1. Introduction
Parkinson’s disease (PD) is a debilitating neurodegenerative disorder that affects millions of people worldwide. It is characterized by the loss of dopamine-producing neurons in the brain, which leads to both motor and non-motor symptoms [
1,
2,
3]. Motor symptoms include tremors, rigidity, bradykinesia, and postural instability, while non-motor symptoms include depression, anxiety, cognitive dysfunction [
4,
5], adjustment disorder (AD), and stress-related disorders.
Recent research has suggested that environmental exposures [
6,
7] throughout an individual’s life, known as the exposome [
8], may contribute to the development and progression of PD through epigenetic modifications [
9] that affect genes encoding for dopamine. These modifications can alter the expression and function of proteins involved in dopamine synthesis, storage, release, and signaling, leading to changes in dopamine levels in the brain and the motor and non-motor symptoms associated with PD, such as depression, anxiety, and adjustment disorders.
Depression is the most frequently observed psychiatric disorder in PD [
10,
11] and is associated with worsening of motor function [
12] and a more severe clinical picture of PD. Studies suggest that depression in PD may result from both neuropathology and psychological and physical limitations of the patient. Anxiety symptoms may also be observed in PD patients, with varying incidence rates, ranging from 3.6% to 40%.
Understanding the correlations between motor and non-motor symptoms in PD is crucial for effective management of the condition [
13]. Recent research has shed light on the potential correlations between adjustment disorder (AD) and the onset and exacerbation of Parkinsonian symptoms [
14,
15]. AD is one of the most commonly diagnosed mental health disorders and is generally conceptualized to be mild and short-lived. Despite the frequent use of AD in clinical settings, little is known about the prognosis of this condition [
16].
Parkinson’s disease patients may exhibit emotional and behavioral symptoms similar to those of AD, including anxiety, depression, irritability, and withdrawal, as they navigate the challenges of their condition, such as mobility problems, tremors, and speech or swallowing difficulties. To investigate these correlations and reciprocal influences between motor and non-motor symptoms in PD, this study employed Neuro-Postural Optimization (NPO) and Neuro-Psycho-Physical Optimization (NPPOs) treatments of Radio Electric Asymmetric Conveyer (REAC) technology. These treatments have been studied to improve neuropsychic and physical performance in the broad field of mood, behavior, and adaptive disorders through a positive remodulation of neuronal endogenous bioelectrical activity (EBA) [
17].
In this paper, we will discuss the potential correlations between environmental exposures, epigenetic modifications, and the onset and progression of PD symptoms, with a particular focus on the non-motor symptoms of depression, anxiety, and adjustment disorder. We will also present the results of our study, which used NPO and NPPOs treatments of REAC technology to investigate the reciprocal influences between motor and non-motor symptoms in PD patients. Our findings have the potential to improve the understanding and management of PD, particularly in terms of the non-motor symptoms that have a significant impact on patients’ quality of life.
2. Materials and Methods
2.1. Study Design
This single-arm trial was conducted at the Health Department’s Laboratory at the Federal University of Amapá (UNIFAP) in Macapá, Brazil. The study was approved by the UNIFAP Ethics Committee in accordance with its opinion 5.232.551.
2.2. Power Analysis
We calculated the statistical sample size using GPower [
18,
19] software version 3.1.9.4. Using the following parameters: effect size of 0.50, α probability error of 0.05, power of 0.95, and the Wilcoxon signed-rank test. Based on these parameters, the sample size calculation yielded a sample size of 34 subjects. To mitigate potential dropouts by some participants, we increased the number of patients in the study.
2.3. Inclusion and Exclusion Criteria
The study included adult male and female participants who had received a clinical diagnosis of Parkinson’s disease at least six months prior to enrollment and demonstrated the willingness and ability to understand and comply with study requirements.
Participants with cognitive and behavioral impairments that may affect comprehension or expression, as well as those with debilitating physical conditions that could impede study participation, were excluded.
2.4. Population
The present study aimed to investigate the multidisciplinary follow-up of patients with neurodegenerative illnesses in two university extension projects. The study enrolled 50 participants, consisting of 14 females aged between 45 and 70 years (mean age ± standard deviation [SD]: 58 ± 8.7 years) and 36 males aged between 38 and 89 years (mean age ± SD: 65 ± 13.2 years), with an overall mean age of 64 years. The majority of participants were retired (70%), and 56% of them were married. Furthermore, 62% of participants identified as having brown skin.
