Low Lung Function Is Associated with Low Baseline Calcaneus Ultrasound T-Score but a Slow Decline in T-Score in a Taiwanese Follow-Up Population with No History of Smoking, Bronchitis, Emphysema, or Asthma
Abstract
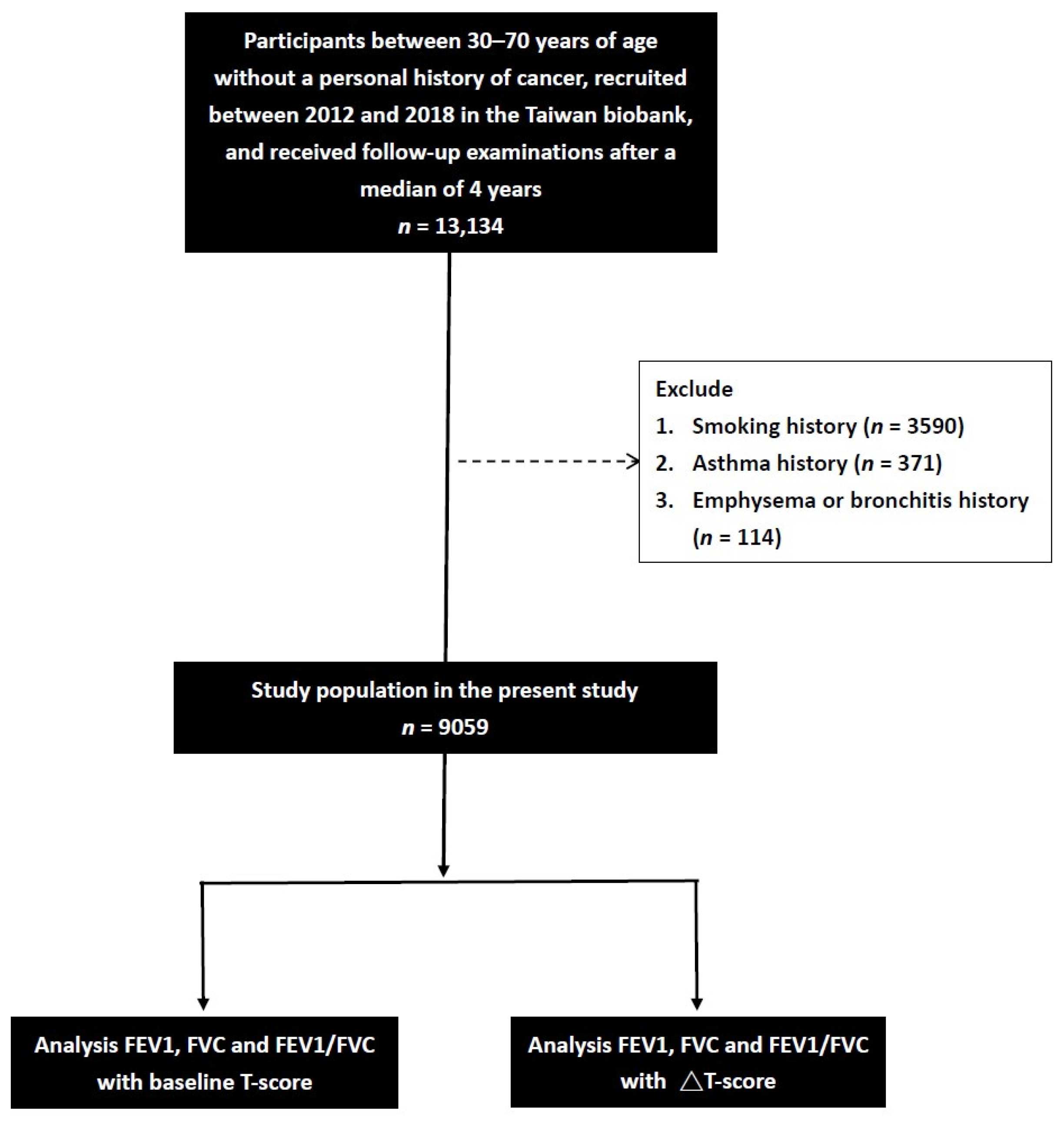
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Taiwan Biobank
2.3. Laboratory, Medical, and Demographic Variables
2.4. Assessment of Cigarette Smoking History
2.5. Calcaneus Ultrasound T-Score Measurements
2.6. Spirometry Measurements
2.7. Statistical Analysis
3. Results
3.1. Comparisons of Clinical Characteristics between the Baseline T-Score Groups
3.2. Determinants of Baseline Calcaneus Ultrasound T-Score in Univariable Linear Regression Analysis
3.3. Determinants of Calcaneus Ultrasound ΔT-Score ≤ −3 in Univariable Binary Logistic Regression Analysis
3.4. Associations of FEV1, FVC, and FEV1/FVC with Calcaneus Ultrasound ΔT-Score ≤ −3 in Multivariable Binary Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ganesan, K.; Jandu, J.S.; Anastasopoulou, C.; Ahsun, S.; Roane, D. Secondary Osteoporosis. In StatPearls; Treasure Island (FL): Tampa, FL, USA, 2022. [Google Scholar]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. WHO Study Group. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Carmona, R.H. Surgeon General reports on bone health. J. Calif. Dent. Assoc. 2005, 33, 9–11. [Google Scholar] [CrossRef]
- Lane, N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef]
- World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases. Available online: https://www.who.int/publications/i/item/global-surveillance-prevention-and-control-of-chronic-respiratory-diseases (accessed on 18 September 2022).
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 18 September 2022).
- Taiwan MoHaWo. 2021 National Death Statistics Results. Available online: https://www.mohw.gov.tw/cp-16-70314-1.html (accessed on 18 September 2022).
- Shahriary, A.; Panahi, Y.; Shirali, S.; Rahmani, H. Relationship of serum levels of interleukin 6, interleukin 8, and C-reactive protein with forced expiratory volume in first second in patients with mustard lung and chronic obstructive pulmonary diseases: Systematic review and meta-analysis. Postep. Derm. Alergol 2017, 34, 192–198. [Google Scholar] [CrossRef]
- Agusti, A.G. COPD, a multicomponent disease: Implications for management. Respir. Med. 2005, 99, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp. Med. 2018, 16, 4553–4561. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, J.H.; Chiang, C.W.K.; Hsiung, C.N.; Wu, P.E.; Chang, L.C.; Chu, H.W.; Chang, J.; Song, I.W.; Yang, S.L.; et al. Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Hum. Mol. Genet. 2016, 25, 5321–5331. [Google Scholar] [CrossRef]
- Fan, C.T.; Hung, T.H.; Yeh, C.K. Taiwan Regulation of Biobanks. J. Law Med. Ethics A J. Am. Soc. Law Med. Ethics 2015, 43, 816–826. [Google Scholar] [CrossRef]
