Development of Predictive Models for the Response of Vestibular Schwannoma Treated with Cyberknife®: A Feasibility Study Based on Radiomics and Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Images Acquisition
2.3. Radiosurgical Treatment
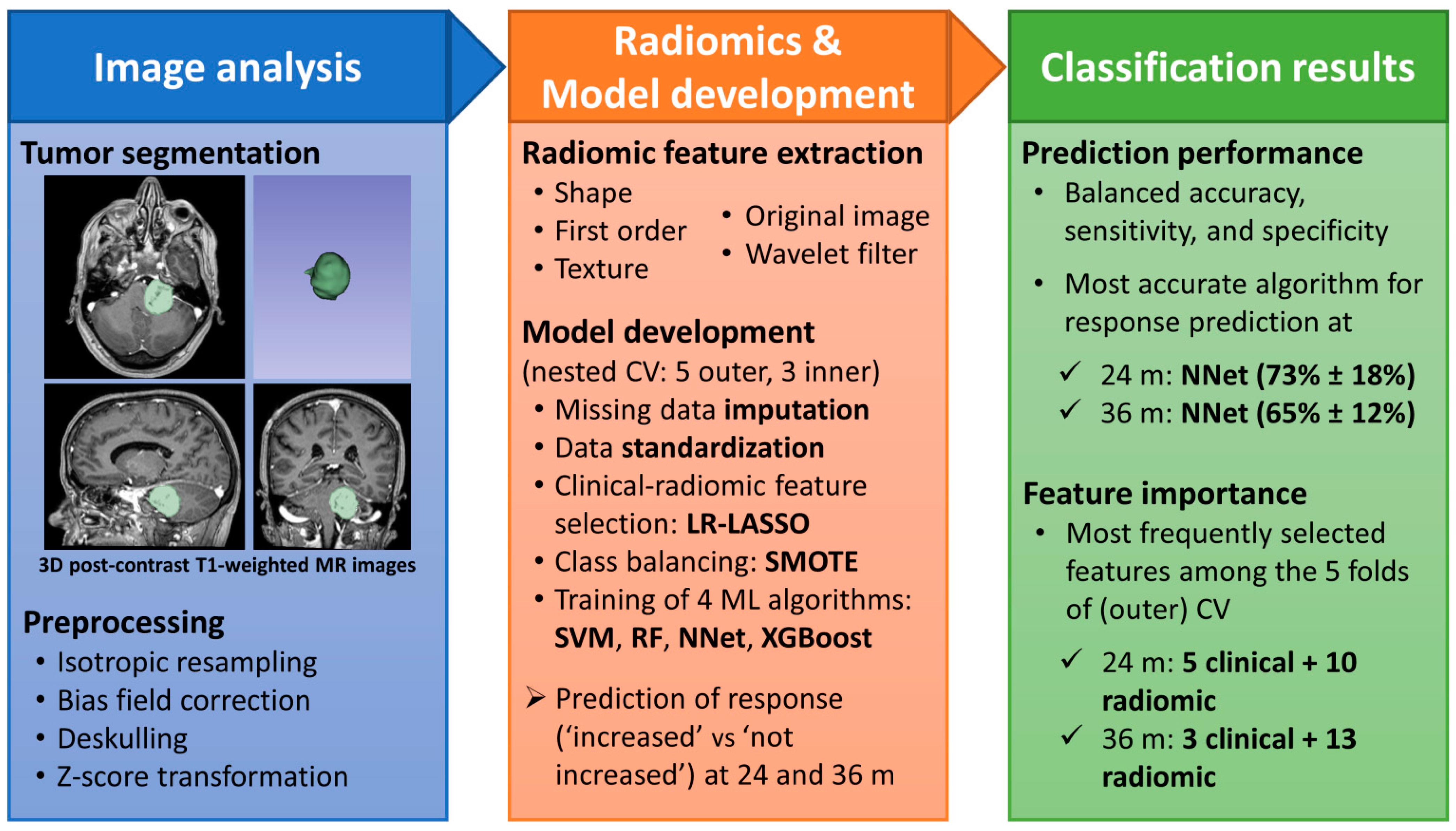
2.4. Segmentation
2.5. Image Pre-Processing
2.6. Features Extraction
2.7. Feature Selection, Model Development and Test
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balossier, A.; Tuleasca, C.; Delsanti, C.; Troude, L.; Thomassin, J.M.; Roche, P.U.; Régis, J. Long-term hearing outcome after radiosurgery for vestibular schwannoma: A systematic review and meta-analysis. Neurosurgery 2023, 3, e002354. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Weller, M.; Regis, J.; Lund-Johansen, M.; Stavrinou, P.; Reuss, D.; Evans, D.G.; Lefranc, F.; Sallabanda, K.; Falini, A.; et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-Oncology 2020, 22, 31–45. [Google Scholar] [CrossRef]
- Forgues, M.; Mehta, R.; Anderson, D.; Morel, C.; Miller, L.; Sevy, A.; Son, L.; Arriaga, M. Non-contrast magnetic resonance imaging for monitoring patients with acoustic neuroma. J. Laryngol. Otol. 2018, 132, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Wolbers, J.G.; Dallenga, A.H.; Romero, A.M.; van Linge, A. What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open 2013, 3, e001345. [Google Scholar] [CrossRef] [PubMed]
- Linkov, F.; Valappil, B.; McAfee, J.; Goughnour, S.L.; Hildrew, D.M.; McCall, A.A.; Linkov, I.; Hirsch, B.; Snyderman, C. Development of an evidence-based decision pathway for vestibular schwannoma treatment options. Am. J. Otolaryngol. 2017, 38, 57–64. [Google Scholar] [CrossRef]
- Rueß, D.; Pöhlmann, L.; Hellerbach, A.; Hamisch, C.; Hoevels, M.; Treuer, H.; Grau, S.; Jablonska, K.; Kocher, M.; Ruge, M.I. (Acoustic neuroma treated with stereotactic radiosurgery: Follow-up of 335 patients. World Neurosurg. 2018, 116, e194–e202. [Google Scholar] [CrossRef] [PubMed]
- Buss, E.J.; Wang, T.J.; Sisti, M.B. Stereotactic radiosurgery for management of vestibular schwannoma: A short review. Neurosurg. Rev. 2021, 44, 901–904. [Google Scholar] [CrossRef]
- Boari, N.; Bailo, M.; Gagliardi, F.; Franzin, A.; Gemma, M.; del Vecchio, A.; Bolognesi, A.; Picozzi, P.; Mortini, P. Gamma Knife radiosurgery for vestibular schwannoma: Clinical results at long-term follow-up in a series of 379 patients. J. Neurosurg. 2014, 121 (Suppl. S2), 123–142. [Google Scholar] [CrossRef]
- Przybylowski, C.J.; Baranoski, J.F.; Paisan, G.M.; Chapple, K.M.; Meeusen, A.J.; Sorensen, S.; Almefty, K.K.; Porter, R.W. CyberKnife radiosurgery for acoustic neuromas: Tumor control and clinical outcomes. J. Clin. Neurosci. 2019, 63, 72–76. [Google Scholar] [CrossRef]
