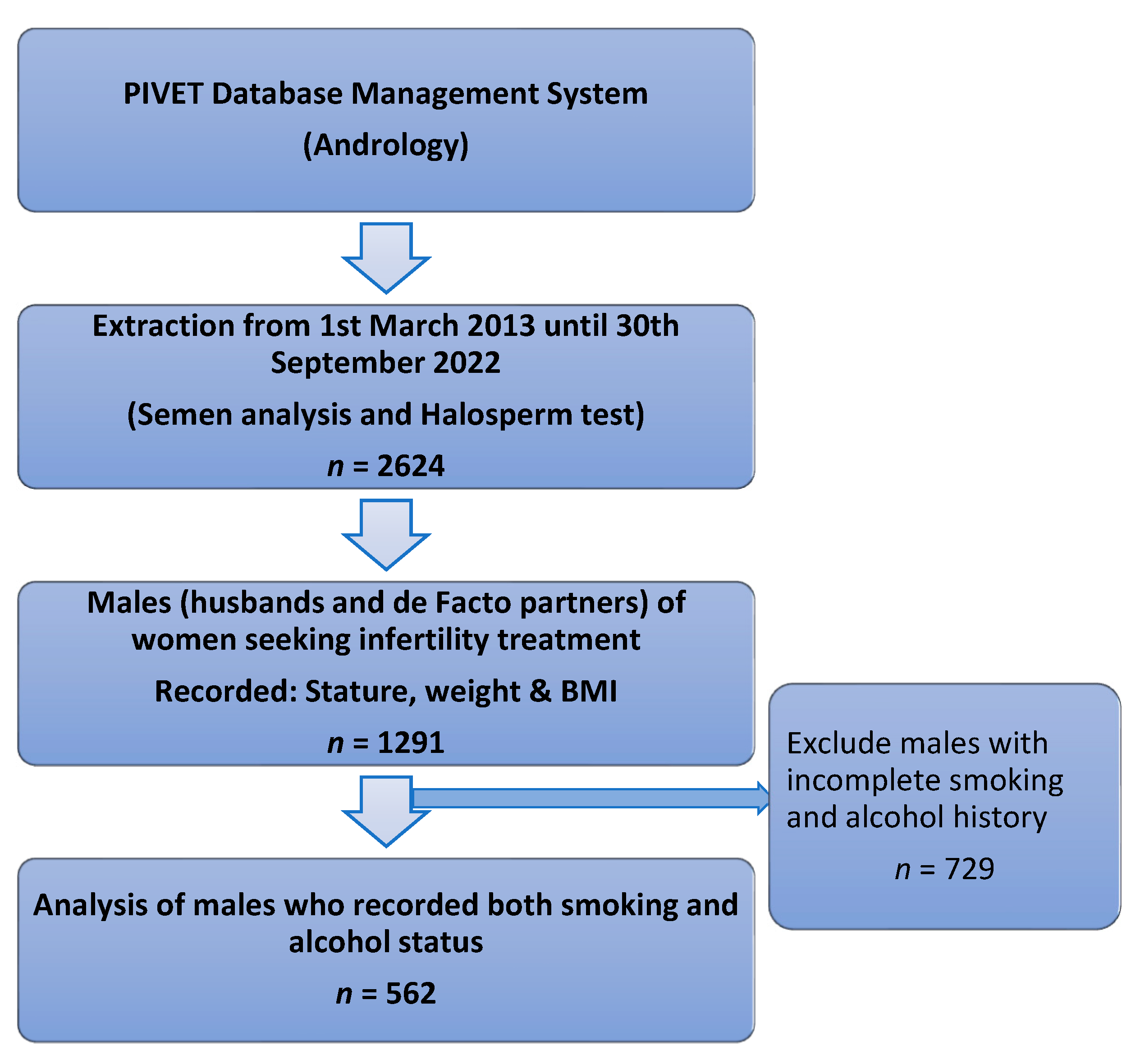
2. Materials and Methods
2.1. Methodology
2.2. Laboratory Procedures
Principle of Sperm Chromatin Dispersion Test—Halosperm® G2
2.3. Validation and Quality Control
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
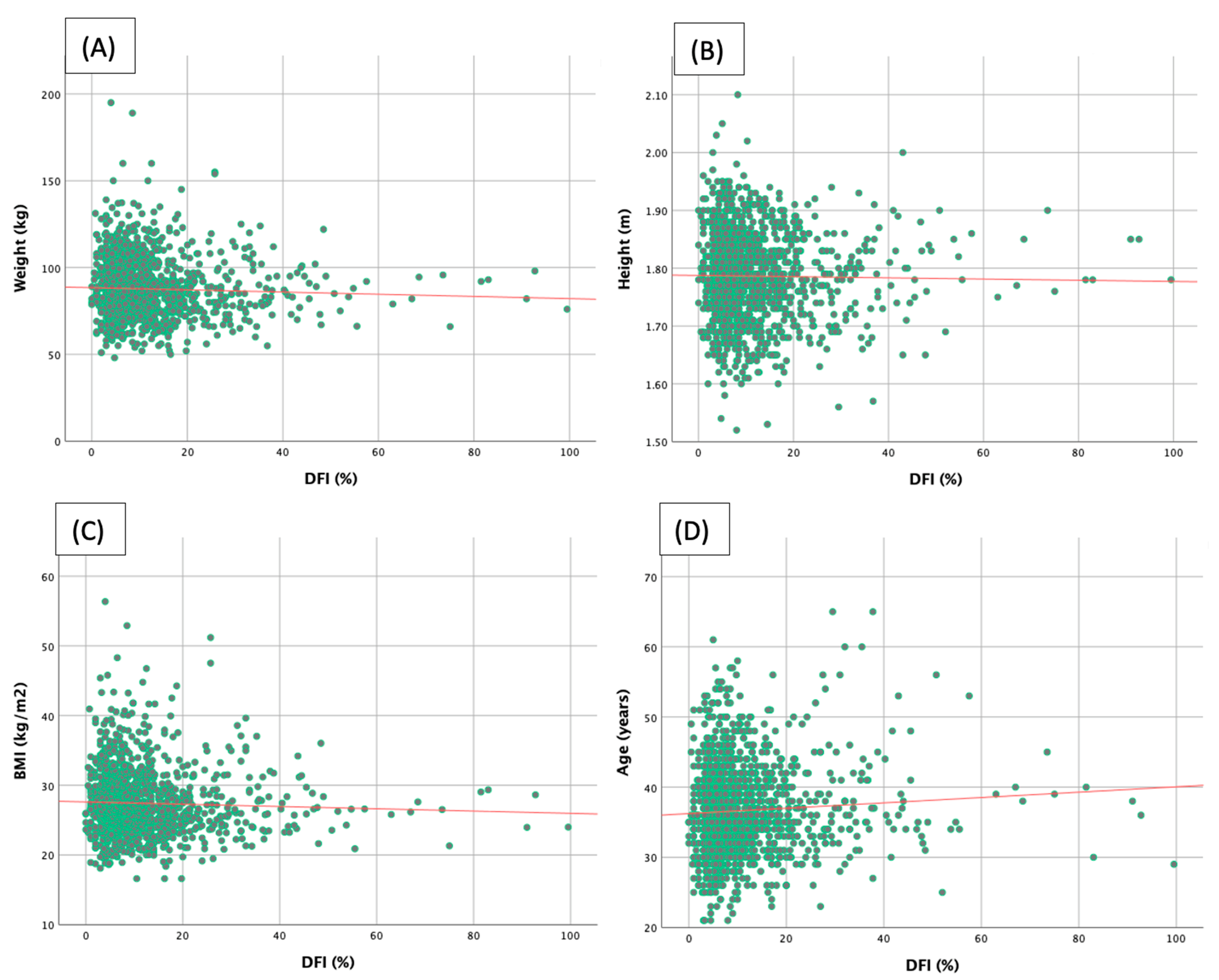
3.2. Correlations between Clinical Parameters and Sperm DFI
3.3. Relationship between Lifestyle and Sperm DFI
3.4. Subgroup Analysis of Various Lifestyle with Sperm DFI ≥ 15%
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.A.; Peña, F.J. Apoptotic Events in Male Germ Cells and in Mature Mammalian Spermatozoa. In Apoptosis: Involvement of Oxidative Stress and Intracellular Ca2+ Homeostasi; Salido, G.M., Rosado, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 165–209. [Google Scholar]
- Muciaccia, B.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; De Rooij, D.G.; Vicini, E. Novel stage classification of human spermatogenesis based on acrosome development. Biol Reprod. 2013, 89, 60. [Google Scholar] [CrossRef] [PubMed]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Baldi, E. Sperm DNA Fragmentation: Mechanisms of Origin. Adv. Exp. Med. Biol. 2019, 1166, 75–85. [Google Scholar] [PubMed]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef] [PubMed]
- Gamete Transport. Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Jequier, A.M.; Cummins, J.M.; Gearon, C.; Apted, S.L.; Yovich, J.M.; Yovich, J.L. A pregnancy achieved using sperm from the epididymal caput in idiopathic obstructive azoospermia. Fertil. Steril. 1990, 53, 1104–1105. [Google Scholar] [CrossRef] [PubMed]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int. J. Reprod. Biomed. 2017, 15, 331–344. [Google Scholar] [CrossRef]
