Relationship between the TGFBR1 Gene and Molar Incisor Hypomineralization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Type of Study and Population
2.2. Inclusion and Exclusion Criteria
2.3. Clinical Examination and Questionnaire
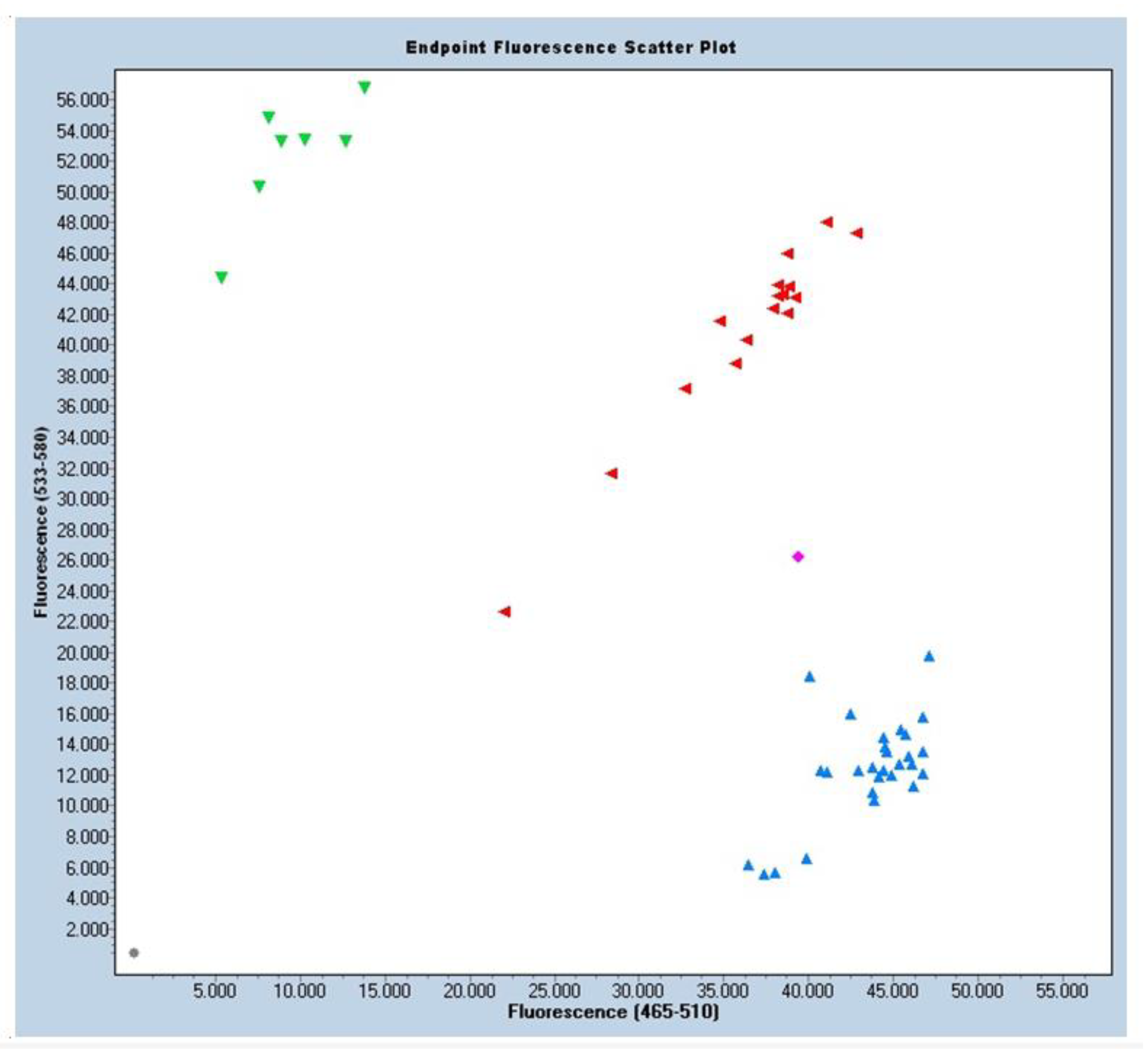
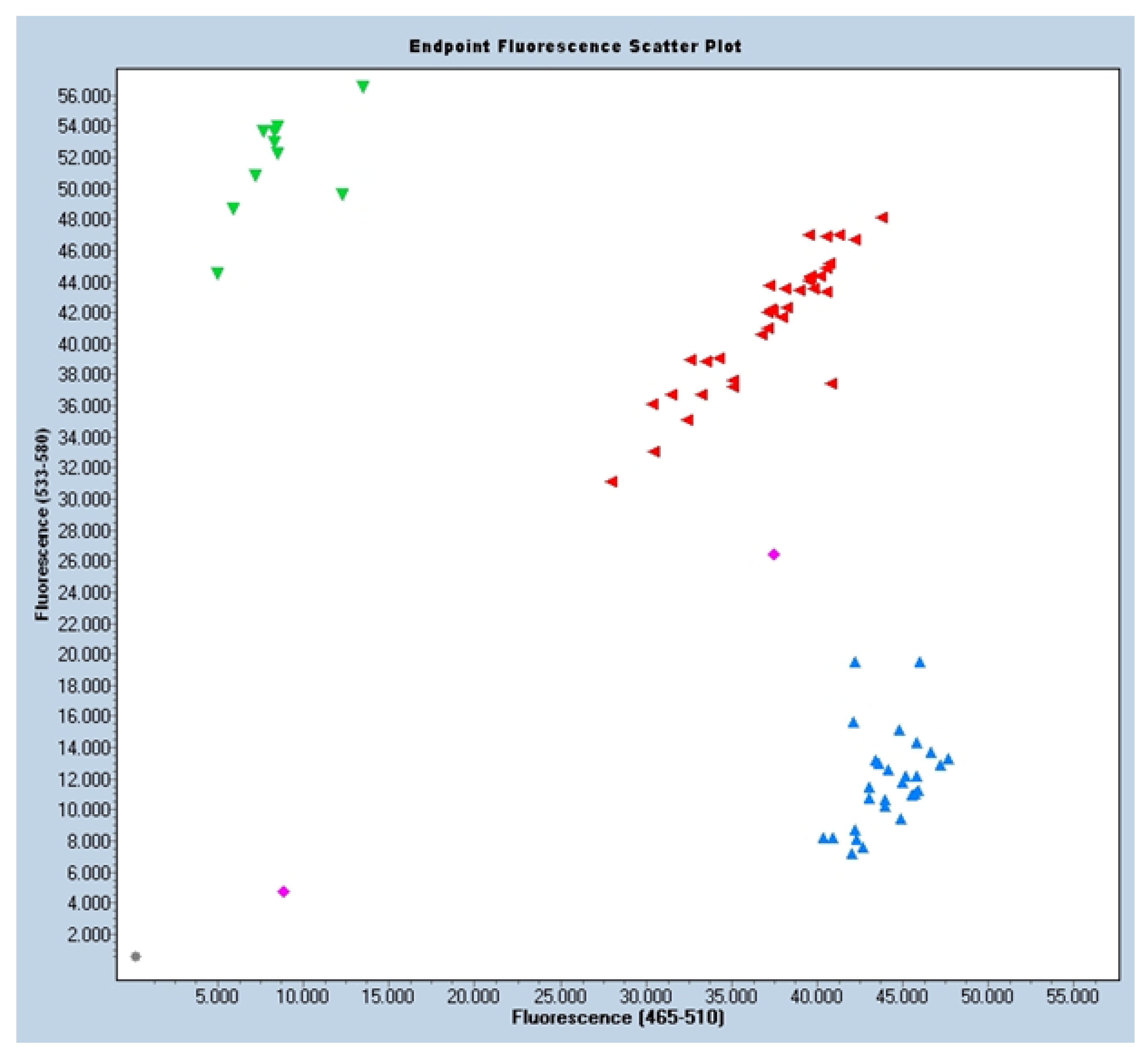
2.4. Sample Collection and Genetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Distribution of the Allelic Frequencies
3.3. Logistic Regression
4. Discussion
4.1. Prenatal Factors
4.2. Perinatal Factors
4.3. Postnatal Factors
4.4. Genetic Influence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mild MIH | Delimited opacification in the zones without occlusal forces Isolated opacifications No enamel loss in the opaque zones No history of dental hypersensitivity Carious lesions are not associated with affected enamel Involvement of the incisors is mild, if present |
| Moderate MIH | Atypical and intact restorations may be present Delimited opacification in the occlusal/incisal third of the tooth, without structural loss after eruption Post-eruptive enamel breakdown and carious lesions limited to 1–2 zones, without participation of the cusps Dental hypersensitivity is typically present Aesthetic concerns are commonly expressed by the patient or parents |
| Severe MIH | Post-eruptive enamel losses are present and generally occur when the tooth erupts History of dental sensitivity Extensive carious lesions are often associated with affected enamel Crown destruction may advance quickly and envelop the dental pulp Presence of defects in atypical restorations Aesthetic concerns are expressed by the patient or parents |
References
- Koch, G.; Hallonsten, A.L.; Ludvigsson, N.; Hansson, B.O.; Holst, A.; Ullbro, C. Epidemiologic study of idiopathic enamel hypomineralization in permanent teeth of Swedish children. Commun. Dent. Oral Epidemiol. 1987, 15, 279–285. [Google Scholar] [CrossRef]
- Bekes, K. Molar Incisor Hypomineralization; Quintessenz Verlag: New Malden, UK, 2022. [Google Scholar]
- Weerheijm, K.L.; Duggal, M.; Mejàre, I.; Papagiannoulis, L.; Koch, G.; Martens, L.C.; Hallonsten, A.L. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiologic studies: A summary of the European meeting on MIH held in Athens, 2003. Eur. J. Paediatr. Dent. 2003, 4, 110–113. [Google Scholar] [PubMed]