The study population demonstrated significant variability in disease progression and therapy strategy-related traits. Sociodemographic factors, including gender, drug type, and comorbidity profile, were analyzed, and presented in
Table 1 and
Table 2.
2.5. Functional Capacity Tests and Quality of Life Assessment
To evaluate the motor functional capacities of the subjects, two assessments were performed. The first assessment utilized the five times sit to stand test, which is a widely used measure to evaluate the functional capacities of individuals. The second assessment aimed to identify dysfunctional motor control by analyzing the presence of asymmetric activations of symmetrical muscle groups, a phenomenon known as functional dysmetria.
For assessing the quality of life, we utilized the 12-item Short-Form Health Survey, a reliable and validated tool that comprehensively evaluates various aspects of an individual’s quality of life.
2.5.1. Five Times Sit to Stand Test
The five times sit to stand test (FTSST) is a common clinical assessment used to evaluate functional mobility and lower extremity strength in patients with Parkinson’s disease (PD) [
20,
21]. Parkinson’s disease is a neurodegenerative disorder that affects movement, and evaluating functional mobility is important for assessing disease progression and monitoring treatment efficacy. The FTSST is a standardized test that involves measuring the time it takes for a patient to rise from a seated position to standing and then sitting back down five times consecutively, with the goal of completing the task as quickly as possible.
One of the primary outcomes measured during the FTSST is the time taken to complete the task. In Parkinson patients, this time is often increased compared to healthy individuals, reflecting impaired functional mobility and lower extremity strength. PD patients have slower sit-to-stand performance due to a combination of factors, including bradykinesia, rigidity, and postural instability. These motor symptoms of PD can affect the coordination and timing of movements required for the FTSST, resulting in longer completion times.
The FTSST is a reliable and valid assessment tool for evaluating functional mobility in Parkinson patients, as it provides a standardized and quantifiable measure of lower extremity strength and motor performance [
21].
2.5.2. Functional Dysmetria Assessment
Functional dysmetria (FD) is a phenomenon characterized by asymmetric activation of homologous muscle groups on opposite sides of the body and a more general asymmetry in neuromotor control [
22]. The term was coined by Rinaldi and Fontani to describe this motor control deficit, which may arise from epigenetic adaptations that results in dysfunction within the neural circuits involved in motor control [
22,
23].
FD is thought to be a consequence of altered neural plasticity due to various factors, such as genetic, environmental, and developmental influences, resulting in an impaired ability to coordinate movements accurately [
22].
Previous studies have investigated the neural mechanisms that underlie functional dysmetria (FD), and they suggest that changes in connectivity and excitability within the cerebellum and cortical motor areas may drive and underpin this phenomenon [
23,
24,
25].
Functional dysmetria (FD) can be evaluated by assessing the activation of the quadriceps muscles during the transition from a supine to sitting position. To measure FD, an operator places their hands symmetrically on the patient’s quadriceps and perceives any asymmetrical activation of these muscle groups during the movement. The measurement of FD is typically given by calculating the difference in alignment between two reference points, which are often the operator’s thumbs, after the patient completes the movement.
2.5.3. Quality of Life Assessment
2.5.4. REAC Technology
The REAC neurobiological technology and treatments aim to modulate endogenous bioelectric activity at a global level by interacting with the cellular bioelectric activity and optimizing underlying mechanisms, such as neurotransmission.
The REAC technology employs low-intensity radio electric fields, asymmetrically conveyed inside the body to stimulate the cellular bioelectric activity and enhance the communication between neurons. This stimulation can lead to changes in the release of neurotransmitters and neuromodulators, which can have beneficial effects on the nervous system’s overall function. The REAC treatments have been shown to have a positive impact on various neurological and psychiatric conditions, including depression, anxiety, stress-related disorders, and chronic pain.
The device used in this study was BENE 110, produced by ASMED Srl in Florence, Italy.
2.5.5. REAC Neuro Postural Optimization
The REAC NPO treatment was developed to induce an initial and stable electrometabolic and functional reorganization in the brain that may have been altered by dysfunctional adaptive processes, including those with an epigenetic basis [
22]. The treatment involves a single administration lasting a few milliseconds that can modify the gradients of endogenous bioelectric activity, leading to a soliton effect that explains the long-term stability of even a single administration [
25].
The most notable clinical effect of the REAC NPO treatment is the induction of stable disappearance of functional dysmetria. This effect may be attributed to the treatment’s ability to modulate the bioelectric activity of neurons and enhance communication between brain regions, leading to improved neural plasticity and functional reorganization.
