Deep Clinical Phenotyping of Schizophrenia Spectrum Disorders Using Data-Driven Methods: Marching towards Precision Psychiatry
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Population
2.2. Measurements
2.3. Data Analyses
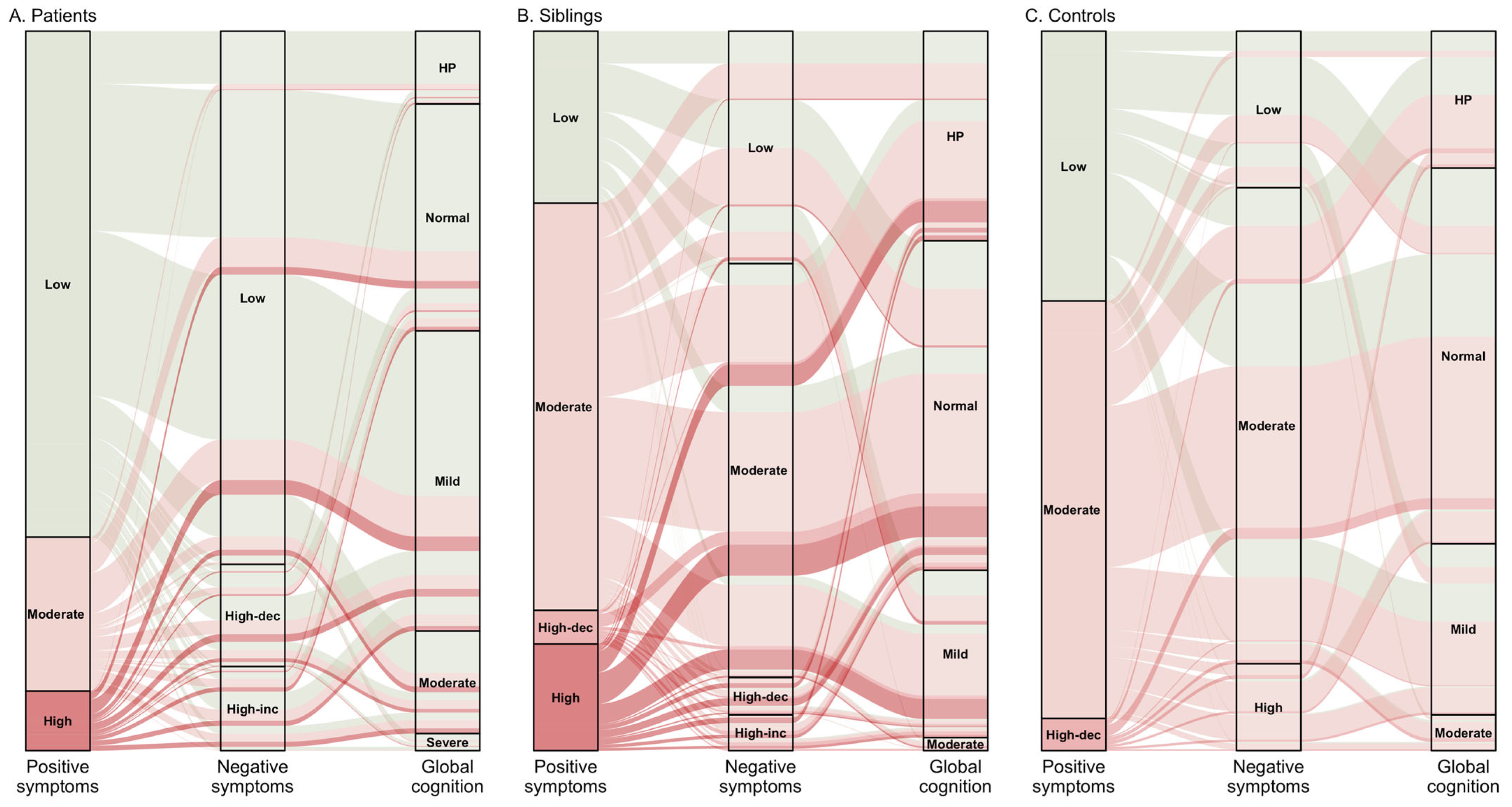
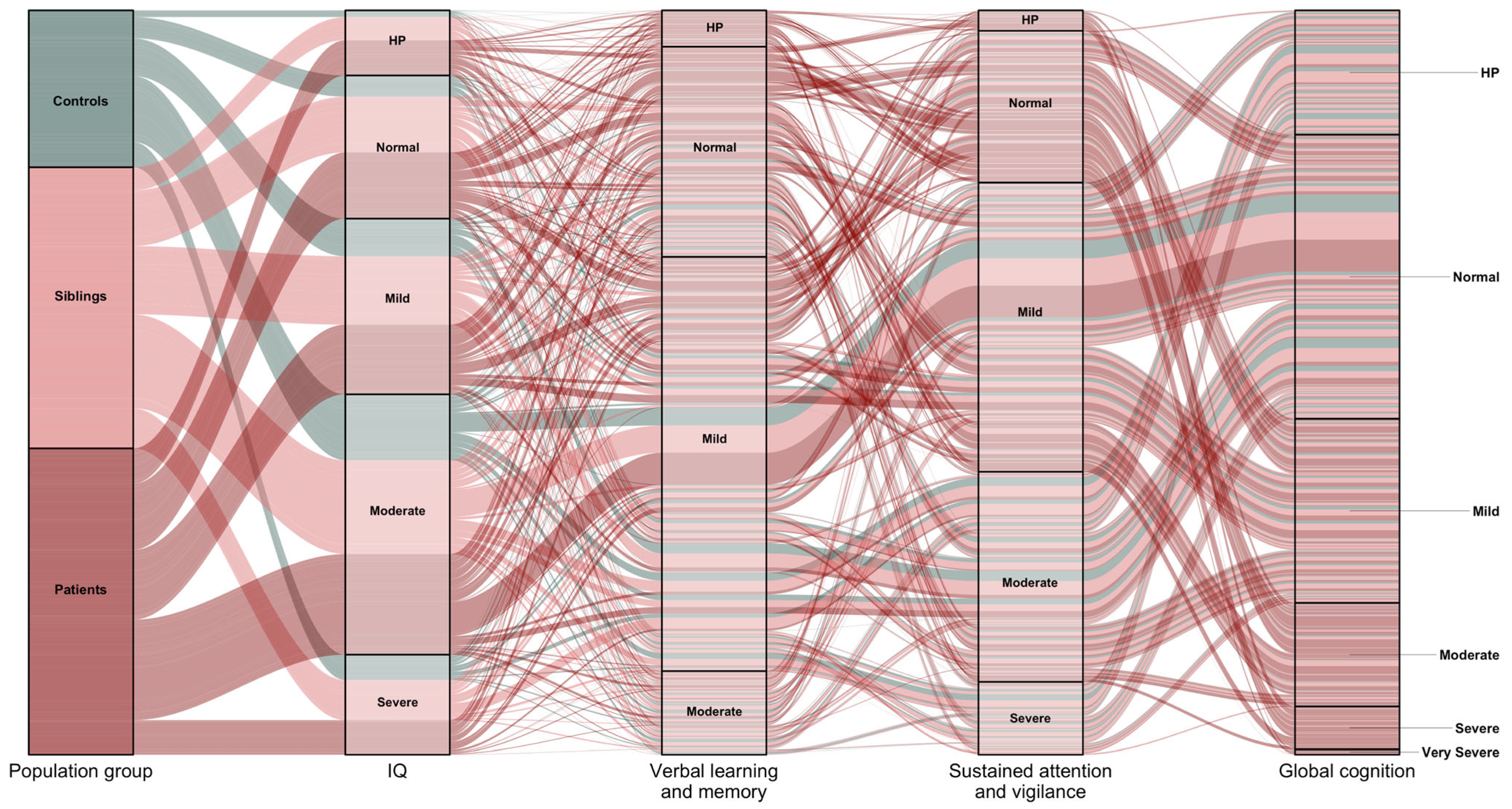
3. Results
3.1. Latent Subtypes
3.2. Longitudinal Courses and Profiles of Subtypes
3.3. Predictors of Latent Subtypes
4. Discussion
5. Strengths and Limitations
6. Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, M.J.; Sawa, A.; Mortensen, P.B. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Charlson, F.J.; Ferrari, A.J.; Santomauro, D.F.; Diminic, S.; Stockings, E.; Scott, J.G.; McGrath, J.J.; Whiteford, H. Global Epidemiology and Burden of Schizophrenia: Findings from the Global Burden of Disease Study 2016. Schizophr. Bull. 2018, 44, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub: Arlington, VA, USA, 2013. [Google Scholar]
- McCutcheon, R.; Marques, T.R.; Howes, O.D. Schizophrenia—An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef]
- Zarogianni, E.; Storkey, A.J.; Johnstone, E.C.; Owens, D.G.; Lawrie, S.M. Improved individualized prediction of schizophrenia in subjects at familial high risk, based on neuroanatomical data, schizotypal and neurocognitive features. Schizophr. Res. 2017, 181, 6–12. [Google Scholar] [CrossRef]
- Keefe, R.S.E.; Fenton, W.S. How Should DSM-V Criteria for Schizophrenia Include Cognitive Impairment? Schizophr. Bull. 2007, 33, 912–920. [Google Scholar] [CrossRef]
- Walker, A.E.; Spring, J.D.; Travis, M.J. Addressing Cognitive Deficits in Schizophrenia: Toward a Neurobiologically Informed Approach. Biol. Psychiatry 2017, 81, e1–e3. [Google Scholar] [CrossRef]
- Lencz, T.; Knowles, E.; Davies, G.; Guha, S.; Liewald, D.C.; Starr, J.M.; Djurovic, S.; Melle, I.; Sundet, K.; Christoforou, A.; et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: A report from the Cognitive Genomics consorTium (COGENT). Mol. Psychiatry 2013, 19, 168–174. [Google Scholar] [CrossRef]
- Blackman, R.K.; Dickinson, D.; Eisenberg, D.P.; Gregory, M.D.; Apud, J.A.; Berman, K.F. Antipsychotic medication-mediated cognitive change in schizophrenia and polygenic score for cognitive ability. Schizophr. Res. Cogn. 2021, 27, 100223. [Google Scholar] [CrossRef]