- Lygidakis, N.A.; Wong, F.; Jälevik, B.; Vierrou, A.M.; Alaluusua, S.; Espelid, I. Best Clinical Practice Guidance for clinicians dealing with children presenting with Molar-Incisor-Hypomineralisation (MIH): An EAPD Policy Document. Eur. Arch. Paediatr. Dent. 2010, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Weerheijm, K.L.; Mejare, I. Molar incisor hypomineralization:a questionnaire inventory of its occurrence in member countries of the European Academy of Paediatric Dentistry. Int. Pediatr. Dent. 2003, 13, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.A.; Dimou, G.; Marinou, D. Molar-incisor-hypomineralisation (MIH). A retrospective clinical study in Greek children. II. Possible medical aetiological factors. Eur. Arch. Paediatr. Dent. 2008, 9, 207–217. [Google Scholar] [CrossRef]
- Allazzam, S.M.; Alaki, S.M.; El Meligy, O.A. Molar incisor hypomineralization, prevalence, and etiology. Int. J. Dent. 2014, 2014, 234508. [Google Scholar] [CrossRef] [Green Version]
- Americano, G.C.; Jacobsen, P.E.; Soviero, V.M.; Haubek, D. A systematic review on the association between molar incisor hypomineralization and dental caries. Int. J. Paediatr. Dent. 2017, 27, 11–21. [Google Scholar] [CrossRef]
- Gurrusquieta, B.J.; Núñez, V.M.; López, M.L. Prevalence of Molar Incisor Hypomineralization in Mexican Children. J. Clin. Pediatr. Dent. 2017, 41, 18–21. [Google Scholar] [CrossRef]
- Hysi, D.; Kuscu, O.O.; Droboniku, E.; Toti, C.; Xhemnica, L.; Caglar, E. Prevalence and aetiology of Molar-Incisor Hypomineralisation among children aged 8-10 years in Tirana, Albania. Eur. J. Paediatr. Dent. 2016, 17, 75–79. [Google Scholar]
- Jälevik, B.; Klingberg, G.; Barregård, L.; Norén, J.G. The prevalence of demarcated opacities in permanent first molars in a group of Swedish children. Acta Odontol. Scand. 2001, 59, 255–260. [Google Scholar] [CrossRef]
- Jeremias, F.; de Souza, J.F.; Silva, C.M.; Cordeiro Rde, C.; Zuanon, A.C.; Santos-Pinto, L. Dental caries experience and Molar-Incisor Hypomineralization. Acta Odontol. Scand. 2013, 71, 870–876. [Google Scholar] [CrossRef]
- Da Costa-Silva, C.M.; Jeremias, F.; de Souza, J.F.; Cordeiro Rde, C.; Santos-Pinto, L.; Zuanon, A.C. Molar incisor hypomineralization: Prevalence, severity and clinical consequences in Brazilian children. Int. J. Paediatr. Dent. 2010, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Crombie, F.; Manton, D.; Kilpatrick, N. Aetiology of molar-incisor hypomineralization: A critical review. Int. J. Paediatr. Dent. 2009, 19, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, F.; Pierri, R.A.; Souza, J.F.; Fragelli, C.M.; Restrepo, M.; Finoti, L.S.; Bussaneli, D.G.; Cordeiro, R.C.; Secolin, R.; Maurer-Morelli, C.V.; et