Uterine Artery Embolization for the Treatment of Symptomatic Uterine Fibroids of Different Sizes: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Peri-Procedural Care
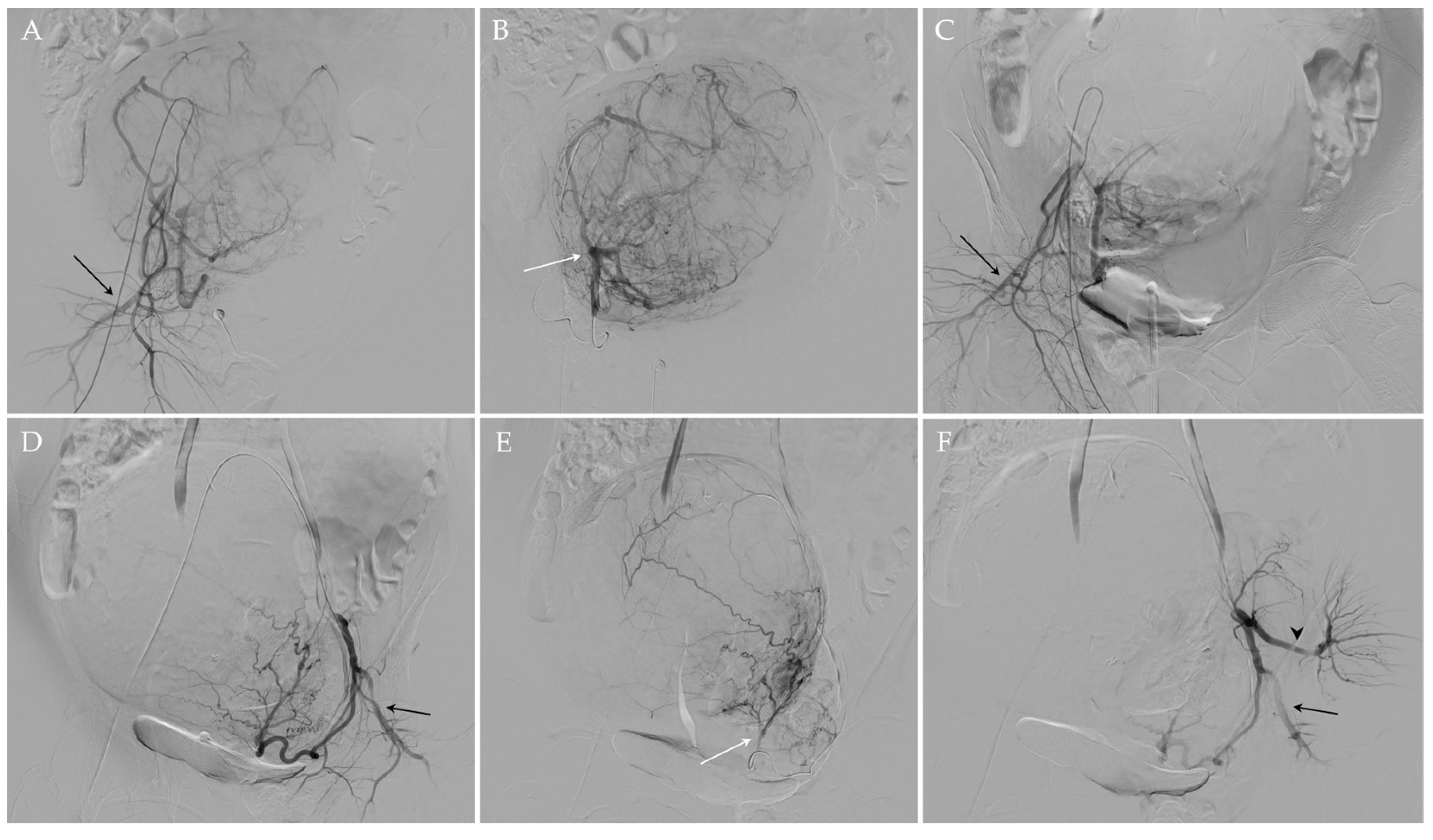
2.3. UAE Procedure
2.4. Post-Procedural Care
2.5. Statistical Analysis
3. Results
3.1. Radiological Evaluation after UAE
3.2. Clinical Evaluation after UAE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Bulun, S.E. Uterine fibroids. N. Engl. J. Med. 2013, 369, 1344–1355. [Google Scholar] [CrossRef] [PubMed]
- Drayer, S.M.; Catherino, W.H. Prevalence, morbidity, and current medical management of uterine leiomyomas. Int. J. Gynaecol. Obstet. 2015, 131, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Vilos, G.A.; Allaire, C.; Laberge, P.Y.; Leyland, N. The management of uterine leiomyomas. J. Obstet. Gynaecol. Can. 2015, 37, 157–178. [Google Scholar] [CrossRef]
- Giuliani, E.; As-Sanie, S.; Marsh, E.E. Epidemiology and management of uterine fibroids. Int. J. Gynaecol. Obstet. 2020, 149, 3–9. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Sogoyan, N.S.; Donnikov, A.J.; Trofimov, D.Y.; Adamyan, L.V.; Mishina, N.D.; Shubina, J.; Zelensky., D.V.; Sukhikh, G.T. Familial Predisposition to Leiomyomata: Searching for Protective Genetic Factors. Biomedicines 2022, 10, 508. [Google Scholar] [CrossRef]
- Awiwi, M.O.; Badawy, M.; Shaaban, A.M.; Menias, C.O.; Horowitz, J.M.; Soliman, M.; Jensen, C.T.; Gaballah, A.H.; Ibarra-Rovira, J.J.; Feldman, M.K.; et al. Review of uterine fibroids: Imaging of typical and atypical features, variants, and mimics with emphasis on workup and FIGO classification. Abdom. Radiol. 2022, 47, 2468–2485. [Google Scholar] [CrossRef]
- Sparić, R. Uterine myomas in pregnancy, childbirth and puerperium. Srp. Arh. Celok. Lek. 2014, 142, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Chen, L.; Wu, C.; Hu, C.; Xiong, Y. Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG Int. J. Obstet. Gynaecol. 2017, 124 (Suppl. S3), 30–35. [Google Scholar] [CrossRef]
- El-Balat, A.; DeWilde, R.L.; Schmeil, I.; Tahmasbi-Rad, M.; Bogdanyova, S.; Fathi, A.; Becker, S. Modern Myoma Treatment in the Last 20 Years: A Review of the Literature. Biomed. Res. Int. 2018, 2018, 4593875. [Google Scholar] [CrossRef]
- Neis, K.J.; Zubke, W.; Fehr, M.; Römer, T.; Tamussino, K.; Nothacker, M. Hysterectomy for Benign Uterine Disease. Dtsch. Arztebl. Int. 2016, 113, 242–249. [Google Scholar] [CrossRef] [PubMed]
- The North American Menopause Society (NAMS). NAMS 2018 Utian Translational Science Symposium, October 2018, San Diego, California New therapies for leiomyomas: When surgery may not be the best option. Menopause 2019, 26, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Duhan, N. Current and emerging treatments for uterine myoma—An update. Int. J. Womens Health 2011, 3, 231–241. [Google Scholar] [CrossRef]