- Cooper, A.M.; Michels, R. Diagnostic and statistical manual of mental disorders, revised (DSM-III-R). Am. J. Psychiatry 1988, 145, 1300–1301. [Google Scholar] [CrossRef]
- Yung, A.R.; Phillips, L.J.; Yuen, H.P.; McGorry, P.D. Risk factors for psychosis in an ultra high-risk group: Psychopathology and clinical features. Schizophr. Res. 2004, 67, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castroman, J.; Leiva-Murillo, J.M.; Cegla-Schvartzman, F.; Blasco-Fontecilla, H.; Garcia-Nieto, R.; Artes-Rodriguez, A.; Morant-Ginestar, C.; Courtet, P.; Blanco, C.; Aroca, F.; et al. Onset of schizophrenia diagnoses in a large clinical cohort. Sci. Rep. 2019, 9, 9865. [Google Scholar] [CrossRef] [PubMed]
- Korth, C.; Fangerau, H. Blood tests to diagnose schizophrenia: Self-imposed limits in psychiatry. Lancet Psychiatry 2020, 7, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M. Antipsychotic treatment in elderly patients on polypharmacy with schizophrenia. Curr. Opin. Psychiatry 2022, 35, 332–337. [Google Scholar] [CrossRef]
- Muthen, B.; Muthen, L.K. Integrating Person-Centered and Variable-Centered Analyses: Growth Mixture Modeling with Latent Trajectory Classes. Alcohol. Clin. Exp. Res. 2000, 24, 882–891. [Google Scholar] [CrossRef]
- Delude, C.M. Deep phenotyping: The details of disease. Nature 2015, 527, S14–S15. [Google Scholar] [CrossRef]
- Robinson, P.N. Deep phenotyping for precision medicine. Hum. Mutat. 2012, 33, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Habtewold, T.D.; Tiles-Sar, N.; Liemburg, E.J.; Sandhu, A.K.; Islam, M.A.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z. Six-year trajectories and associated factors of positive and negative symptoms in schizophrenia patients, siblings, and controls: The GROUP study. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Stiekema, A.P.; Islam, A.; Liemburg, E.J.; Castelein, S.; Heuvel, E.R.V.D.; van Weeghel, J.; Aleman, A.; Bruggeman, R.; van der Meer, L. Long-term course of negative symptom subdomains and relationship with outcome in patients with a psychotic disorder. Schizophr. Res. 2018, 193, 173–181. [Google Scholar] [CrossRef]