- Timmer, F.C.; van Haren, A.E.; Mulder, J.J.; Hanssens, P.E.; van Overbeeke, J.J.; Cremers, C.W.; Graamans, K. Quality of life after gamma knife radiosurgery treatment in patients with a vestibular schwannoma: The patient’s perspective. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 867–873. [Google Scholar] [CrossRef]
- Di Maio, S.; Akagami, R. Prospective comparison of quality of life before and after observation, radiation, or surgery for vestibular schwannomas. J. Neurosurg. 2009, 111, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Troude, L.; Boucekine, M.; Balossier, A.; Baucher, G.; Lavieille, J.P.; Régis, J.D.; Roche, P.H. Is salvage surgery for large vestibular schwannomas after failed gamma knife radiosurgery more challenging? Neurosurg. Rev. 2022, 45, 751–761. [Google Scholar] [CrossRef]
- Lohmann, P.; Galldiks, N.; Kocher, M.; Heinzel, A.; Filss, C.P.; Stegmayr, C.; Mottaghy, F.M.; Fink, G.R.; Jon Shah, N.; Langen, K.J. Radiomics in neuro-oncology: Basics, workflow, and applications. Methods 2021, 188, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Abdel Razek AA, K.; Alksas, A.; Shehata, M.; AbdelKhalek, A.; Abdel Baky, K.; El-Baz, A.; Helmy, E. Clinical applications of artificial intelligence and radiomics in neuro-oncology imaging. Insights Into Imaging 2021, 12, 152. [Google Scholar] [CrossRef]
- Kocher, M.; Ruge, M.I.; Galldiks, N.; Lohmann, P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther. Und Onkol. 2020, 196, 856–867. [Google Scholar] [CrossRef]
- Langenhuizen, P.P.; Zinger, S.; Leenstra, S.; Kunst, H.P.; Mulder, J.J.; Hanssens, P.E.; Verheul, J.B. Radiomics-based prediction of long-term treatment response of vestibular schwannomas following stereotactic radiosurgery. Otol. Neurotol. 2020, 41, e1321–e1327. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Lee, W.K.; Wu, C.C.; Lu, C.F.; Yang, H.C.; Chen, Y.W.; Chung, W.Y.; Hu, Y.S.; Wu, H.M.; Wu, Y.T.; et al. Applying artificial intelligence to longitudinal imaging analysis of vestibular schwannoma following radiosurgery. Sci. Rep. 2021, 11, 3106. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Wu, C.C.; Lee, C.C.; Huang, H.E.; Lee, W.K.; Chung, W.Y.; Wu, H.M.; Guo, W.Y.; Wu, Y.T.; Lu, C.F. Prediction of pseudoprogression and long-term outcome of vestibular schwannoma after Gamma Knife radiosurgery based on preradiosurgical MR radiomics. Radiother. Oncol. 2021, 155, 123–130. [Google Scholar] [CrossRef]
- Narayanasamy, G.; Zhang, G.; Siegel, E.; Campbell, G.; Moros, E.G.; Galhardo, E.P.; Morrill, S.; Day, J.; Penagaricano, J. Radiomic assessment of the progression of acoustic neuroma after gamma knife stereotactic radiosurgery. J. Solid Tumors 2019, 9, 1. [Google Scholar] [CrossRef]