- Vargas, M.V.; Moawad, G.N.; Sievers, C.; Opoku-Anane, J.; Marfori, C.Q.; Tyan, P.; Robinson, J.K. Feasibility, Safety, and Prediction of Complications for Minimally Invasive Myomectomy in Women With Large and Numerous Myomata. J. Minim. Invasive Gynecol. 2017, 24, 315–322. [Google Scholar] [CrossRef]
- Ravina, J.H.; Herbreteau, D.; Ciraru-Vigneron, N.; Bouret, J.M.; Houdart, E.; Aymard, A.; Merland, J.J. Arterial embolisation to treat uterine myomata. Lancet 1995, 346, 671–672. [Google Scholar] [CrossRef]
- Mas, A.; Tarazona, M.; Dasí Carrasco, J.; Estaca, G.; Cristóbal, I.; Monleón, J. Updated approaches for management of uterine fibroids. Int. J. Womens Health 2017, 9, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Athreya, S. Systematic review of uterine artery embolisation practice guidelines: Are all the guidelines on the same page? Clin. Radiol. 2018, 73, 507.e9–507.e15. [Google Scholar] [CrossRef]
- Marret, H.; Fritel, X.; Ouldamer, L.; Bendifallah, S.; Brun, J.-L.; De Jesus, I.; Derrien, J.; Giraudet, G.; Kahn, V.; Koskas, M.; et al. Therapeutic management of uterine fibroid tumors: Updated French guidelines. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ngeh, N.; Belli, A.M.; Morgan, R.; Manyonda, I. Pre-myomectomy uterine artery embolisation minimises operative blood loss. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1139–1140. [Google Scholar] [CrossRef]
- Goodwin, S.C.; Spies, J.B.; Worthington-Kirsch, R.; Peterson, E.; Pron, G.; Li, S.; Myers, E.R. Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: Long-term outcomes from the FIBROID Registry. Obstet. Gynecol. 2008, 111, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Worthington-Kirsch, R.; Spies, J.B.; Myers, E.R.; Mulgund, J.; Mauro, M.; Pron, G.; Peterson, E.D.; Goodwin, S. FIBROID Investigators. The Fibroid Registry for outcomes data (FIBROID) for uterine embolization: Short-term outcomes. Obstet. Gynecol. 2005, 106, 52–59. [Google Scholar] [CrossRef]
- Voogt, M.J.; Arntz, M.J.; Lohle, P.N.; Mali, W.P.; Lampmann, L.E. Uterine fibroid embolisation for symptomatic uterine fibroids: A survey of clinical practice in Europe. Cardiovasc. Intervent Radiol. 2011, 34, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Tomislav, S.; Josip, M.; Liana, C.S.; Marko, V.; Marko, J.; Ante, R.; Dzenis, J.; Leo, G.; Ivica, S.; Marijan, T.; et al. Uterine artery embolization as nonsurgical treatment of uterine myomas. ISRN Obstet. Gynecol. 2011, 2011, 489281. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.B.; Scialli, A.R.; Jha, R.C.; Imaoka, I.; Ascher, S.M.; Fraga, V.M.; Barth, K.H. Initial results from uterine fibroid embolization for symptomatic leiomyomata. J. Vasc. Interv. Radiol. 1999, 10, 1149–1157. [Google Scholar] [CrossRef]
- de Bruijn, A.M.; Ankum, W.M.; Reekers, J.A.; Birnie, E.; van der Kooij, S.M.; Volkers, N.A.; Hehenkamp, W.J. Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am. J. Obstet. Gynecol. 2016, 215, 745.e1–745.e12. [Google Scholar] [CrossRef]