- Ho, T.W.; Tsai, H.H.; Lai, J.F.; Chu, S.M.; Liao, W.C.; Chiu, H.M. Physical fitness cognition, assessment, and promotion: A cross-sectional study in Taiwan. PLoS ONE 2020, e0240137. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Al-Bashaireh, A.M.; Haddad, L.G.; Weaver, M.; Chengguo, X.; Kelly, D.L.; Yoon, S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J. Osteoporos. 2018, 2018, 1206235. [Google Scholar] [CrossRef]
- Wilson-Barnes, S.L.; Lanham-New, S.A.; Lambert, H. Modifiable risk factors for bone health & fragility fractures. Best Pr. Res. Clin. Rheumatol. 2022, 36, 101758. [Google Scholar] [CrossRef]
- Hlebichuk, J.L.; Gretebeck, R.J.; Garnier-Villarreal, M.; Piacentine, L.B.; Singh, M.; Gretebeck, K.A. Physical Activity, Inflammation, and Physical Function in Older Adults: Results From the Health & Retirement Study. Biol. Res. Nurs. 2023, 25, 24–32. [Google Scholar] [CrossRef]
- Lin, C.W.; Chen, Y.Y.; Chen, Y.J.; Liang, C.Y.; Lin, M.S.; Chen, W. Prevalence, risk factors, and health-related quality of life of osteoporosis in patients with COPD at a community hospital in Taiwan. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 1493–1500. [Google Scholar] [CrossRef]
- Xiong, Z.; Leme, A.S.; Ray, P.; Shapiro, S.D.; Lee, J.S. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-alpha and IL-6 and promote cigarette smoke-induced emphysema. J. Immunol. 2011, 186, 3206–3214. [Google Scholar] [CrossRef]
- Vitenberga, Z.; Pilmane, M.; Babjoniseva, A. The evaluation of inflammatory, anti-inflammatory and regulatory factors contributing to the pathogenesis of COPD in airways. Pathol. Res. Pr. 2019, 215, 97–105. [Google Scholar] [CrossRef]
- Ugay, L.; Kochetkova, E.; Nevzorova, V.; Maistrovskaia, Y. Role of Osteoprotegerin and Receptor Activator of Nuclear Factor-kappaB Ligand in Bone Loss Related to Advanced Chronic Obstructive Pulmonary Disease. Chin. Med. J. 2016, 129, 1696–1703. [Google Scholar] [CrossRef]
- Li, Y.; Gao, H.; Zhao, L.; Wang, J. Osteoporosis in COPD patients: Risk factors and pulmonary rehabilitation. Clin. Respir. J. 2022, 16, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhu, B.; Xiao, C.; Zheng, Z. Vitamin D deficiency is associated with the severity of COPD: A systematic review and meta-analysis. Int. J. Chron. Obs. Pulmon. Dis. 2015, 10, 1907–1916. [Google Scholar] [CrossRef]
- Persson, L.J.; Aanerud, M.; Hiemstra, P.S.; Hardie, J.A.; Bakke, P.S.; Eagan, T.M. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS ONE 2012, 7, e38934. [Google Scholar] [CrossRef] [PubMed]
- Forli, L.; Halse, J.; Haug, E.; Bjortuft, O.; Vatn, M.; Kofstad, J.; Boe, J. Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J. Intern. Med. 2004, 256, 56–62. [Google Scholar] [CrossRef]