- Habtewold, T.D.; Liemburg, E.J.; Islam, A.; de Zwarte, S.M.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z.; Luykx, J.J.; Rutten, B.P.; van Winkel, R.; et al. Association of schizophrenia polygenic risk score with data-driven cognitive subtypes: A six-year longitudinal study in patients, siblings and controls. Schizophr. Res. 2020, 223, 135–147. [Google Scholar] [CrossRef]
- Islam, A.; Habtewold, T.D.; van Es, F.D.; Quee, P.J.; Heuvel, E.R.V.D.; Alizadeh, B.Z.; Bruggeman, R.; GROUP Investigators. Long-term cognitive trajectories and heterogeneity in patients with schizophrenia and their unaffected siblings. Acta Psychiatr. Scand. 2018, 138, 591–604. [Google Scholar] [CrossRef]
- Quee, P.J.; Alizadeh, B.Z.; Aleman, A.; Heuvel, E.R.V.D.; GROUP Investigators. Cognitive subtypes in non-affected siblings of schizophrenia patients: Characteristics and profile congruency with affected family members. Psychol. Med. 2014, 44, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Tiles-Sar, N.; Liemburg, E.J.; Habtewold, T.D.; Bruggeman, R.; van der Meer, L.; Alizadeh, B.Z. Multidimensional social inclusion and its prediction in schizophrenia spectrum disorder. Res. Sq. 2023. [Google Scholar]
- Quee, P.J.; GROUP Investigators; Meijer, J.H.; Islam, A.; Aleman, A.; Alizadeh, B.Z.; Meijer, C.J.; Heuvel, E.R.V.D. Premorbid adjustment profiles in psychosis and the role of familial factors. J. Abnorm. Psychol. 2014, 123, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Tiles-Sar, N.; Habtewold, T.D.; Liemburg, E.J.; van der Meer, L.; Bruggeman, R.; Alizadeh, B.Z.; van Amelsvoort, T.; Bartels-Velthuis, A.; de Haan, L.; Schirmbeck, F.; et al. Understanding Lifelong Factors and Prediction Models of Social Functioning After Psychosis Onset Using the Large-Scale GROUP Cohort Study. Schizophr. Bull. 2023, sbad046. [Google Scholar] [CrossRef]
- Korver, N.; Quee, P.J.; Boos, H.B.M.; Simons, C.J.P.; de Haan, L.; GROUP Investigators. Genetic Risk and Outcome of Psychosis (GROUP), a multi site longitudinal cohort study focused on gene-environment interaction: Objectives, sample characteristics, recruitment and assessment methods. Int. J. Methods Psychiatr. Res. 2012, 21, 205–221. [Google Scholar] [CrossRef]
- Bell, C.C. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. JAMA 1994, 272, 828–829. [Google Scholar] [CrossRef]
- Habtewold, T. Data-Driven Subphenotypic Dissection of the Clinical Heterogeneity of Schizophrenia Spectrum Disorders. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2021. [Google Scholar]