- Allameh, Z.; Afzali, S.; Jafarpisheh, M.; Movahedi, M.; Mousavi Seresht, L. Evaluation of the Efficacy and Complications of Uterine Artery Embolization in Comparison with Laparotomy-Myomectomy in the Treatment of Uterine Myomas: A Randomized Clinical Trial. Med. J. Islam. Repub. Iran. 2022, 36, 87. [Google Scholar] [CrossRef]
- Flynn, M.; Jamison, M.; Datta, S.; Myers, E. Health care resource use for uterine fibroid tumors in the United States. Am. J. Obstet. Gynecol. 2006, 195, 955–964. [Google Scholar] [CrossRef]
- Wu, O.; Briggs, A.; Dutton, S.; Hirst, A.; Maresh, M.; Nicholson, A.; McPherson, K. Uterine artery embolisation or hysterectomy for the treatment of symptomatic uterine fibroids: A cost-utility analysis of the HOPEFUL study. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1352–1362. [Google Scholar] [CrossRef]
- Moss, J.G.; Cooper, K.G.; Khaund, A.; Murray, L.S.; Murray, G.D.; Wu, O.; Craig, L.E.; Lumsden, M.A. Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 936–944. [Google Scholar] [CrossRef]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef] [PubMed]
- Laughlin-Tommaso, S.K. Alternatives to Hysterectomy: Management of Uterine Fibroids. Obstet. Gynecol. Clin. N. Am. 2016, 43, 397–413. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Huff, T.J.; Shanahan, M.M.; Stavas, J.M. Pregnancy success and outcomes after uterine fibroid embolization: Updated review of published literature. Br. J. Radiol. 2020, 93, 20190551. [Google Scholar] [CrossRef] [PubMed]
- Mara, M.; Maskova, J.; Fucikova, Z.; Kuzel, D.; Belsan, T.; Sosna, O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc. Intervent Radiol. 2008, 31, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Firouznia, K.; Ghanaati, H.; Sanaati, M.; Jalali, A.H.; Shakiba, M. Pregnancy after uterine artery embolization for symptomatic fibroids: A series of 15 pregnancies. AJR Am. J. Roentgenol. 2009, 192, 1588–1592. [Google Scholar] [CrossRef] [PubMed]
- Homer, H.; Saridogan, E. Uterine artery embolization for fibroids is associated with an increased risk of miscarriage. Fertil. Steril. 2010, 94, 324–330. [Google Scholar] [CrossRef]
- Karlsen, K.; Hrobjartsson, A.; Korsholm, M.; Mogensen, O.; Humaidan, P.; Ravn, P. Fertility after uterine artery embolization of fibroids: A systematic review. Arch. Gynecol. Obstet. 2018, 297, 13–25. [Google Scholar] [CrossRef]
- Serres-Cousine, O.; Kuijper, F.M.; Curis, E.; Atashroo, D. Clinical investigation of fertility after uterine artery embolization. Am. J. Obstet. Gynecol. 2021, 225, 403.e1–403.e22. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Gynecology. Management of Symptomatic Uterine Leiomyomas: ACOG Practice Bulletin, Number 228. Obstet. Gynecol. 2021, 137, e100–e115. [Google Scholar] [CrossRef]
