Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Tissue Samples
2.2. Radiotherapy
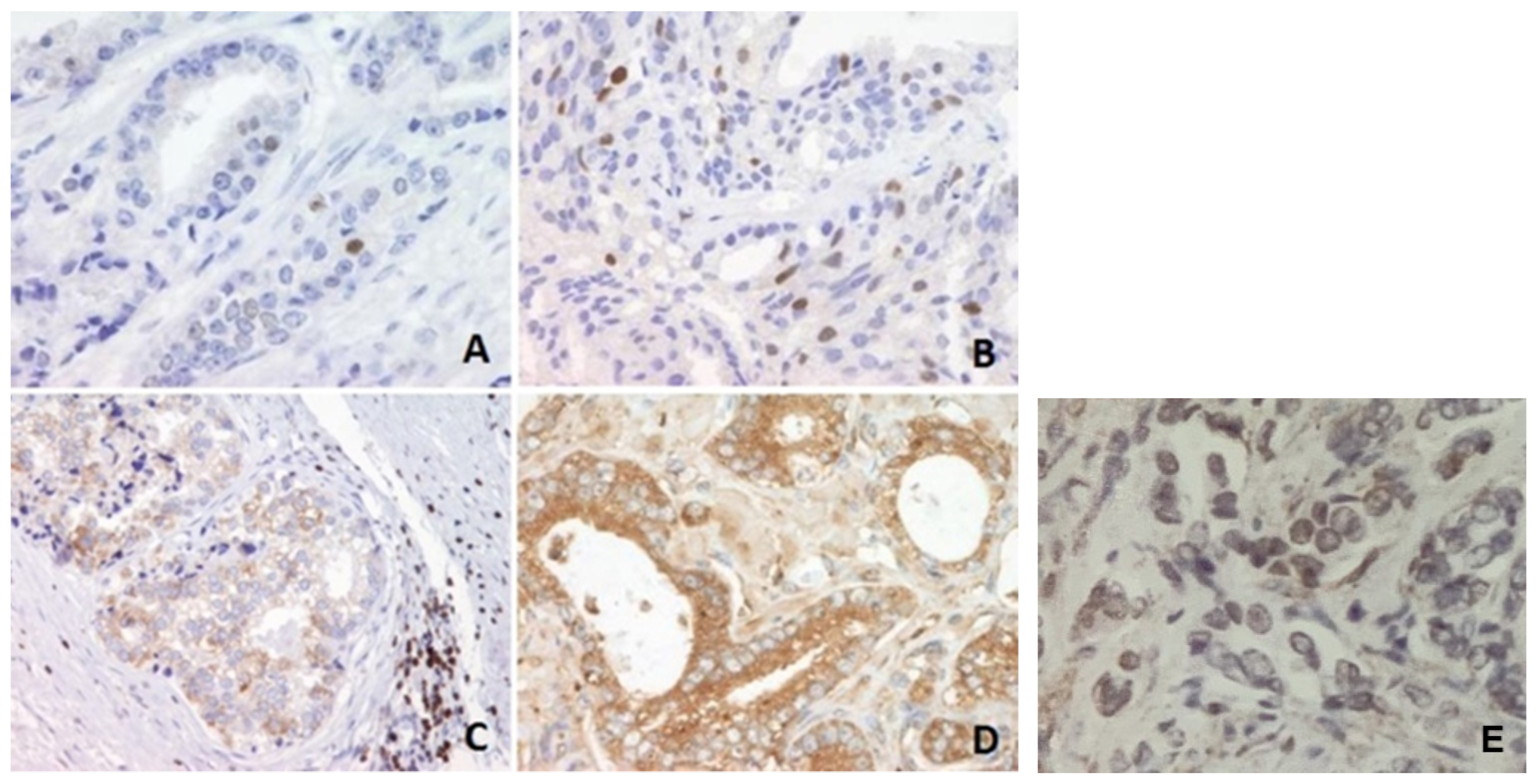
2.3. Sample Preparation and Immunohistochemistry
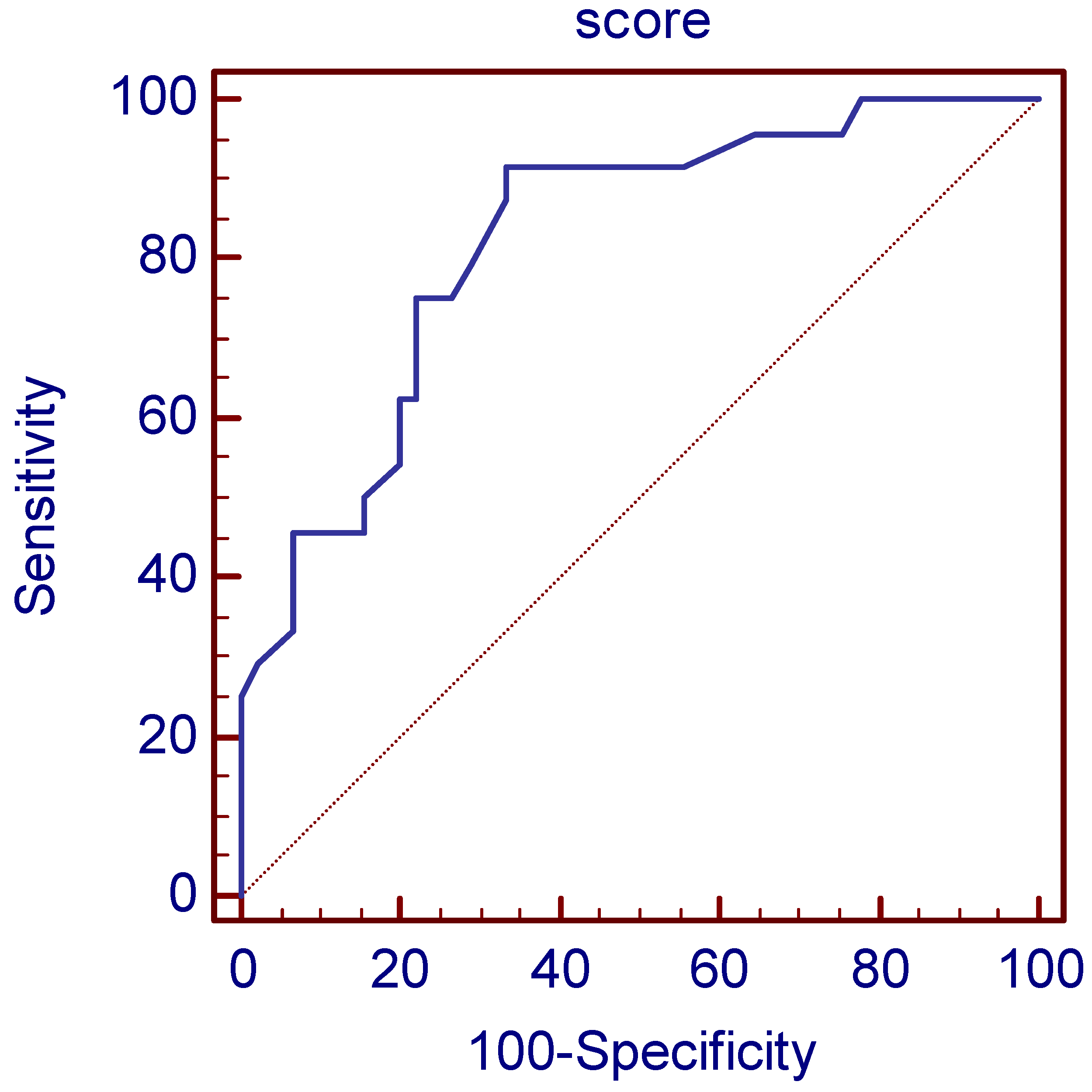
2.4. Statistical Analysis
3. Results
Clinicopathological Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Brockman, J.A.; Alanee, S.; Vickers, A.; Scardino, P.T.; Wood, D.P.; Kibel, A.S.; Lin, D.W.; Bianco, F.J., Jr.; Rabah, D.; Klein, E.A.; et al. Nomogram predicting prostate cancer–specific mortality for men with biochemical recurrence after radical prostatectomy. Eur. Urol. 2015, 67, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, A.M.; Kweldam, C.F.; Van Leenders, G.J.L.H. Prognostic histopathological and molecular markers on prostate cancer needle-biopsies: A Review. BioMed Res. Int. 2014, 2014, 341324. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.G.; Millar, J.L.; Dally, M.J.; Sia, S.; Miles, W.; Duchesne, G.M. What defines intermediate-risk prostate cancer? Variability in published prognostic models. Int. J. Radiat. Oncol. 2004, 58, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.J.; Oxley, J.; Young, G.J.; Lane, J.A.; Metcalfe, C.; Davis, M.; Turner, E.L.; Martin, R.M.; Goepel, J.R.; Varma, M.; et al. The ProtecT trial: Analysis of the patient cohort, baseline risk stratification and disease progression. BJU Int. 2020, 125, 506–514. [Google Scholar] [CrossRef]
- Shu, K.-X.; Li, B.; Wu, L.-X. The p53 network: p53 and its downstream genes. Colloids Surf. B Biointerfaces 2007, 55, 10–18. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Green, D.R. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006, 13, 994–1002. [Google Scholar] [CrossRef]
- Ecke, T.H.; Schlechte, H.H.; Schiemenz, K.; Sachs, M.D.; Lenk, S.V.; Rudolph, B.D.; Loening, S.A. TP53 gene mutations in prostate cancer progression. Anticancer Res. 2010, 30, 1579–1586. [Google Scholar]
- Simone, C.B.; John-Aryankalayil, M.; Palayoor, S.T.; Makinde, A.Y.; Cerna, D.; Falduto, M.T.; Magnuson, S.R.; Coleman, C.N. mRNA expression profiles for prostate cancer following fractionated irradiation are influenced by p53 status. Transl. Oncol. 2013, 6, 573–585. [Google Scholar] [CrossRef]