- Habtewold, T.D.; Rodijk, L.H.; Liemburg, E.J.; Sidorenkov, G.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl. Psychiatry 2020, 10, 224. [Google Scholar] [CrossRef]
- Hall, M.-H.; Holton, K.M.; Öngür, D.; Montrose, D.; Keshavan, M.S. Longitudinal trajectory of early functional recovery in patients with first episode psychosis. Schizophr. Res. 2019, 209, 234–244. [Google Scholar] [CrossRef]
- Chang, W.C.; Chu, A.O.K.; Kwong, V.W.Y.; Wong, C.S.M.; Hui, C.L.; Chan, K.W.; Lee, H.M.E.; Chen, E. Patterns and predictors of trajectories for social and occupational functioning in patients presenting with first-episode non-affective psychosis: A three-year follow-up study. Schizophr. Res. 2018, 197, 131–137. [Google Scholar] [CrossRef]
- Kam, C.T.K.; Chang, W.C.; Kwong, V.W.Y.; Lau, E.S.K.; Chan, G.H.K.; Jim, O.T.T.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chen, E.Y.H. Patterns and predictors of trajectories for subjective quality of life in patients with early psychosis: Three-year follow-up of the randomized controlled trial on extended early intervention. Aust. N. Z. J. Psychiatry 2021, 55, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Krumholz, H.M.; Allore, H.G. Using Latent Class Analysis to Identify Hidden Clinical Phenotypes. In JAMA Guide to Statistics and Methods; Livingston, E.H., Lewis, R.J., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Hayes, A.M.; Feldman, G. Clarifying the construct of mindfulness in the context of emotion regulation and the process of change in therapy. Clin. Psychol. Sci. Pract. 2004, 11, 255–262. [Google Scholar] [CrossRef]
- Hayes, S.C.; Hofmann, S.G. The third wave of cognitive behavioral therapy and the rise of process-based care. World Psychiatry 2017, 16, 245–246. [Google Scholar] [CrossRef]
- Zanardi, R.; Prestifilippo, D.; Fabbri, C.; Colombo, C.; Maron, E.; Serretti, A. Precision psychiatry in clinical practice. Int. J. Psychiatry Clin. Pract. 2020, 25, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Snitz, B.; MacDonald, A.W., III; Carter, C.S. Cognitive Deficits in Unaffected First-Degree Relatives of Schizophrenia Patients: A Meta-analytic Review of Putative Endophenotypes. Schizophr. Bull. 2005, 32, 179–194. [Google Scholar] [CrossRef]