- Brincat, M.; Gambin, J.; Brincat, M.; Calleja-Agius, J. The role of vitamin D in osteoporosis. Maturitas 2015, 80, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr. Osteoporos. Rep. 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Caramori, G.; Ruggeri, P.; Arpinelli, F.; Salvi, L.; Girbino, G. Long-term use of inhaled glucocorticoids in patients with stable chronic obstructive pulmonary disease and risk of bone fractures: A narrative review of the literature. Int. J. Chron. Obs. Pulmon. Dis. 2019, 14, 1085–1097. [Google Scholar] [CrossRef]
- Gurevitch, O.; Slavin, S. The hematological etiology of osteoporosis. Med. Hypotheses. 2006, 67, 729–735. [Google Scholar] [CrossRef]
- Fujimoto, H.; Fujimoto, K.; Ueda, A.; Ohata, M. Hypoxemia is a risk factor for bone mass loss. J. Bone Min. Metab. 1999, 17, 211–216. [Google Scholar] [CrossRef]
- Jorgensen, C.; Khoury, M. Musculoskeletal Progenitor/Stromal Cell-Derived Mitochondria Modulate Cell Differentiation and Therapeutical Function. Front. Immunol. 2021, 12, 606781. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Zhou, Z.; Wu, C.; Xing, Y.; Zou, X.; Tian, W.; Zhang, C. HIF-1alpha inhibits Wnt signaling pathway by activating Sost expression in osteoblasts. PLoS ONE 2013, 8, e65940. [Google Scholar] [CrossRef]
- Gorissen, B.; de Bruin, A.; Miranda-Bedate, A.; Korthagen, N.; Wolschrijn, C.; de Vries, T.J.; van Weeren, R.; Tryfonidou, M.A. Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis. J. Cell. Physiol. 2018, 234, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Graat-Verboom, L.; Wouters, E.F.; Smeenk, F.W.; van den Borne, B.E.; Lunde, R.; Spruit, M.A. Current status of research on osteoporosis in COPD: A systematic review. Eur. Respir. J. 2009, 34, 209–218. [Google Scholar] [CrossRef]
- King, P.T. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin. Transl. Med. 2015, 4, 68. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.; Scott, A.; Newton Ede, M.; Jones, S.W. The impact of E-cigarette vaping and vapour constituents on bone health. J. Inflamm. 2021, 18, 16. [Google Scholar] [CrossRef]
- Sritharan, S.S.; Ostergaard, E.B.; Callesen, J.; Elkjaer, M.; Sand, L.; Hilberg, O.; Skaarup, S.H.; Lokke, A. Barriers toward Physical Activity in COPD: A Quantitative Cross-Sectional, Questionnaire-Based Study. COPD 2021, 18, 272–280. [Google Scholar] [CrossRef]
- McEvoy, C.E.; Ensrud, K.E.; Bender, E.; Genant, H.K.; Yu, W.; Griffith, J.M.; Niewoehner, D.E. Association between corticosteroid use and vertebral fractures in older men with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 704–709. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological Reaction in TNF-α-Mediated Osteoclast Formation and Bone Resorption In Vitro and In Vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef]