al. Family-Based Genetic Association for Molar-Incisor Hypomineralization. Caries Res. 2016, 50, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussaneli, D.G.; Restrepo, M.; Fragelli, C.M.B.; Santos-Pinto, L.; Jeremias, F.; Cordeiro, R.C.L.; Bezamat, M.; Vieira, A.R.; Scarel-Caminaga, R.M. Genes Regulating Immune Response and Amelogenesis Interact in Increasing the Susceptibility to Molar-Incisor Hypomineralization. Caries Res. 2019, 53, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bravo Pérez, M.; Almerich Silla, J.M.; Ausina Márquez, V.; Avilés Gutiérrez, P.; Blanco González, J.M.; Canorea Díaz, E.; Casals Peidró, E.; Gómez Santos, G.; Hita Iglesias, C.; Llodra Calvo, J.C.; et al. Encuesta de Salud Oral en España 2015. RCOE 2016, 21 (Suppl. 1), 8–48. [Google Scholar]
- Mathu-Muju, K.; Wright, J.T. Diagnosis and treatment of molar incisor hypomineralization. Compend. Contin. Educ. Dent. 2006, 27, 604–610, quiz 611. [Google Scholar]
- Spielman, R.S.; McGinnis, R.E.; Ewens, W.J. Transmission test for linkage disequilibrium: The insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am. J. Hum. Genet. 1993, 52, 506–516. [Google Scholar]
- Vieira, A.R.; Meira, R.; Modesto, A.; Murray, J.C. MSX1, PAX9, and TGFA contribute to tooth agenesis in humans. J. Dent. Res. 2004, 83, 723–727. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, R.J.P.B.; Andrade, N.S.; Queiroz, L.C.C.; Mendes, F.M.; Moura, M.S.; Moura, L.F.A.D.; Lima, M.D.M. Exploring the association between genetic and environmental factors and molar incisor hypomineralization: Evidence from a twin study. Int. J. Paediatr. Dent. 2018, 28, 198–206. [Google Scholar] [CrossRef]
- Bansal, V.; Libiger, O.; Torkamani, A.; Schork, N.J. Statistical analysis strategies for association studies involving rare variants. Nat. Rev. Genet. 2010, 11, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado-Peralvo, A.; Peralvo-García, V.; Torres, A.; Mateos-moreno, V.; Ribas-Pérez, D.; Castano-Séiquer, A. Prevalencia del síndrome de hipomineralización incisivo-molar: Revisión de la literatura. Odontol. Pediátr. 2016, 24, 134–148. [Google Scholar]
- Kevrekidou, A.; Kosma, I.; Arapostathis, K.; Kotsanos, N. Molar incisor hypomineralization of Eight- and 14- year-old children: Prevalence, severity and defect characteristics. Pediatr. Dent. 2015, 37, 455–461. [Google Scholar]
- Jasulaityte, L.; Weerheijm, K.L.; Veerkamp, J.S. Prevalence of molarincisor-hypomineralisation among children participating in the Dutch National Epidemiological Survey (2003). Eur. Arch. Paediatr. Dent. 2008, 9, 218–223. [Google Scholar] [CrossRef]