- Kröncke, T.; David, M. Uterine Artery Embolization (UAE) for Fibroid Treatment—Results of the 7th Radiological Gynecological Expert Meeting. Rofo 2019, 191, 630–634. [Google Scholar]
- Pérez-López, F.R.; Ornat, L.; Ceausu, I.; Depypere, H.; Erel, C.T.; Lambrinoudaki, I.; Schenck-Gustafsson, K.; Simoncini, T.; Tremollieres, F.; Rees, M.; et al. EMAS position statement: Management of uterine fibroids. Maturitas 2014, 79, 106–116. [Google Scholar] [CrossRef]
- Jha, R.C.; Ascher, S.M.; Imaoka, I.; Spies, J.B. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology 2000, 217, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pelage, J.P.; Le Dref, O.; Soyer, P.; Kardache, M.; Dahan, H.; Abitbol, M.; Merland, J.J.; Ravina, J.H.; Rymer, R. Fibroid-related menorrhagia: Treatment with superselective embolization of the uterine arteries and midterm follow-up. Radiology 2000, 215, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Worthington-Kirsch, R.L.; Popky, G.L.; Hutchins, F.L., Jr. Uterine arterial embolization for the management of leiomyomas: Quality-of-life assessment and clinical response. Radiology 1998, 208, 625–629. [Google Scholar] [CrossRef]
- Naguib, N.N.; Mbalisike, E.; Nour-Eldin, N.E.; Jost, A.; Lehnert, T.; Ackermann, H.; Vogl, T.J. Leiomyoma volume changes at follow-up after uterine artery embolization: Correlation with the initial leiomyoma volume and location. J. Vasc. Interv. Radiol. 2010, 21, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Czuczwar, P.; Woźniak, S.; Szkodziak, P.; Woźniakowska, E.; Paszkowski, M.; Wrona, W.; Milart, P.; Paszkowski, T.; Popajewski, M. Predicting the results of uterine artery embolization: Correlation between initial intramural fibroid volume and percentage volume decrease. Prz. Menopauzalny 2014, 13, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Katsumori, T.; Yoshikawa, T.; Miura, H. Insufficient Leiomyoma Infarction in Uterine Artery Embolization: Relationship with Tumor Location. J. Vasc. Interv. Radiol. 2019, 30, 668–675.e1. [Google Scholar] [CrossRef] [PubMed]
- Firouznia, K.; Ghanaati, H.; Sanaati, M.; Jalali, A.H.; Shakiba, M. Uterine artery embolization in 101 cases of uterine fibroids: Do size, location, and number of fibroids affect therapeutic success and complications? Cardiovasc. Intervent Radiol. 2008, 31, 521–526. [Google Scholar] [CrossRef]
- Spies, J.B.; Roth, A.R.; Jha, R.C.; Gomez-Jorge, J.; Levy, E.B.; Chang, T.C.; Ascher, S.A. Leiomyomata treated with uterine artery embolization: Factors associated with successful symptom and imaging outcome. Radiology 2002, 222, 45–52. [Google Scholar] [CrossRef]