2.5.6. REAC Neuro Psycho Physical Optimization
REAC Neuro Psycho Physical Optimization (NPPO) are a cutting-edge neuromodulation treatment that maximizes human performance by considering the interconnectedness between the nervous system, psychological state, and physical body.
REAC NPPOs treatments have demonstrated efficacy in managing a range of mood and behavioral disorders, including those caused by epigenetic modifications such as in autism spectrum disorder [
26,
27].
2.6. Data Analysis
To assess the normality of the variables before and after the therapy, the Shapiro–Wilk Test was employed. For paired samples with normal distribution, Student’s “T” parametric test was used to compare pre- and post-therapy measurements. In contrast, for variables that did not follow a normal distribution, the Wilcoxon’s non-parametric test was utilized. A significance level of p < 0.05 was chosen for both tests.
3. Results
3.1. Functional Capacity Tests
3.1.1. Functional Dysmetria
The participants in the study underwent an evaluation for functional dysmetria (FD) prior to receiving REAC NPO treatment. The mean FD measurement at baseline was found to be 2 cm. The data were then analyzed using a paired
t-test to compare the measurements before and after treatment. The analysis revealed a highly significant statistical difference, with a
p-value of ≤0.001. Notably, following the administration of REAC NPO treatment, the levels of FD in all participants approached zero, as depicted in
Figure 1.
3.1.2. Five Times Sit to Stand Test
The five times sit to stand test (FTSST) was administered both before NPO and after 18 treatment cycles of REAC NPPO to assess the impact of the treatments on the variables pre-FTSST and post-FTSST. The results revealed a significant difference between these variables, as evidenced by a Wilcoxon statistic value (V) of 0.79616 and a corresponding p-value of 0.0003671. Specifically, a significant decrease in the median time to complete the task was observed from FTSST pre 21.98 s,
± SD 12.18, to FTSST post 17.15 s ± SD 7.26, with a reduction of 4.83 s (
Figure 2).
3.2. Quality of Life Assessment
4. Discussion
The aim of this study was to evaluate the impact of REAC NPO and a single cycle of REAC NPPO 18 treatment on the physical and mental well-being of individuals diagnosed with Parkinson’s disease. Specifically, we aimed to improve their functional capacity and quality of life through REAC neuromodulation treatments targeting mood and behavioral disorders.
The results regarding functional capabilities are generally intriguing and affirmative, warranting a detailed discussion to comprehend the underlying reasons for the observed outcomes.
Functional dysmetria is a complex maladaptive condition that affects neurological, psychological, and physical functioning and is likely influenced by epigenetic factors, as evidenced by changes in fluctuating asymmetry of morphological asymmetries. REACNPO treatment offers a promising approach to address this condition by promoting a more efficient and functional encephalic electro-metabolic reorganization [
25] that can optimize the body’s response to epigenetic decay.
In this study, we have shown that NPO can effectively reduce functional dysmetria in individuals with Parkinson’s disease, highlighting the dysfunctional component of the condition that can be addressed through this treatment approach.
Moreover, the results of the study suggest that REAC NPO and NPPOs treatments have a positive effect on the functional capacity of individuals with Parkinson’s disease, as demonstrated by the significant improvement in their ability to complete the FTSST. Specifically, the median time to complete the FTSST was significantly reduced after the treatments, which suggests that the REAC treatments may have improved in individuals with Parkinson’s disease strength, balance, and overall physical function.
The findings are consistent with previous studies that have investigated the effects of REAC treatments on physical function in older adults [
28].