- Jasulaityte, L.; Veerkamp, J.S.; Weerheijm, K.L. Molar incisor hypomineralizartion: Review and prevalence data from the study of primary school in Kaunas/Lithuania. Eur. Arch. Paediatr. Dent. 2007, 8, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Lygidakis, N.A.; Garot, E.; Somani, C.; Taylor, G.D.; Rouas, P.; Wong, F.S.L. Best clinical practice guidance for clinicians dealing with children presenting with molar-incisor-hypomineralisation (MIH): An updated European Academy of Paediatric Dentistry policy document. Eur. Arch. Paediatr. Dent. 2022, 23, 3–21. [Google Scholar] [CrossRef]
- Silva, M.J.; Scurrah, K.J.; Craig, J.M.; Manton, D.J.; Kilpatrick, N. Etiology of molar incisor hypomineralization—A systematic review. Commun. Dent. Oral Epidemiol. 2016, 44, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Garot, E.; Rouas, P.; Somani, C.; Taylor, G.D.; Wong, F.; Lygidakis, N.A. An update of the aetiological factors involved in molar incisor hypomineralisation (MIH): A systematic review and meta-analysis. Eur. Arch. Paediatr. Dent. 2022, 23, 23–38. [Google Scholar] [CrossRef]
- Nørrisgaard, P.E.; Haubek, D.; Kühnisch, J.; Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Association of High-Dose Vitamin D Supplementation During Pregnancy With the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 924–930. [Google Scholar] [CrossRef]
- Kühnisch, J.; Mach, D.; Thiering, E.; Brockow, I.; Hoffmann, U.; Neumann, C.; Heinrich-Weltzien, R.; Bauer, C.P.; Berdel, D.; von Berg, A.; et al. GINI Plus 10 Study Group. Respiratory diseases are associated with molar-incisor hypomineralizations. Swiss. Dent. J. 2014, 124, 286–293. [Google Scholar]
- Xavier, G.M.; Sharpe, P.T.; Cobourne, M.T. Scube1 is expressed during facial development in the mouse. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312B, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Hočevar, L.; Kovač, J.; Podkrajšek, K.T.; Battelino, S.; Pavlič, A. The possible influence of genetic aetiological factors on molar-incisor hypomineralisation. Arch. Oral Biol. 2020, 118, 104848. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Li, X.; Wang, K.; Tao, Y.; Cui, T.; Xu, Q.; Lin, H. Interactions with the aquaporin 5 gene increase the susceptibility to molar-incisor hypomineralization. Arch. Oral Biol. 2020, 111, 104637. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, D.; Han, T.; Sun, Y.; Zhang, J. TGF-beta1 and TGFBR1 are expressed in ameloblasts and promote MMP20 expression. Anat. Rec. 2009, 292, 885–890. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.J.; Kang, J.; Shin, T.J.; Hyun, H.K.; Lee, S.H.; Lee, Z.H.; Kim, J.W. A novel AMELX mutation causes hypoplastic amelogenesis imperfecta. Arch. Oral Biol. 2017, 76, 61–65. [Google Scholar] [CrossRef]