- Kurban, L.A.S.; Metwally, H.; Abdullah, M.; Kerban, A.; Oulhaj, A.; Alkoteesh, J.A. Uterine Artery Embolization of Uterine Leiomyomas: Predictive MRI Features of Volumetric Response. AJR Am. J. Roentgenol. 2021, 216, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Burn, P.R.; McCall, J.M.; Chinn, R.J.; Vashisht, A.; Smith, J.R.; Healy, J.C. Uterine fibroleiomyoma: MR imaging appearances before and after embolization of uterine arteries. Radiology 2000, 214, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Sipola, P.; Ruuskanen, A.; Yawu, L.; Husso, M.; Vanninen, R.; Hippeläinen, M.; Manninen, H. Preinterventional quantitative magnetic resonance imaging predicts uterus and leiomyoma size reduction after uterine artery embolization. J. Magn. Reson. Imaging 2010, 31, 617–624. [Google Scholar] [CrossRef]
- Golfieri, R.; Garzillo, G.; Ascanio, S.; Renzulli, M. Focal lesions in the cirrhotic liver: Their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig. Dis. 2014, 32, 696–704. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; FIGO Menstrual Disorders Working Group. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil. Steril. 2011, 95, 2204–2208.e22083. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Martin, J.; Bhanot, K.; Athreya, S. Complications and reinterventions in uterine artery embolization for symptomatic uterine fibroids: A literature review and meta analysis. Cardiovasc. Intervent Radiol. 2013, 36, 395–402. [Google Scholar] [CrossRef] [PubMed]
- van Overhagen, H.; Reekers, J.A. Uterine Artery Embolization for Symptomatic Leiomyomata. Cardiovasc. Intervent Radiol. 2015, 38, 536–542. [Google Scholar] [CrossRef]
- Ierardi, A.M.; Carnevale, A.; Pellegrino, F.; Stefano, G.D.; Bonelli, C.; Renzulli, M.; Giganti, M.; Carrafiello, G. Uterine Myomas: Extravascular Treatment. Semin. Ultrasound CT MR 2021, 42, 56–74. [Google Scholar] [CrossRef]
- Das, R.; Wale, A.; Renani, S.A.; Ratnam, L.; Mailli, L.; Chun, J.Y.; Das, S.; Duggal, B.; Manyonda, I.; Belli, A.M. Randomised Controlled Trial of Particles Used in Uterine fibRoid Embolisation (PURE): Non-Spherical Polyvinyl Alcohol Versus Calibrated Microspheres. Cardiovasc. Intervent Radiol. 2022, 45, 207–215. [Google Scholar] [CrossRef]
- Koesters, C.; Powerski, M.J.; Froeling, V.; Kroencke, T.J.; Scheurig-Muenkler, C. Uterine artery embolization in single symptomatic leiomyoma: Do anatomical imaging criteria predict clinical presentation and long-term outcome? Acta Radiol. 2014, 55, 441–449. [Google Scholar] [CrossRef]
- Chung, Y.J.; Kang, S.Y.; Chun, H.J.; Rha, S.E.; Cho, H.H.; Kim, J.H.; Kim, M.R. Development of a Model for the Prediction of Treatment Response of Uterine Leiomyomas after Uterine Artery Embolization. Int. J. Med. Sci. 2018, 15, 1771–1777. [Google Scholar] [CrossRef]
- Laios, A.; Baharuddin, N.; Iliou, K.; Gubara, E.; O’Sullivan, G. Uterine artery embolization for treatment of symptomatic fibroids; a single institution experience. Hippokratia 2014, 18, 258–261. [Google Scholar] [PubMed]
- Katsumori, T.; Nakajima, K.; Mihara, T. Is a large fibroid a high-risk factor for uterine artery embolization? AJR Am. J. Roentgenol. 2003, 181, 1309–1314. [Google Scholar] [CrossRef]