- Greenwood, T.A.; Lazzeroni, L.C.; Calkins, M.E.; Freedman, R.; Green, M.F.; Gur, R.E.; Gur, R.C.; Light, G.A.; Nuechterlein, K.H.; Olincy, A.; et al. Genetic assessment of additional endophenotypes from the Consortium on the Genetics of Schizophrenia Family Study. Schizophr. Res. 2016, 170, 30–40. [Google Scholar] [CrossRef]
- van Os, J.; Guloksuz, S. A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry 2017, 16, 200–206. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Pan, C.-H.; Chang, C.-K.; Chen, P.-H.; Chang, H.-M.; Tai, M.-H.; Su, S.-S.; Tsai, S.-Y.; Chen, C.-C.; Kuo, C.-J. Physical Illnesses Before Diagnosed as Schizophrenia: A Nationwide Case-Control Study. Schizophr. Bull. 2020, 46, 785–794. [Google Scholar] [CrossRef]
- Eack, S.M.; Keshavan, M.S. Cognition, negative symptoms, and functional outcome in psychosis. Schizophr. Res. 2020, 224, 22–23. [Google Scholar] [CrossRef]
- Harvey, P.D.; Koren, D.; Reichenberg, A.; Bowie, C.R. Negative Symptoms and Cognitive Deficits: What Is the Nature of Their Relationship? Schizophr. Bull. 2006, 32, 250–258. [Google Scholar] [CrossRef]
- Carrà, G.; Crocamo, C.; Angermeyer, M.; Brugha, T.; Toumi, M.; Bebbington, P. Positive and negative symptoms in schizophrenia: A longitudinal analysis using latent variable structural equation modelling. Schizophr. Res. 2019, 204, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Roffman, J.L. Endophenotype Research in Psychiatry—The Grasshopper Grows Up. JAMA Psychiatry 2019, 76, 1230. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.B. Polygenic risk scores in schizophrenia: Ready for the real world? Am. Psychiatr. Assoc. 2019, 174, 783–784. [Google Scholar] [CrossRef]
- Zheutlin, A.B.; Dennis, J.; Linnér, R.K.; Moscati, A.; Restrepo, N.; Straub, P.; Ruderfer, D.; Castro, V.M.; Chen, C.-Y.; Ge, T.; et al. Penetrance and Pleiotropy of Polygenic Risk Scores for Schizophrenia in 106,160 Patients Across Four Health Care Systems. Am. J. Psychiatry 2019, 176, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, K.; Read, J.; Corcoran, R.; Kinderman, P. Heterogeneity in psychiatric diagnostic classification. Psychiatry Res. 2019, 279, 15–22. [Google Scholar] [CrossRef]