- Asadi, A.; Ghahremani, R.; Abdolmaleki, A.; Rajaei, F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021, 19, 493–504. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. 2012, 4, 746–754. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Men’s Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- John Aitken, R.; Clarkson, J.S.; Fishel, S. Generation of Reactive Oxygen Species, Lipid Peroxidation, and Human Sperm Function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Venkatesh, S.; Riyaz, A.M.; Shamsi, M.B.; Kumar, R.; Gupta, N.P.; Mittal, S.; Malhotra, N.; Sharma, R.K.; Agarwal, A.; Dada, R. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia 2009, 41, 251–256. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2018, 50, e12926. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Niaki, M.T.; Sheikhha, M.H.; Khalili, M.A.; Fesahat, F.; Nabi, A.; Izadi, M.; Esmailabad, S.G.; Talebi, A.R. Possible Harmful Effects of Smoking Hookah on Sperm DNA Fragmentation Index and Protamine Genes Expression in Normozoospermic Men. Subst. Abuse Res. Treat. 2023, 17, 11782218221144547. [Google Scholar]
- Omolaoye, T.S.; El Shahawy, O.; Skosana, B.T.; Boillat, T.; Loney, T.; du Plessis, S.S. The mutagenic effect of tobacco smoke on male fertility. Environ. Sci. Pollut. Res. 2022, 29, 62055–62066. [Google Scholar] [CrossRef]
- Szabo, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle-, environmental-, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef]
- Shen, H.-M.; Chia, S.-E.; Ni, Z.-Y.; New, A.-L.; Lee, B.-L.; Ong, C.-N. Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reprod. Toxicol. 1997, 11, 675–680. [Google Scholar] [CrossRef]
- Sergerie, M.; Ouhilal, S.; Bissonnette, F.; Brodeur, J.; Bleau, G. Lack of association between smoking and DNA fragmentation in the spermatozoa of normal men. Hum. Reprod. 2000, 15, 1314–1321. [Google Scholar] [CrossRef]