- Sardanelli, F.; Alì, M.; Hunink, M.G.; Houssami, N.; Sconfienza, L.M.; Di Leo, G. To share or not to share? Expected pros and cons of data sharing in radiological research. Eur. Radiol. 2018, 28, 2328–2335. [Google Scholar] [CrossRef]
- Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol. Head Neck Surg. 1995, 113, 179–180. [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Juntu, J.; Sijbers, J.; Dyck, D.V.; Gielen, J. Bias field correction for MRI images. In Computer Recognition Systems; Springer: Berlin/Heidelberg, Germany, 2005; pp. 543–551. [Google Scholar]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Soda, P. A multi-objective optimisation approach for class imbalance learning. Pattern Recognit. 2011, 44, 1801–1810. [Google Scholar] [CrossRef]
- Huang, P.; Liu, X.; Huang, Y. Data Augmentation for Medical MR Image Using Generative Adversarial Networks. arXiv 2021, arXiv:2111.14297. [Google Scholar]
- Zhang, Z.; Yang, J.; Ho, A.; Jiang, W.; Logan, J.; Wang, X.; Brown, P.D.; McGovern, S.L.; Guha-Thakurta, N.; Ferguson, S.D.; et al. A predictive model for distinguishing radiation necrosis from tumour progression after gamma knife radiosurgery based on radiomic features from MR images. Eur. Radiol. 2018, 28, 2255–2263. [Google Scholar] [CrossRef]
| Variable | Value (No. or Mean) | |
|---|---|---|
| Age, mean (range) | 61 years (34–83) | |
| Sex | Female | 62 |
| Male | 46 | |
| Symptoms | 77 | |
| Chronic diseases | 41 | |
| 5th or 7th cranial nerve deficit | 16 | |
| Audiometric data | A | 1 |
| B | 13 | |
| C | 45 | |
| D | 20 | |
| Previous surgery | 19 | |
| Intra- or extra-canalicular disease | Intra | 17 |
| Extra | 13 | |
| Intra and Extra | 78 | |
| Tumor volume, mean (range) mm3 | 3108 (154–16,239) | |
| Tumor laterality | Left | 52 |
| Right | 56 | |
| Characteristics | Magnetic Resonance System | ||
|---|---|---|---|
| CDI Centro Diagnostico Italiano | IRCCS Istituto Carlo Besta | ||
| GE Signa Excite 1.5 T | Philips Achieva 1.5 T | Philips Achieva 1.5 T | |
| Pulse sequence | T1-w 3D | ST1w-3D-Iso sense | T1 3D TFE mdc |
| TR [ms] | 15.27 | 25 | 7.16 |
| TE [ms] | 6.93 | 4.5 | 3.21 |
| Slice thickness [mm] | 1 | 1 | 1 |
| Slice spacing | 0 | 0 | 0 |
| Pixel spacing [mm/mm] | 0.97/0.97 | 1/1 | 1/1 |
| Feature Type | Most Frequently Selected Features for Predicting Response to Treatment: | |||
|---|---|---|---|---|
| at 24 Months | at 36 Months | |||
| Name | Frequency | Name | Frequency | |
| Clinical | Age Laterality right Laterality left Total dose Deficit 5–7th Extra-intra canalicular | 5 5 4 2 2 2 | Isodose Deficit 5–7th Age | 3 3 2 |
| Radiomic Shape | - | - | Flatness | 3 |
| Radiomic First order | HHL-Median HHH-Median LHL-Minimum LLH-Energy | 4 2 2 2 | LHL-Skewness HHL-Maximum LLH-Energy HLH-Kurtosis LLH-90Percentile | 4 3 2 2 2 |