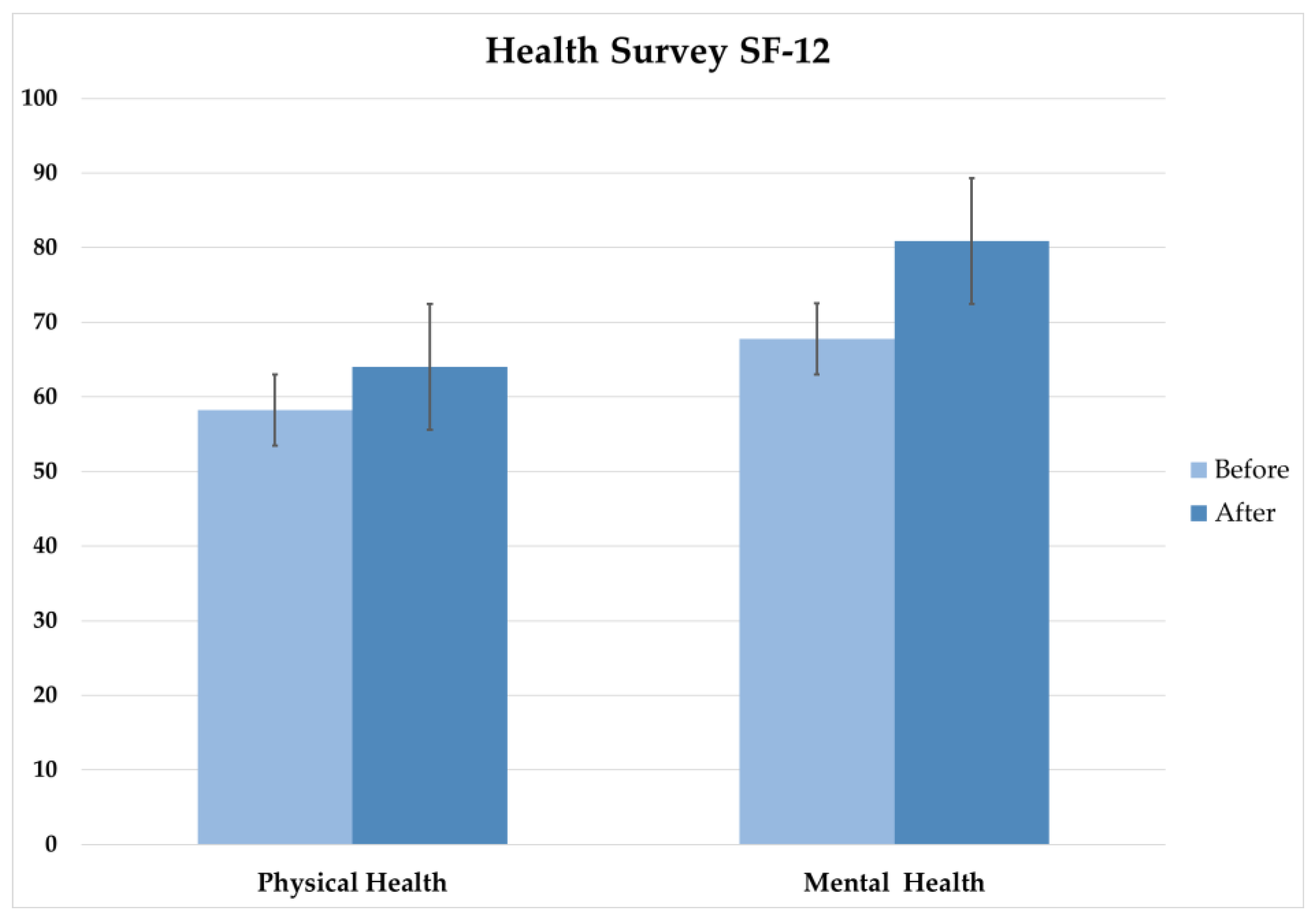
The results relating to the quality-of-life assessment obtained using the 12-item Short-Form Health Survey (SF-12) questionnaire was used to measure physical and mental health components before and after the intervention.
The statistical analysis showed significant improvements in both physical and mental health following the REAC NPO and NPPOs treatments. The Wilcoxon signed-rank test showed a significant improvement in physical health, while the paired t-test showed a significant improvement in mental health. These findings are consistent with previous studies that have shown the positive effects of REAC on physical and mental health.
The implications of these SF-12 results are significant, as the findings suggest that the REAC NPO and NPPOs treatment protocols can be an effective intervention results for improving both physical and mental health. This is particularly relevant in the context of the post-COVID-19 pandemic effects, where mental health issues have become increasingly prevalent.
The findings of this study have important implications, suggesting that REAC NPO and NPPOs neuromodulation treatments may be an effective intervention for improving physical function in older adults and individuals with Parkinson’s disease. This is particularly relevant given the increasing prevalence of age-related conditions that can lead to functional decline.
In addition to improving specific ailments, the beneficial impact of REAC NPO and NPPOs treatments on physical function can extend to enhancing overall health and wellbeing in older adults. This improvement can help them maintain their independence and quality of life. By aiding in the recovery and preservation of physical function, REAC treatments can contribute to a better quality of life, enabling older adults and individuals with Parkinson’s disease to engage in daily activities and maintain their sense of autonomy. This highlights the potential of REAC NPO and NPPOs treatments approach to promoting healthy aging and addressing the challenges faced by older adults also with Parkinson’s disease.