- Martini, A.C.; Molina, R.I.; Estofán, D.; Senestrari, D.; de Cuneo, M.F.; Ruiz, R.D. Effects of alcohol and cigarette consumption on human seminal quality. Fertil. Steril. 2004, 82, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, F.F.; Sobreiro, B.P.; Hallak, J.; Pasqualotto, E.B.; Lucon, A.M. Cigarette smoking is related to a decrease in semen volume in a population of fertile men. BJU Int. 2006, 97, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Trummer, H.; Habermann, H.; Haas, J.; Pummer, K. The impact of cigarette smoking on human semen parameters and hormones. Hum. Reprod. 2002, 17, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Teijón, M.L.; Garcia, F.; Serra, O.; Moragas, M.; Rabanal, A.; Olivares, R.; Alvarez, J. Semen quality in a population of volunteers from the province of Barcelona. Reprod. Biomed. Online 2007, 15, 434–444. [Google Scholar] [CrossRef]
- Jensen, T.K.; Swan, S.; Jørgensen, N.; Toppari, J.; Redmon, B.; Punab, M.; Drobnis, E.Z.; Haugen, T.B.; Zilaitiene, B.; Sparks, A.E.; et al. Alcohol and male reproductive health: A cross-sectional study of 8344 healthy men from Europe and the USA. Hum. Reprod. 2014, 29, 1801–1809. [Google Scholar] [CrossRef]
- Yang, H.; Li, G.; Jin, H.; Guo, Y.; Sun, Y. The effect of sperm DNA fragmentation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Transl. Androl. Urol. 2019, 8, 356–365. [Google Scholar] [CrossRef]
- Jensen, T.K.; Gottschau, M.; Madsen, J.O.B.; Andersson, A.-M.; Lassen, T.H.; Skakkebaek, N.E.; Swan, S.; Priskorn, L.; Juul, A.; Jørgensen, N. Habitual alcohol consumption associated with reduced semen quality and changes in reproductive hormones; a cross-sectional study among 1221 young Danish men. BMJ Open 2014, 4, e005462. [Google Scholar] [CrossRef]
- Boeri, L.; Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Pederzoli, F.; Cazzaniga, W.; Chierigo, F.; Dehò, F.; Montanari, E.; Montorsi, F. Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J. Androl. 2019, 21, 478–485. [Google Scholar]
- Ricci, E.; Beitawi, S.; Cipriani, S.; Candiani, M.; Chiaffarino, F.; Viganò, P.; Noli, S.; Parazzini, F. Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod. Biomed. Online 2017, 34, 38–47. [Google Scholar] [CrossRef]