- Incognito, L.S.; Cazares, L.; Schellhammer, P.F.; A Kuban, D.; O Van Dyk, E.; Moriarty, R.P.; Wright, G.L.; Somers, K.D. Overexpression of p53 in prostate carcinoma is associated with improved overall survival but not predictive of response to radiotherapy. Int. J. Oncol. 2000, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Catz, S.D.; Johnson, J.L. BCL-2 in prostate cancer: A minireview. Apoptosis 2003, 8, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Kallakury, B.V.; Sheehan, C.E.; Fisher, H.A.; Kaufman, R.P., Jr.; Kaur, P.; Gray, K.; Stringer, B. Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: Correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin. Cancer Res. 2004, 10, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Margaryan, N.V.; Loessner, D.; Collins, A.; Kerr, K.M.; Turner, M.; Seftor, E.A.; Stephens, C.R.; Lai, J.; Postovit, L.-M.; et al. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate 2011, 71, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Waldman, F.; Pollack, A. Predictive models in external beam radiotherapy for clinically localized prostate cancer. Cancer 2009, 115, 3112–3120. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of tumours of the urinary system and male genital organs—Part B: Prostate and bladder tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Hilton, J.F.; Carroll, P.R. The CAPRA-S score: A straightforwardtool for improved prediction of outcomes after radicalprostatectomy. Cancer 2011, 117, 5039–5046. [Google Scholar] [CrossRef]
- Takeuchi, S.; Iinuma, K.; Nakano, M.; Kawase, M.; Kato, D.; Kawase, K.; Takai, M.; Nakane, K.; Ito, M.; Kumano, T.; et al. Patient age as a predictive factor in biochemical recurrence following brachytherapy: Oncological outcomes at a single center. Prostate Int. 2022, 10, 224–228. [Google Scholar] [CrossRef]
- Godtman, R.A.; Stinesen Kollberg, K.; Pihl, C.G.; Månsson, M.; Hugosson, J. The association between age, prostate cancer risk, and higher gleason score in a long-term screening program: Results from the goteborg-1 prostate cancer screening trial. Eur. Urol. 2022, 82, 311–317. [Google Scholar] [CrossRef]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.