- Khosla, S. Minireview: The OPG/RANKL/RANK system. Endocrinology 2001, 142, 5050–5055. [Google Scholar] [CrossRef]
- Liang, B.; Feng, Y. The association of low bone mineral density with systemic inflammation in clinically stable COPD. Endocrine 2012, 42, 190–195. [Google Scholar] [CrossRef]
- Bai, P.; Sun, Y.; Jin, J.; Hou, J.; Li, R.; Zhang, Q.; Wang, Y. Disturbance of the OPG/RANK/RANKL pathway and systemic inflammation in COPD patients with emphysema and osteoporosis. Respir. Res. 2011, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.J.; Simon, M.C.; Johnson, R. The biology of hypoxia: The role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004, 18, 2183–2194. [Google Scholar] [CrossRef]
- Ramachandran, K.; Mani, S.K.; Gopal, G.K.; Rangasami, S. Prevalence of Bone Mineral Density Abnormalities and Factors Affecting Bone Density in Patients with Chronic Obstructive Pulmonary Disease in a Tertiary Care Hospital in Southern India. J. Clin. Diagn. Res. 2016, 10, OC32–OC34. [Google Scholar] [CrossRef] [PubMed]
- Dery, M.A.; Michaud, M.D.; Richard, D.E. Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell. Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Klimova, T.; Chandel, N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008, 15, 660–666. [Google Scholar] [CrossRef]
- Hu, C.J.; Wang, L.Y.; Chodosh, L.A.; Keith, B.; Simon, M.C. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 2003, 23, 9361–9374. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Kaluz, S.; Kaluzova, M.; Stanbridge, E.J. Regulation of gene expression by hypoxia: Integration of the HIF-transduced hypoxic signal at the hypoxia-responsive element. Clin. Chim. Acta. 2008, 395, 6–13. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.S.; Rameshwar, P.; Chang, V.; Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 2002, 99, 394. [Google Scholar] [CrossRef] [PubMed]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Shomento, S.H.; Wan, C.; Cao, X.; Faugere, M.C.; Bouxsein, M.L.; Clemens, T.L.; Riddle, R.C. Hypoxia-inducible factors 1alpha and 2alpha exert both distinct and overlapping functions in long bone development. J. Cell. Biochem. 2010, 109, 196–204. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, K.H.; Yu, H.G.; Kook, E.; Song, W.H.; Lee, G.; Koh, J.T.; Shin, H.I.; Choi, J.Y.; Huh, Y.H.; et al. Controlling hypoxia-inducible factor-2alpha is critical for maintaining bone homeostasis in mice. Bone Res. 2019, 7, 14. [Google Scholar] [CrossRef]
- Jin, N.; Lin, S.; Zhang, Y.; Chen, F. Assess the discrimination of Achilles InSight calcaneus quantitative ultrasound device for osteoporosis in Chinese women: Compared with dual energy X-ray absorptiometry measurements. Eur. J. Radiol. 2010, 76, 265–268. [Google Scholar] [CrossRef]