5. Conclusions
PD is a complex disorder with both motor and non-motor symptoms that can significantly impact daily functioning. Understanding the correlations between motor and non-motor symptoms is important for the effective management of the condition, and the use of advanced technologies, such as REAC NPO and NPPO neuromodulation treatments may offer new avenues for improving neuropsychic and physical performance in PD patients. More research is needed to explore the effects of multiple courses of REAC NPPOs treatments in individuals with Parkinson’s disease to optimize treatment protocols for maximum benefit.
Author Contributions
Author Contributions: Conceptualization, C.R., V.F., S.R., A.R.B. and C.B.L.; methodology, C.B.L., M.I.V., L.d.S.N., R.G.G., A.V.C. and D.D.; validation, C.B.L., D.D., M.I.V.; formal analysis, C.R., V.F., S.R., A.R.B. and C.B.L.; investigation A.V.C., J.D.O., D.D., L.d.S.N.; resources, J.D.O. and R.G.G.; data curation, A.V.C., R.G.G. and L.d.S.N.; writing—original draft preparation, C.R., V.F., S.R., A.R.B. and C.B.L.; writing—review and editing, C.R., V.F., S.R., A.R.B. and C.B.L.; visualization, M.I.V., J.D.O. and A.V.C.; supervision, C.R., C.B.L., M.I.V. and A.R.B.; project administration, A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by the International Scientific Society of Neuro Psycho Physical Optimization with REAC Technology.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the UNIFAP Ethics Committee in accordance with its opinion 5.232.551.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All study data are present in the manuscript.
Conflicts of Interest
C.R. is the daughter of S.R. and V.F. authors of the REAC technology patent. The other authors declare no conflict of interest.
References
- Barreto, K.S.; Oliveira, J.; Reis, L.D.; Ribeiro, T.G.; Kauark, R.B.G. Non-motor symptoms fluctuations in patients with Parkinson’s disease at the Clinical Hospital of Salvador, Bahia. Dement. Neuropsychol. 2022, 16, 213–219. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, S.; Singh, V.K.; Pathak, A.; Chaurasia, R.N.; Mishra, V.N.; Joshi, D. Assessment of Non-Motor Symptoms of Parkinson’s Disease and Their Impact on the Quality of Life: An Observatiobnal Study. Ann. Indian Acad. Neurol. 2022, 25, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Si, Q.; Tong, R.; Zhang, X.; Li, C.; Mao, S. Abnormal dynamic functional connectivity changes correlated with non-motor symptoms of Parkinson’s disease. Front. Neurosci. 2023, 17, 1116111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.Y.; Park, S.; Kim, R.O.; Lee, E.J.; Lee, M. Associations of cognitive dysfunction with motor and non-motor symptoms in patients with de novo Parkinson’s disease. Sci. Rep. 2022, 12, 11461. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, E.; Zucchella, C.; Argyriou, A.A.; Tamburin, S. Treatment for cognitive and neuropsychiatric non-motor symptoms in Parkinson’s disease: Current evidence and future perspectives. Expert. Rev. Neurother. 2023, 23, 25–43. [Google Scholar] [CrossRef]
- Schaffner, S.L.; Kobor, M.S. DNA methylation as a mediator of genetic and environmental influences on Parkinson’s disease susceptibility: Impacts of alpha-Synuclein, physical activity, and pesticide exposure on the epigenome. Front. Genet. 2022, 13, 971298. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Greco, A.; Seripa, D. Genetic Profile, Environmental Exposure, and Their Interaction in Parkinson’s Disease. Parkinsons Dis. 2016, 2016, 6465793. [Google Scholar] [CrossRef] [PubMed]
- Talavera Andujar, B.; Aurich, D.; Aho, V.T.E.; Singh, R.R.; Cheng, T.; Zaslavsky, L.; Bolton, E.E.; Mollenhauer, B.; Wilmes, P.; Schymanski, E.L. Studying the Parkinson’s disease metabolome and exposome in biological samples through different analytical and cheminformatics approaches: A pilot study. Anal. Bioanal. Chem. 2022, 414, 7399–7419. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Paudel, Y.N.; Papageorgiou, S.G.; Piperi, C. Environmental Impact on the Epigenetic Mechanisms Underlying Parkinson’s Disease Pathogenesis: A Narrative Review. Brain Sci. 2022, 12, 175. [Google Scholar] [CrossRef]