-I.; Ganswindt, U. The role of cancer stem cells in radiation resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumor progression. J. Biomed. Sci. 2018, 25, 26. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Saga, R.; Monzen, S.; Tsuruga, E.; Hasegawa, K.; Hosokawa, Y. Understanding the mechanism underlying the acquisition of radioresistance in human prostate cancer cells. Oncol. Lett. 2019, 17, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Yang, T.; Liu, R.; Xu, Y. Overexpression levels of cripto-1 predict poor prognosis in patients with prostate cancer following radical prostatectomy. Oncol. Lett. 2019, 18, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Grignon, D.J.; Sarkar, F.H.; Forman, J.D.; Caplan, R.; Pajak, T.F.; Lawton, C.A.; Hammond, E.H.; Pilepich, M.V.; Mesic, J.; Fu, K.K.; et al. p53 status and prognosis of locally advanced prostatic adenocarcinoma: A study based on RTOG 8610. Gynecol. Oncol. 1997, 89, 158–165. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Halabi, S.; Vollmer, R.; Loffredo, M.; McMahon, E.; Sanford, B.; Archer, L.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. p53 protein expression status and recurrence in men treated with radiation and androgen suppression therapy for higher-risk prostate cancer: A prospective phase II Cancer and Leukemia Group B Study (CALGB 9682). Urology 2008, 71, 933–937. [Google Scholar] [CrossRef]
- Pollack, A.; Cowen, D.; Troncoso, P.; Zagars, G.K.; Von Eschenbach, A.C.; Meistrich, M.L.; McDonnell, T. Molecular markers of outcome after radiotherapy in patients with prostate carcinoma: Ki-67, bcl-2, bax, and bcl-x. Cancer 2003, 97, 1630–1638. [Google Scholar] [CrossRef]
- Buhmedia, A.; Pyrhönen, S.; Laato, M.; Collan, Y. Prognostic factors in prostate cancer. Diagn. Pathol. 2006, 1, 4. [Google Scholar] [CrossRef]
- Brewster, S.F.; Oxley, J.D.; Trivella, M.; Abbott, C.D.; Gillatt, D.A. Preoperative p53, bcl-2, CD44 and E-cadherin immunohistochemistry as predictors of biochemical relapse after radical prostatectomy. J. Urol. 1999, 161, 1238–1243. [Google Scholar] [CrossRef]
- Kotera, Y.; Shimizu, K.; Mulé, J.J. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001, 61, 8105–8109. [Google Scholar]