- Das, R.; Champaneria, R.; Daniels, J.P.; Belli, A.M. Comparison of embolic agents used in uterine artery embolisation: A systematic review and meta-analysis. Cardiovasc. Intervent Radiol. 2014, 37, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Kurjak, A.; Kupesic-Urek, S.; Miric, D. The assessment of benign uterine tumor vascularization by transvaginal color Doppler. Ultrasound Med. Biol. 1992, 18, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Pelage, J.P.; Cazejust, J.; Pluot, E.; Le Dref, O.; Laurent, A.; Spies, J.B.; Chagnon, S.; Lacombe, P. Uterine fibroid vascularization and clinical relevance to uterine fibroid embolization. Radiographics 2005, 25 (Suppl. S1), S99–S117. [Google Scholar] [CrossRef]
- Aziz, A.; Petrucco, O.M.; Makinoda, S.; Wikholm, G.; Svendsen, P.; Brännström, M.; Janson, P.O. Transarterial embolization of the uterine arteries: Patient reactions and effects on uterine vasculature. Acta Obstet. Gynecol. Scand. 1998, 77, 334–340. [Google Scholar] [CrossRef]
- Zlotnik, E.; de Lorenzo Messina, M.; Nasser, F.; Affonso, B.B.; Baroni, R.H.; Wolosker, N.; Baracat, E.C. Predictive factors for pelvic magnetic resonance in response to arterial embolization of a uterine leiomyoma. Clinics 2014, 69, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.B.; Myers, E.R.; Worthington-Kirsch, R.; Mulgund, J.; Goodwin, S.; Mauro, M.; FIBROID Registry Investigators. The FIBROID Registry: Symptom and quality-of-life status 1 year after therapy. Obstet. Gynecol. 2005, 106, 1309–1318. [Google Scholar] [CrossRef]
- Lacayo, E.A.; Richman, D.L.; Acord, M.R.; Wolfman, D.J.; Caridi, T.M.; Desale, S.Y.; Spies, J.B. Leiomyoma Infarction after Uterine Artery Embolization: Influence of Embolic Agent and Leiomyoma Size and Location on Outcome. J. Vasc. Interv. Radiol. 2017, 28, 1003–1010. [Google Scholar] [CrossRef]
- Stewart, J.K. Uterine Artery Embolization for Uterine Fibroids: A Closer Look at Misperceptions and Challenges. Tech. Vasc. Interv. Radiol. 2021, 24, 100725. [Google Scholar] [CrossRef]
- Fonseca, M.C.M.; Castro, R.; Machado, M.; Conte, T.; Girao, M.J.B.C. Uterine Artery Embolization and Surgical Methods for the Treatment of Symptomatic Uterine Leiomyomas: A Systemic Review and Meta-analysis Followed by Indirect Treatment Comparison. Clin. Ther. 2017, 39, 1438–1455.e2. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, T.; David, M. Uterine Artery Embolization (UAE) for Fibroid Treatment: Results of the 6th Radiological Gynecological Expert Meeting. Rofo 2017, 189, 511–514. [Google Scholar] [PubMed]
- Roth, A.R.; Spies, J.B.; Walsh, S.M.; Wood, B.J.; Gomez-Jorge, J.; Levy, E.B. Pain after uterine artery embolization for leiomyomata: Can its severity be predicted and does severity predict outcome? J. Vasc. Interv. Radiol. 2000, 11, 1047–1052. [Google Scholar] [CrossRef]