- Fariello, R.M.; Pariz, J.R.; Spaine, D.M.; Cedenho, A.P.; Bertolla, R.P.; Fraietta, R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. BJU Int. 2012, 110, 863–867. [Google Scholar] [CrossRef]
- Pearce, K.L.; Hill, A.; Tremellen, K.P. Obesity related metabolic endotoxemia is associated with oxidative stress and impaired sperm DNA integrity. Basic Clin. Androl. 2019, 29, 6. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Maleki-Hajiagha, A.; Maroufizadeh, S.; Rezaeinejad, M.; Almasi-Hashiani, A.; Razavi, M. The effect of body mass index on sperm DNA fragmentation: A systematic review and meta-analysis. Int. J. Obes. 2020, 44, 549–558. [Google Scholar] [CrossRef]
- Chua, S.C.; Yovich, S.J.; Hinchliffe, P.M.; Yovich, J.L. How Well Do Semen Analysis Parameters Correlate with Sperm DNA Fragmentation? A Retrospective Study from 2567 Semen Samples Analyzed by the Halosperm Test. J. Pers. Med. 2023, 13, 518. [Google Scholar]
- Mustafa, K.B.; Yovich, J.L.; Marjanovich, N.; Yovich, S.J.; Keane, K.N. IVF-ICSI Split Insemination Reveals those Cases of Unexplained Infertility benefitting from ICSI even when the DNA fragmentation index is reduced to 15% or even 5%. Androl. Gynecol. Curr. Res. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Sedó, C.A.; Bilinski, M.; Lorenzi, D.; Uriondo, H.; Noblía, F.; Longobucco, V.; Lagar, E.V.; Nodar, F. Effect of sperm DNA fragmentation on embryo development: Clinical and biological aspects. JBRA Assist. Reprod. 2017, 21, 343–350. [Google Scholar] [CrossRef]
- Bungum, M.; Humaidan, P.; Axmon, A.; Spano, M.; Bungum, L.; Erenpreiss, J.; Giwercman, A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum. Reprod. 2007, 22, 174–179. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management-meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010; p. 271.