- Lee, R.J.; Tzou, K.S.; Heckman, M.G.; Hobbs, C.J.; Rawal, B.; Diehl, N.N.; Peterson, J.L.; Paryani, N.N.; Ko, S.J.; Daugherty, L.C.; et al. Proposed prognostic scoring system evaluating risk factors for biochemical recurrence of prostate cancer after salvage radiation therapy. BJU Int. 2016, 118, 236–242. [Google Scholar] [CrossRef]
- Freedland, S.J.; Gerber, L.; Reid, J.; Welbourn, W.; Tikishvili, E.; Park, J.; Younus, A.; Gutin, A.; Sangale, Z.; Lanchbury, J.S.; et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int. J. Radiat. Oncol. 2013, 86, 848–853. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | |
|---|---|
| Age of patients (years, mean ± SD) | 65.08 ± 6.93 |
| Level of serum PSA at the time of the dg of Pca (ng/mL, mean ± SD) | 24.11 ± 29.42 |
| Pathologic stage (%) | |
| cT1 | 52.17 |
| cT2 | 4.35 |
| cT3 | 43.48 |
| Gleason score (%) | |
| ≤6 | 50.72 |
| ≥8 | 49.28 |
| Ki 67 (mean ± SD) | 8.78 ± 14.08 |
| Parameter | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | AUC (95% CI) |
|---|---|---|---|
| Age (years) (>72) | 24.24 (11.1–42.3) | 92.31 (81.5–97.9) | 0.51 (0.39–0.62) |
| PSA (nmol/L) (>8.3) | 88.89 (73.9–96.9) | 60.00 (45.2–73.6) | 0.71 (0.60–0.80) |
| Gleason score (≥7) | 72.22 (54.8–85.8) | 59.26 (45.0–72.4) | 0.71 (0.60–0.08) |
| grade group 1 | 25.00 (12.1–42.2) | 42.59 (29.2–56.8) | 0.34 (0.24–0.45) |
| grade group 2 | 44.00 (24.4–65.1) | 61.54 (48.6–73.3) | 0.53 (0.42–063) |
| grade group 3 | 66.67 (9.4–99.2) | 60.92 (49.9–71.2) | 0.64 (0.53–0.74) |
| grade group 4 | 44.44 (13.7–78.8) | 60.49 (49.0–71.2) | 0.53 (0.42–0.63) |
| grade group 5 | 76.92 (46.2–95.0) | 66.23 (54.6–76.6) | 0.72 (0.61–0.81) |
| prognostic group 1 | 5.56 (0.7–18.7) | 82.69 (69.7–91.8) | 0.44 (0.34–0.55) |
| prognostic group 2 | 25.00 (12.1–42.2) | 75.00 (61.1–86.0) | 0.50 (0.39–0.61) |
| prognostic group 3 | 2.78 (0.07–14.5) | 98.08 (89.7–100.0) | 0.50 (0.40–0.61) |
| prognostic group 4 | 22.22 (10.1–39.1) | 55.77 (41.3–69.5) | 0.39 (0.29–0.50) |
| prognostic group 5 | 72.73 (49.8–89.3) | 69.70 (57.1–80.4) | 0.71 (0.61–0.80) |
| cripto1 (>0.25) | 64.00 (42.5–82.0) | 48.98 (34.4–63.7) | 0.53 (0.41–0.65) |