- Armstrong, C.; Caird, L. Fibroid embolisation: A technique not without significant complications. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 132. [Google Scholar] [CrossRef]
- Vashisht, A.; Studd, J.; Carey, A.; Burn, P. Fatal septicaemia after fibroid embolisation. Lancet 1999, 354, 307–308. [Google Scholar] [CrossRef]
- Parthipun, A.A.; Taylor, J.; Manyonda, I.; Belli, A.M. Does size really matter? Analysis of the effect of large fibroids and uterine volumes on complication rates of uterine artery embolisation. Cardiovasc. Intervent Radiol. 2010, 33, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.J.; Nijenhuis, R.J.; van Rooij, W.J.; Weimar, E.A.; Boekkooi, P.F.; Lampmann, L.E.; Vervest, H.A.; Lohle, P.N. Uterine artery embolization in patients with a large fibroid burden: Long-term clinical and MR follow-up. Cardiovasc. Intervent Radiol. 2010, 33, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Society of Interventional Radiology. Available online: https://www.sirweb.org/globalassets/aasociety-of-interventional-radiology-home-page/patient-center/fibroid/sir_report_final.pdf (accessed on 10 March 2023).
- Alreshidi, M.N.; Alshubrmi, D.; Alrashidi, I.; Arabi, M. Awareness of uterine artery embolization as a treatment for fibroids among Saudi women. Arab. J. Intervent Radiol. 2020, 4, 79–82. [Google Scholar] [CrossRef]
- Plaskos, N.P.; Kachura, J.R. Survey of gynecologists’ and interventional radiologists’ opinions of uterine fibroid embolization. Can. Assoc. Radiol. J. 2006, 57, 140–146. [Google Scholar]




| Total (n = 62) | |
|---|---|
| Age (mean (±SD)) | 45 (±3.4) |
| Symptoms (n (%)) | |
| Menorrhagia | 62 (100%) |
| Dysmenorrhea | 31 (%) |
| Pain | 10 (%) |
| Bulky symptoms | 54 (%) |
| Obstructive symptoms | 4 (%) |
| Number of uterine fibroids (n (%)) | |
| Mean number (mean (±SD)) | 2.3 (±1.3) |
| Solitary | 23 (37.1%) |
| Multiple | 39 (62.9%) |
| Diameter of uterine fibroids (mean (±SD)) | |
| Total | 74 (±30.1) |
| Solitary | 85 (±29.8) |
| Multiple (dominant) | 67.5 (±28.6) |
| Dimension of uterine fibroids (n (%)) | |
| Group 1 (<50 mm) | 12 (19.4%) |
| Group 2 (≥50 but ≤80 mm) | 25 (40.3%) |
| Group 3 (>80 mm) | 25 (40.3%) |
| Class of Myoma According to the FIGO Classification | ||||||
|---|---|---|---|---|---|---|
| Class 0 | Class 1 | Class 2–5 | Class 6 | Class 7 | Class 8 | |
| Type of uterine fibroids (mean (SD)) | ||||||
| Total | 1 (1.6%) | 2 (3.2%) | 43 (68.25%) | 16 (25.4%) | 0 (0%) | 0 (0%) |
| Solitary | 0 (0%) | 0 (0%) | 21 (91.3%) | 2 (8.7%) | 0 (0%) | 0 (0%) |
| Multiple | 1 (2.6%) | 2 (5%) | 22 (56.4%) | 14 (35.9%) | 0 (0%) | 0 (0%) |
| Pre-UAE Diameter in mm (Mean (±SD)) | p | Post-UAE Diameter in mm (Mean (±SD)) | p | Percentage of Reduction (% (±SD%)) | p | |
|---|---|---|---|---|---|---|
| Total | 74 (±30.1) | 42.2 (±30.1) | 42.6% (±21.6%) | |||
| Number of uterine fibroids | 0.026 | 0.011 | n.s. | |||