- Yen, C.C.; Lin, W.C.; Wang, T.H.; Chen, G.F.; Chou, D.Y.; Lin, D.M.; Lin, S.Y.; Chan, M.H.; Wu, J.M.; Tseng, C.D.; et al. Pre-screening for osteoporosis with calcaneus quantitative ultrasound and dual-energy X-ray absorptiometry bone density. Sci. Rep. 2021, 11, 15709. [Google Scholar] [CrossRef]
| Characteristics | T-Score ≥ −2.5 (n = 8501) | T-Score < −2.5 (n = 558) | p |
|---|---|---|---|
| Age (year) | 50.6 ± 10.2 | 58.2 ± 7.5 | <0.001 |
| Male gender (%) | 20.0 | 21.0 | 0.565 |
| DM (%) | 4.3 | 4.7 | 0.650 |
| Hypertension (%) | 11.1 | 14.3 | 0.018 |
| BMI (kg/m2) | 23.7 ± 3.4 | 23.1 ± 3.4 | <0.001 |
| Regular exercise habit (%) | 48.8 | 58.2 | <0.001 |
| Menopause in female (%) | 53.5 | 90.0 | <0.001 |
| T-score | −0.16 ± 1.52 | −3.09 ± 0.53 | <0.001 |
| ΔT-score | −0.35 ± 1.00 | 0.25 ± 1.31 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (mg/dL) | 94.6 ± 17.3 | 96.5 ± 20.2 | 0.013 |
| Hemoglobin (g/dL) | 13.4 ± 1.5 | 13.5 ± 1.3 | 0.097 |
| Triglyceride (mg/dL) | 106.2 ± 73.5 | 102.7 ± 60.6 | 0.270 |
| Total cholesterol (mg/dL) | 195.8 ± 35.2 | 202.3 ± 34.9 | <0.001 |
| HDL cholesterol (mg/dL) | 56.0 ± 13.0 | 57.4 ± 13.8 | 0.017 |
| LDL cholesterol (mg/dL) | 121.3 ± 31.2 | 124.9 ± 30.0 | 0.007 |
| eGFR (mL/min/1.73 m2) | 111.0 ± 25.1 | 109.8 ± 27.5 | 0.292 |
| Uric acid (mg/dL) | 5.2 ± 1.3 | 5.2 ± 1.3 | 0.284 |
| Lung function | |||
| FEV1 (L) | 1.95 ± 0.74 | 1.76 ± 0.71 | <0.001 |
| FVC (L) | 2.66 ± 0.71 | 2.41 ± 0.72 | <0.001 |
| FEV1/FVC (%) | 72.8 ± 18.4 | 72.7 ± 18.3 | 0.936 |
| Parameters | Baseline T-Score | ||
|---|---|---|---|
| Univariable | |||
| Unstandardized Coefficient β | 95% CI | p | |
| Age (per 1 year) | −0.059 | −0.062, −0.056 | <0.001 |
| Male (vs. female) | −0.262 | −0.346, −0.178 | <0.001 |
| DM | −0.289 | −0.455, −0.1123 | 0.001 |
| Hypertension | −0.477 | −0.583, −0.371 | <0.001 |
| Height (per 1 cm) | 0.014 | 0.010, 0.019 | <0.001 |
| BMI (per 1 kg/m2) | 0.031 | 0.021, 0.041 | <0.001 |
| Regular exercise habits | −0.273 | −0.340, −0.206 | <0.001 |
| Menopause in female | −1.390 | −1.461, −1.319 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (per 1 mg/dL) | −0.007 | −0.009, −0.005 | <0.001 |
| Hemoglobin (per 1 g/dL) | −0.073 | −0.096, −0.050 | <0.001 |
| Triglyceride (per 1 mg/dL) | −0.001 | −0.002, −0.001 | <0.001 |
| Total cholesterol (per 1 mg/dL) | −0.004 | −0.005, −0.003 | <0.001 |
| HDL cholesterol (per 1 mg/dL) | 0.001 | −0.001, 0.004 | 0.369 |
| LDL cholesterol (per 1 mg/dL) | −0.003 | −0.004, −0.002 | <0.001 |
| eGFR (per 1 mL/min/1.73 m2) | 0.004 | 0.003, 0.006 | <0.001 |
| Uric acid (per 1 mg/dL) | −0.054 | −0.080, −0.028 | <0.001 |
| Lung function | |||
| FEV1 (per 1 L) | 0.288 | 0.243, 0.333 | <0.001 |
| FVC (per 1 L) | 0.337 | 0.290, 0.383 | <0.001 |
| FEV1/FVC (per 1%) | 0.003 | 0.002, 0.005 | <0.001 |
| Lung Function | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | Unstandardized Coefficient β (95% CI) | p | Unstandardized Coefficient β (95% CI) | p | |