| Radiomic Texture | HHL-GLSZM- SmallAreaHighGrayLevel-Emphasis HLH-GLSZM- HighGrayLevelZoneEmphasis HHL-GLSZM- SmallAreaLowGrayLevel-Emphasis HHH-GLSZM-ZoneEntropy Original-GLCM-MCC HHL-GLCM-MCC | 4 3 2 2 2 2 | HHH-GLSZM-ZoneEntropy HLH-GLRLM-RunEntropy HHL-GLCM-ClusterShade HHL-GLRLM-ShortRunHigh- GrayLevelEmphasis HHL-GLSZM-SmallAreaHigh- GrayLevelEmphasis HHH-GLSZM-SmallArea-Emphasis HHH-GLCM-MCC | 3 3 3 2 2 2 2 |
| Time Point | Classification Metric | Machine Learning Algorithms | |||
|---|---|---|---|---|---|
| SVM | RF | NNet | XGBoost | ||
| 24 months | Balanced Accuracy % | 56.5 ± 15.9 | 55.3 ± 14.1 | 72.6± 17.7 | 57.4 ± 22.2 |
| Sensitivity % | 20.0 ± 27.4 | 20.0 ± 27.4 | 60.0 ± 41.8 | 30.0 ± 44.7 | |
| Specificity % | 92.9 ± 7.7 | 90.6 ± 3.2 | 84.7 ± 12.2 | 84.7 ± 12.2 | |
| 36 months | Balanced Accuracy % | 52.4 ± 13.2 | 56.9 ± 17.8 | 64.9± 11.8 | 51.7 ± 10.5 |
| Sensitivity % | 13.3 ± 18.3 | 20.0 ± 29.8 | 46.7 ± 27.4 | 16.7 ± 23.6 | |
| Specificity % | 91.5 ± 09.2 | 93.9 ± 8.7 | 83.2 ± 9.4 | 86.7 ± 13.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossi Zanetti, I.; De Martin, E.; Pascuzzo, R.; D’Amico, N.C.; Morlino, S.; Cane, I.; Aquino, D.; Alì, M.; Cellina, M.; Beltramo, G.; et al. Development of Predictive Models for the Response of Vestibular Schwannoma Treated with Cyberknife®: A Feasibility Study Based on Radiomics and Machine Learning. J. Pers. Med. 2023, 13, 808. https://doi.org/10.3390/jpm13050808
Bossi Zanetti I, De Martin E, Pascuzzo R, D’Amico NC, Morlino S, Cane I, Aquino D, Alì M, Cellina M, Beltramo G, et al. Development of Predictive Models for the Response of Vestibular Schwannoma Treated with Cyberknife®: A Feasibility Study Based on Radiomics and Machine Learning. Journal of Personalized Medicine. 2023; 13(5):808. https://doi.org/10.3390/jpm13050808
Chicago/Turabian StyleBossi Zanetti, Isa, Elena De Martin, Riccardo Pascuzzo, Natascha Claudia D’Amico, Sara Morlino, Irene Cane, Domenico Aquino, Marco Alì, Michaela Cellina, Giancarlo Beltramo, and et al. 2023. "Development of Predictive Models for the Response of Vestibular Schwannoma Treated with Cyberknife®: A Feasibility Study Based on Radiomics and Machine Learning" Journal of Personalized Medicine 13, no. 5: 808. https://doi.org/10.3390/jpm13050808
APA StyleBossi Zanetti, I., De Martin, E., Pascuzzo, R., D’Amico, N. C., Morlino, S., Cane, I., Aquino, D., Alì, M., Cellina, M., Beltramo, G., & Fariselli, L. (2023). Development of Predictive Models for the Response of Vestibular Schwannoma Treated with Cyberknife®: A Feasibility Study Based on Radiomics and Machine Learning. Journal of Personalized Medicine, 13(5), 808. https://doi.org/10.3390/jpm13050808