| Solitary | 85 (±29.9) | 51.1 (±24.5) | 40.5% (±20.7%) | |||
| Multiple | 67.5 (±28.7) | 37 (±18) | 43.9% (±16.4%) | |||
| Type of uterine fibroids | n.s | n.s | n.s. | |||
| Class 0 (pedunculated intracavitary) | 115 | 84 | 31% | |||
| Class 1 (submucosal <50% intramural) | 44.5 (±6.4) | 21 (±1.4) | 23.5% (±4.9%) | |||
| Class 2–5 (>50% intramural) | 70.6 (±24) | 41.6 (±20.9) | 29.1% (±19.5%) | |||
| Class 6 (subserosal < 50% intramural) | 84.3 (±41.2) | 42.2 (±21.6) | 40.3% (±22.6%) |
| Diameter Reduction in mm (Mean (±SD)) | p | Percentage of Reduction (% (±SD%)) | p | |
|---|---|---|---|---|
| Size of uterine fibroids | ||||
| Group 1 (<50 mm) | 15.8 (±7.7) | <0.01 | 42.7% (±15.1%) | n.s |
| Group 2 (≥50 but ≤80 mm) | 28 (±13.4) | 43.3% (±17.8%) | ||
| Group 3 (>80 mm) | 43.2 (±23.8) | 41.9 (±20.1%) |
| Clinical Outcomes Following UAE | |
|---|---|
| Length of hospital stay (mean days (±SD)) | 4.5 (±1.5) |
| UAE-related complications (n (%)) | |
| Minor | 2 (3.2%) |
| Major | 0 |
| Clinical outcomes following UAE (n (%)) | |
| Disappearance of bleeding | 51 (81%) |
| Regularization of menstrual cycle | 48 (76.1%) |
| Disappearance of bulky symptoms | 54 (100%) |
| Persistence of symptoms | 12 (19%) |
| Subsequent hysterectomy for inadequate symptom control | 4 (6.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappelli, A.; Mosconi, C.; Cocozza, M.A.; Brandi, N.; Bartalena, L.; Modestino, F.; Galaverni, M.C.; Vara, G.; Paccapelo, A.; Pizzoli, G.; et al. Uterine Artery Embolization for the Treatment of Symptomatic Uterine Fibroids of Different Sizes: A Single Center Experience. J. Pers. Med. 2023, 13, 906. https://doi.org/10.3390/jpm13060906
Cappelli A, Mosconi C, Cocozza MA, Brandi N, Bartalena L, Modestino F, Galaverni MC, Vara G, Paccapelo A, Pizzoli G, et al. Uterine Artery Embolization for the Treatment of Symptomatic Uterine Fibroids of Different Sizes: A Single Center Experience. Journal of Personalized Medicine. 2023; 13(6):906. https://doi.org/10.3390/jpm13060906
Chicago/Turabian StyleCappelli, Alberta, Cristina Mosconi, Maria Adriana Cocozza, Nicolò Brandi, Laura Bartalena, Francesco Modestino, Maria Cristina Galaverni, Giulio Vara, Alexandro Paccapelo, Gloria Pizzoli, and et al. 2023. "Uterine Artery Embolization for the Treatment of Symptomatic Uterine Fibroids of Different Sizes: A Single Center Experience" Journal of Personalized Medicine 13, no. 6: 906. https://doi.org/10.3390/jpm13060906
APA StyleCappelli, A., Mosconi, C., Cocozza, M. A., Brandi, N., Bartalena, L., Modestino, F., Galaverni, M. C., Vara, G., Paccapelo, A., Pizzoli, G., Villa, G., Seracchioli, R., & Renzulli, M. (2023). Uterine Artery Embolization for the Treatment of Symptomatic Uterine Fibroids of Different Sizes: A Single Center Experience. Journal of Personalized Medicine, 13(6), 906. https://doi.org/10.3390/jpm13060906