- Laird, N.M.; Horvath, S.; Xu, X. Implementing a unified approach to family-based tests of association. Genet. Epidemiol. 2000, 19 (Suppl. 1), S36–S42. [Google Scholar] [CrossRef]
- Preusser, S.E.; Ferring, V.; Wleklinski, C.; Wetzel, W.E. Prevalence and severity of molar incisor hypomineralization in a region of Germany—A brief communication. J. Public Health Dent. 2007, 67, 148–150. [Google Scholar] [CrossRef]
- Sahlberg, C.; Pavlic, A.; Ess, A.; Lukinmaa, P.L.; Salmela, E.; Alaluusua, S. Combined effect of amoxicillin and sodium fluoride on the structure of developing mouse enamel in vitro. Arch. Oral Biol. 2013, 58, 1155–1164. [Google Scholar] [CrossRef]
- Weerheijm, K.L.; Groen, H.J.; Beentjes, V.E.; Poorterman, J.H. Prevalence of cheese molars in eleven-year-old Dutch children. ASDC J. Dent. Child. 2001, 68, 259–262, 229. [Google Scholar]
- Ott, J.; Kamatani, Y.; Lathrop, M. Family-based designs for genome-wide association studies. Nat. Rev. Genet. 2011, 12, 465–474. [Google Scholar] [CrossRef]
| Frequency | Percentage | ||
|---|---|---|---|
| Sex | Male | 28 | 56% |
| Female | 22 | 44% | |
| MIH Severity | Mild | 10 | 20% |
| Moderate | 11 | 22% | |
| Severe | 29 | 58% |
| Logistic Regression AA vs. AG + GG Sex Value | Logistic Regression GG vs. AG + AA Sex Value |
|---|---|
| Step 0 Sig. 0.000 | Step 0 Sig. 0.000 |
| Step 1 Sig. 0.001 | Step 1 Sig. 0.000 |
| Step 2 Sig. 0.001 | Step 2 Sig. 0.000 |
| Step 3 Sig. 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgina-Pérez, L.; Ribas-Pérez, D.; Dehesa-Santos, A.; Mendoza-Mendoza, A. Relationship between the TGFBR1 Gene and Molar Incisor Hypomineralization. J. Pers. Med. 2023, 13, 777. https://doi.org/10.3390/jpm13050777
Georgina-Pérez L, Ribas-Pérez D, Dehesa-Santos A, Mendoza-Mendoza A. Relationship between the TGFBR1 Gene and Molar Incisor Hypomineralization. Journal of Personalized Medicine. 2023; 13(5):777. https://doi.org/10.3390/jpm13050777
Chicago/Turabian StyleGeorgina-Pérez, Laura, David Ribas-Pérez, Alexandra Dehesa-Santos, and Asunción Mendoza-Mendoza. 2023. "Relationship between the TGFBR1 Gene and Molar Incisor Hypomineralization" Journal of Personalized Medicine 13, no. 5: 777. https://doi.org/10.3390/jpm13050777