- Khedr, E.M.; Abdelrahman, A.A.; Elserogy, Y.; Zaki, A.F.; Gamea, A. Depression and anxiety among patients with Parkinson’s disease: Frequency, risk factors, and impact on quality of life. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 116. [Google Scholar] [CrossRef]
- Marsh, L. Depression and Parkinson’s disease: Current knowledge. Curr. Neurol. Neurosci. Rep. 2013, 13, 409. [Google Scholar] [CrossRef]
- Papapetropoulos, S.; Ellul, J.; Argyriou, A.A.; Chroni, E.; Lekka, N.P. The effect of depression on motor function and disease severity of Parkinson’s disease. Clin. Neurol. Neurosurg. 2006, 108, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Prange, S.; Klinger, H.; Laurencin, C.; Danaila, T.; Thobois, S. Depression in Patients with Parkinson’s Disease: Current Understanding of its Neurobiology and Implications for Treatment. Drugs Aging 2022, 39, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.; Farkas, D.K.; Gradus, J.L.; Lash, T.L.; Sorensen, H.T. Adjustment disorder and risk of Parkinson’s disease. Eur. J. Neurol. 2016, 23, 751–756. [Google Scholar] [CrossRef]
- Barer, Y.; Chodick, G.; Glaser Chodick, N.; Gurevich, T. Risk of Parkinson Disease Among Adults With vs Without Posttraumatic Stress Disorder. JAMA Netw. Open 2022, 5, e2225445. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Kelber, M.S.; Bellanti, D.M.; Beech, E.H.; Boyd, C.; Galloway, L.; Ojha, S.; Garvey Wilson, A.L.; Otto, J.; Belsher, B.E. Outcomes and prognosis of adjustment disorder in adults: A systematic review. J. Psychiatr. Res. 2022, 156, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Kang, H. Sample size determination and power analysis using the G*Power software. J. Educ. Eval. Health Prof. 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Munoz, M.; Barrios-Fernandez, S.; Garcia-Gordillo, M.A.; Perez-Gomez, J.; Carlos-Vivas, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Leddy, A.L.; Earhart, G.M. Five times sit-to-stand test performance in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2011, 92, 1431–1436. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, A.; Rinaldi, C.; Araldi, L.; Azzara, A.; Carta, A.M.; Casale, N.; Castagna, A.; Del Medico, M.; Di Stasio, M.; et al. Long-Lasting Efficacy of Radio Electric Asymmetric Conveyer Neuromodulation Treatment on Functional Dysmetria, an Adaptive Motor Behavior. Cureus 2022, 14, e25768. [Google Scholar] [CrossRef]
- Mura, M.; Castagna, A.; Fontani, V.; Rinaldi, S. Preliminary pilot fMRI study of neuropostural optimization with a noninvasive asymmetric radioelectric brain stimulation protocol in functional dysmetria. Neuropsychiatr. Dis. Treat. 2012, 8, 149–154. [Google Scholar] [CrossRef]
- Rinaldi, S.; Fontani, V.; Castagna, A. Brain activity modification produced by a single radioelectric asymmetric brain stimulation pulse: A new tool for neuropsychiatric treatments. Preliminary fMRI study. Neuropsychiatr. Dis. Treat. 2011, 7, 649–654. [Google Scholar] [CrossRef]
- Rinaldi, S.; Mura, M.; Castagna, A.; Fontani, V. Long-lasting changes in brain activation induced by a single REAC technology pulse in Wi-Fi bands. Randomized double-blind fMRI qualitative study. Sci. Rep. 2014, 4, 5668. [Google Scholar] [CrossRef]
- Rinaldi, A.; Maioli, M.; Marins Martins, M.C.; de Castro, P.C.F.; de Oliveira Silva, N.A.P.; de Mattos, J.A.V.; Fontani, V.; Rinaldi, S. REAC Non-invasive Neurobiological Stimulation for Mitigating the Impact of Internalizing Disorders in Autism Spectrum Disorder. Adv. Neurodev. Disord. 2021, 5, 446–456. [Google Scholar] [CrossRef]
- Rinaldi, A.; Martins, M.C.M.; Maioli, M.; Rinaldi, S.; Fontani, V. REAC Noninvasive Neurobiological Stimulation in Autism Spectrum Disorder for Alleviating Stress Impact. Adv. Neurodev. Disord. 2023, 7, 244–251. [Google Scholar] [CrossRef]
- Fontani, V.; Rinaldi, S.; Castagna, A.; Margotti, M.L. Noninvasive radioelectric asymmetric conveyor brain stimulation treatment improves balance in individuals over 65 suffering from neurological diseases: Pilot study. Ther. Clin. Risk Manag. 2012, 8, 73–78. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).