| p53 (>0.169) | 22.22 (10.1–39.2) | 94.44 (84.6–98.8) | 0.55 (0.44–0.65) |
| bcl2 (>0.1256) | 35.29 (19.7–53.5) | 83.33 (70.7–92.1) | 0.58 (0.47–0.69) |
| NFKb (>0.309) | 79.41 (62.1–91.3) | 47.17 (33.3–61.4) | 0.62 (0.52–0.73) |
| Ki67 (>0.0445) | 60.00 (42.1–76.1) | 75.93 (62.4–86.5) | 0.69 (0.58–0.78) |
| Parameter | Cramer’s V Coefficient | Cramer’s V Ratio | Points |
|---|---|---|---|
| Age (>72) | 0.2316 | 2.29 | 9 |
| PSA (>8.3) | 0.4933 | 4.88 | 20 |
| Gleason score (≥7) | 0.3091 | 3.06 | 12 |
| grade group 1 | −0.3195 | −3.16 | −13 |
| grade group 2 | 0.0506 | 0 | 0 |
| grade group 3 | 0.1011 | 1 | 4 |
| grade group 4 | 0.0302 | 0 | 0 |
| grade group 5 | 0.3097 | 3.06 | 12 |
| prognostic group 1 | −0.1747 | −1.73 | −7 |
| prognostic group 2 | 0.0000 | 0 | 0 |
| prognostic group 3 | 0.0282 | 0 | 0 |
| prognostic group 4 | −0.2265 | −2.24 | −9 |
| prognostic group 5 | 0.3736 | 3.7 | 15 |
| cripto1 (>0.25) | 0.1235 | 1.22 | 5 |
| p53 (>0.169) | 0.2493 | 2.47 | 10 |
| bcl2 (>0.1256) | 0.2128 | 2.10 | 8 |
| NFKb (>0.309) | 0.2690 | 2.66 | 11 |
| Ki67 (>0.0445) | 0.3612 | 3.57 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesar, E.C.; Mikolasevic, I.; Skocilic, I.; Redjovic, A.; Vucinic, D.; Marusic, J.; Djordjevic, G. Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease. J. Pers. Med. 2023, 13, 870. https://doi.org/10.3390/jpm13050870
Tesar EC, Mikolasevic I, Skocilic I, Redjovic A, Vucinic D, Marusic J, Djordjevic G. Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease. Journal of Personalized Medicine. 2023; 13(5):870. https://doi.org/10.3390/jpm13050870
Chicago/Turabian StyleTesar, Eleonora Cini, Ivana Mikolasevic, Iva Skocilic, Arnela Redjovic, Damir Vucinic, Jasna Marusic, and Gordana Djordjevic. 2023. "Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease" Journal of Personalized Medicine 13, no. 5: 870. https://doi.org/10.3390/jpm13050870
APA StyleTesar, E. C., Mikolasevic, I., Skocilic, I., Redjovic, A., Vucinic, D., Marusic, J., & Djordjevic, G. (2023). Prostate Cancer Scoring Index for Risk of Progression of Radioresistant Disease. Journal of Personalized Medicine, 13(5), 870. https://doi.org/10.3390/jpm13050870