- halosperm® G2. Available online: https://www.halotechdna.com/wp-content/uploads/2014/06/IU-halosperm-G2.pdf (accessed on 17 April 2023).
- Word Bank Group. Fertility Rate, Total (Births Per Woman). 2023. Available online: http://data.worldbank.org/indicator/SP.DYN.TFRT.IN (accessed on 17 April 2023).
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Manthey, J.; Shield, K.D.; Rylett, M.; Hasan, O.S.M.; Probst, C.; Rehm, J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: A modelling study. Lancet 2019, 393, 2493–2502. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Insights Into Australian Smokers 2021–2022. 2022. Available online: https://www.abs.gov.au/articles/insights-australian-smokers-2021-22 (accessed on 16 April 2023).
- Schäfer, A.A.; Santos, L.P.; Quadra, M.R.; Dumith, S.C.; Meller, F.O. Alcohol Consumption and Smoking During Covid-19 Pandemic: Association with Sociodemographic, Behavioral, and Mental Health Characteristics. J. Community Health 2022, 47, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Viloria, T.; Meseguer, M.; Martínez-Conejero, J.A.; O’Connor, J.; Remohí, J.; Pellicer, A.; Garrido, N. Cigarette smoking affects specific sperm oxidative defenses but does not cause oxidative DNA damage in infertile men. Fertil. Steril. 2010, 94, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.K.; Song, P.I.; Kim, M.-H.; Kim, J.S.; Hyun, J.-W.; Choi, S.-J.; Yoon, S.P.; Chung, M.H.; Chang, I.-Y.; You, H.J. Human 8-oxoguanine DNA glycosylase suppresses the oxidative stress induced apoptosis through a p53-mediated signaling pathway in human fibroblasts. Mol. Cancer Res. 2007, 5, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. The Amoroso Lecture. The human spermatozoon—A cell in crisis? J. Reprod. Fertil. 1999, 115, 1–7. [Google Scholar] [CrossRef]
- Aitken, R.J. Molecular mechanisms regulating human sperm function. Mol. Hum. Reprod. 1997, 3, 169–173. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Reactive oxygen species (ROS) and reproduction. Adv. Exp. Med. Biol. 1994, 366, 185–197. [Google Scholar]
- Gagnon, C.; Iwasaki, A.; DE Lamirande, E.; Kovalski, N. Reactive Oxygen Species and Human Spermatozoa. Ann. N. Y. Acad. Sci. 1991, 637, 436–444. [Google Scholar] [CrossRef]
- Zini, A.; de Lamirande, E.; Gagnon, C. Low levels of nitric oxide promote human sperm capacitation in vitro. J. Androl. 1995, 16, 424–431. [Google Scholar]
- Dikshit, R.K.; Buch, J.G.; Mansuri, S.M. Effect of tobacco consumption on semen quality of a population of hypofertile males. Fertil. Steril. 1987, 48, 334–336. [Google Scholar] [CrossRef]
- Hassa, H.; Yildirim, A.; Can, C.; Turgut, M.; Tanir, H.M.; Senses, T.; Sahin-Mutlu, F. Effect of smoking on semen parameters of men attending an infertility clinic. Clin. Exp. Obstet. Gynecol. 2006, 33, 19–22. [Google Scholar]
- Li, Y.; Lin, H.; Ma, M.; Li, L.; Cai, M.; Zhou, N.; Han, X.; Bao, H.; Huang, L.; Zhu, C.; et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Hum. Reprod. 2008, 24, 459–469. [Google Scholar] [CrossRef]