| FEV1 (per L) | 0.127 (0.075, 0.180) | <0.001 | - | - | - | - |
| FVC (per 1 L) | - | - | 0.203 (0.128, 0.278) | <0.001 | - | - |
| FEV1/FVC (per 1%) | - | - | - | - | 0.002 (0, 0.004) | 0.013 |
| Parameters | ΔT-Score ≤ −3 | ||
|---|---|---|---|
| Univariable | |||
| Odds Ratio | 95% CI | p | |
| Age (per 1 year) | 1.012 | 1.007–1.016 | <0.001 |
| Male (vs. female) | 0.703 | 0.634–0.780 | <0.001 |
| DM | 1.143 | 0.932–1.403 | 0.199 |
| Hypertension | 0.937 | 0.823–1.068 | 0.328 |
| Height (per 1 cm) | 0.989 | 0.983–0.994 | <0.001 |
| BMI (per 1 kg/m2) | 0.981 | 0.969–0.993 | 0.001 |
| Regular exercise habits | 1.097 | 1.010–1.191 | 0.028 |
| Menopause in female | 1.273 | 1.160–1.397 | <0.001 |
| Laboratory parameters | |||
| Fasting glucose (per 1 mg/dL) | 1.001 | 0.999–1.004 | 0.217 |
| Hemoglobin (per 1 g/dL) | 0.992 | 0.964–1.020 | 0.566 |
| Triglyceride (per 1 mg/dL) | 1.000 | 0.999–1.000 | 0.735 |
| Total cholesterol (per 1 mg/dL) | 1.003 | 1.001–1.004 | <0.001 |
| HDL cholesterol (per 1 mg/dL) | 1.010 | 1.007–1.014 | <0.001 |
| LDL cholesterol (per 1 mg/dL) | 1.001 | 1.000–1.003 | 0.036 |
| eGFR (per 1 mL/min/1.73 m2) | 1.000 | 0.999–1.002 | 0.584 |
| Uric acid (per 1 mg/dL) | 0.977 | 0.947–1.008 | 0.149 |
| Lung function | |||
| FEV1 (per 1 L) | 0.960 | 0.907–1.015 | 0.149 |
| FVC (per 1 L) | 0.871 | 0.822–0.923 | <0.001 |
| FEV1/FVC (per 1%) | 1.003 | 1.001–1.006 | 0.003 |
| Lung Function | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| FEV1 (per 1 L) | 1.146 (1.067–1.229) | 0.001 | - | - | - | - |
| FVC (per 1 L) | - | - | 1.110 (1.004–1.228) | 0.042 | - | - |
| FEV1/FVC (per 1%) | - | - | - | - | 1.004 (1.002–1.006) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-L.; Wang, H.-P.; Wu, D.-W.; Huang, J.-C.; Wu, P.-Y.; Chen, S.-C. Low Lung Function Is Associated with Low Baseline Calcaneus Ultrasound T-Score but a Slow Decline in T-Score in a Taiwanese Follow-Up Population with No History of Smoking, Bronchitis, Emphysema, or Asthma. J. Pers. Med. 2023, 13, 795. https://doi.org/10.3390/jpm13050795
Tsai Y-L, Wang H-P, Wu D-W, Huang J-C, Wu P-Y, Chen S-C. Low Lung Function Is Associated with Low Baseline Calcaneus Ultrasound T-Score but a Slow Decline in T-Score in a Taiwanese Follow-Up Population with No History of Smoking, Bronchitis, Emphysema, or Asthma. Journal of Personalized Medicine. 2023; 13(5):795. https://doi.org/10.3390/jpm13050795
Chicago/Turabian StyleTsai, Yu-Lin, Hao-Ping Wang, Da-Wei Wu, Jiun-Chi Huang, Pei-Yu Wu, and Szu-Chia Chen. 2023. "Low Lung Function Is Associated with Low Baseline Calcaneus Ultrasound T-Score but a Slow Decline in T-Score in a Taiwanese Follow-Up Population with No History of Smoking, Bronchitis, Emphysema, or Asthma" Journal of Personalized Medicine 13, no. 5: 795. https://doi.org/10.3390/jpm13050795