- Hansen, M.L.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J.; Håkonsen, L.B.; Ramlau-Hansen, C.H. Does last week’s alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod. Toxicol. 2012, 34, 457–462. [Google Scholar]
- Ricci, E.; Noli, S.; Ferrari, S.; La Vecchia, I.; Cipriani, S.; De Cosmi, V.; Somigliana, E.; Parazzini, F. Alcohol intake and semen variables: Cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology 2018, 6, 690–696. [Google Scholar] [CrossRef]
- Wogatzky, J.; Wirleitner, B.; Stecher, A.; Vanderzwalmen, P.; Neyer, A.; Spitzer, D.; Schuff, M.; Schechinger, B.; Zech, N.H.; Schuff, M. The combination matters-distinct impact of lifestyle factors on sperm quality: A study on semen analysis of 1683 patients according to MSOME criteria. Reprod. Biol. Endocrinol. 2012, 10, 115. [Google Scholar] [CrossRef]
- Finelli, R.; Mottola, F.; Agarwal, A. Impact of Alcohol Consumption on Male Fertility Potential: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 328. [Google Scholar] [CrossRef]
- Mongioì, L.M.; Perelli, S.; Condorelli, R.A.; Barbagallo, F.; Crafa, A.; Cannarella, R.; La Vignera, S.; Calogero, A.E. The Role of Resveratrol in Human Male Fertility. Molecules 2021, 26, 2495. [Google Scholar] [CrossRef]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef]
- Ferramosca, A.; Di Giacomo, M.; Moscatelli, N.; Zara, V. Obesity and Male Infertility: Role of Fatty Acids in the Modulation of Sperm Energetic Metabolism. Eur. J. Lipid Sci. Technol. 2018, 120, 1700451. [Google Scholar] [CrossRef]
- Andersen, J.M.; Herning, H.; Aschim, E.L.; Hjelmesæth, J.; Mala, T.; Hanevik, H.I.; Bungum, M.; Haugen, T.B.; Witczak, O. Body Mass Index Is Associated with Impaired Semen Characteristics and Reduced Levels of Anti-Müllerian Hormone across a Wide Weight Range. PLoS ONE 2015, 10, e0130210. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.D.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef]
- Dupont, C.; Faure, C.; Sermondade, N.; Boubaya, M.; Eustache, F.; Clément, P.; Briot, P.; Berthaut, I.; Levy, V.; Cedrin-Durnerin, I.; et al. Obesity leads to higher risk of sperm DNA damage in infertile patients. Asian J. Androl. 2013, 15, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Louis, G.M.B. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2014, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Mattila, M.; Comar, V.; Ricci, J.; Dieamant, F.; Oliveira, J.B.A.; et al. The effects of male age on sperm DNA damage: An evaluation of 2178 semen samples. JBRA Assist. Reprod. 2018, 22, 323–330. [Google Scholar] [PubMed]
- Aitken, R.J. The role of free oxygen radicals and sperm function. Int. J. Androl. 1989, 12, 95–97. [Google Scholar] [CrossRef]
- Paoli, D.; Gallo, M.; Rizzo, F.; Spanò, M.; Leter, G.; Lombardo, F.; Lenzi, A.; Gandini, L. Testicular cancer and sperm DNA damage: Short- and long-term effects of antineoplastic treatment. Andrology 2015, 3, 122–128. [Google Scholar] [CrossRef]
- Maselli, J.; Hales, B.F.; Chan, P.; Robaire, B. Exposure to bleomycin, etoposide, and cis-platinum alters rat sperm chromatin integrity and sperm head protein profile. Biol. Reprod. 2012, 86, 166. [Google Scholar] [CrossRef]
- Durairajanayagam, D. Lifestyle causes of male infertility. Arab. J. Urol. 2018, 16, 10–20. [Google Scholar] [CrossRef]

| Clinical Parameters | Mean ± SD | p-Value | |
|---|---|---|---|
| SDF < 15% (n = 990) | SDF ≥ 15% (n = 301) | ||
| Age (years) | 36.49 ± 6.38 | 37.30 ± 6.84 | 0.06 |
| Stature (m) | 1.79 ± 0.08 | 1.78 ± 0.07 | 0.33 |
| Weight (kg) | 88.2 ± 16.72 | 85.84 ± 16.04 | 0.03 |
| BMI (kg/m2) | 27.56 ± 4.65 | 26.96 ± 4.56 | 0.05 |
| SDF (%) | 7.13 ± 3.39 | 26.25 ± 13.67 | <0.0001 |
| Sperm concentration (106/mL) | 65.25 ± 48.92 | 60.35 ± 50.67 | 0.13 |
| Normal morphology (%) | 8.04 ± 0.45 | 8.01 ± 0.27 | 0.27 |
| Total motility (%) | 42.37 ± 32.60 | 35.31 ± 31.71 | 0.001 |
| Progressive motility (%) | 39.51 ± 31.05 | 32.31 ± 29.73 | <0.001 |
| Seminal volume (mL) | 3.45 ± 1.55 | 3.64 ± 1.75 | 0.08 |
| Clinical Parameters | Pearson Correlation (r) | p-Value |
|---|---|---|
| Age (years) | 0.064 | 0.022 |
| Stature (m) | −0.015 | 0.588 |
| Weight (kg) | −0.041 | 0.139 |
| BMI (kg/m2) | −0.038 | 0.168 |
| Lifestyle | SDF Levels | p Value | Odds Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Non-smoker Smoker Current smoker Ex-smoker | 10.06 ± 5.94 (74) | 0.03 a 0.03 a | 0.99 (0.96–1.02) 1.0 (0.94–1.06) | 0.39 1 0.97 1 |
| Nil alcohol consumer Alcohol consumer Rarely Monthly Weekly Daily | 11.37 (120) 10.64 11.88 10.59 | 0.09 a 0.71 b |
| Lifestyle | DFI < 15% | DFI ≥ 15% | Relative Risk | p Value |
|---|---|---|---|---|
| Smoker Non-smoker Current smoker Ex-smoker | 135/166 (81.3%) 293/396 (74.0%) 63/74 (85.1%) 72/92 (78.3%) | 31/166 (18.7%) 103/396 (26.0%) 11/74 (14.9%) 20/92 (21.7%) | 0.72 0.69 | 0.06 a 0.32 b |
| Alcohol consumer Nil alcohol consumer | 370/481 (76.9%) 58/81 (71.6%) | 111/481 (23.1%) 23/81 (28.4%) | 0.81 | 0.30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, S.C.; Yovich, S.J.; Hinchliffe, P.M.; Yovich, J.L. Male Clinical Parameters (Age, Stature, Weight, Body Mass Index, Smoking History, Alcohol Consumption) Bear Minimal Relationship to the Level of Sperm DNA Fragmentation. J. Pers. Med. 2023, 13, 759. https://doi.org/10.3390/jpm13050759
Chua SC, Yovich SJ, Hinchliffe PM, Yovich JL. Male Clinical Parameters (Age, Stature, Weight, Body Mass Index, Smoking History, Alcohol Consumption) Bear Minimal Relationship to the Level of Sperm DNA Fragmentation. Journal of Personalized Medicine. 2023; 13(5):759. https://doi.org/10.3390/jpm13050759
Chicago/Turabian StyleChua, Shiao Chuan, Steven John Yovich, Peter Michael Hinchliffe, and John Lui Yovich. 2023. "Male Clinical Parameters (Age, Stature, Weight, Body Mass Index, Smoking History, Alcohol Consumption) Bear Minimal Relationship to the Level of Sperm DNA Fragmentation" Journal of Personalized Medicine 13, no. 5: 759. https://doi.org/10.3390/jpm13050759