- Feczko, E.; Miranda-Dominguez, O.; Marr, M.; Graham, A.M.; Nigg, J.T.; Fair, D.A. The Heterogeneity Problem: Approaches to Identify Psychiatric Subtypes. Trends Cogn. Sci. 2019, 23, 584–601. [Google Scholar] [CrossRef]
- Twisk, J.; Hoekstra, T. Classifying developmental trajectories over time should be done with great caution: A comparison between methods. J. Clin. Epidemiol. 2012, 65, 1078–1087. [Google Scholar] [CrossRef]
- Quiroz, M.; Villani, M. Dynamic mixture-of-experts models for longitudinal and discrete-time survival data. Riksbank Res. Pap. Ser. 2013, 99, 39. [Google Scholar] [CrossRef]
- Ram, N.; Grimm, K.J. Methods and Measures: Growth mixture modeling: A method for identifying differences in longitudinal change among unobserved groups. Int. J. Behav. Dev. 2009, 33, 565–576. [Google Scholar] [CrossRef]
- Glymour, C.; Zhang, K.; Spirtes, P. Review of Causal Discovery Methods Based on Graphical Models. Front. Genet. 2019, 10, 524. [Google Scholar] [CrossRef]
- Rohrer, J.M. Thinking Clearly About Correlations and Causation: Graphical Causal Models for Observational Data. Adv. Methods Prac. Psychol. Sci. 2018, 1, 27–42. [Google Scholar] [CrossRef]
- Fanous, A.H.; Kendler, K.S. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: Searching for a framework. Mol. Psychiatry 2005, 10, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J.; Heron, J.; Hammerton, G.; Stochl, J.; Jones, P.B.; Cannon, M.; Smith, G.D.; Holmans, P.; Lewis, G.; Linden, D.E.J.; et al. Investigating the genetic architecture of general and specific psychopathology in adolescence. Transl. Psychiatry 2018, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; German, C.A.; Jensen, A.; Shen, J.; Wang, A.; Mehrotra, D.V.; Sun, Y.V.; Sinsheimer, J.S.; Zhou, H.; Zhou, J.J. GWAS of longitudinal trajectories at biobank scale. Am. J. Hum. Genet. 2022, 109, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, T.B.; Peterson, R.E.; Ripke, S.; Bacanu, S.-A.; Amdur, R.L.; Gejman, P.V.; Levinson, D.F.; Riley, B.R.; St. Clair, D.; Rietschel, M.; et al. Genome-wide association study of clinical features in the schizophrenia psychiatric genomics consortium: Confirmation of polygenic effect on negative symptoms. bioRxiv 2017. [Google Scholar] [CrossRef]
- Jonas, K.G.; Lencz, T.; Li, K.; Malhotra, A.K.; Perlman, G.; Fochtmann, L.J.; Bromet, E.J.; Kotov, R. Schizophrenia polygenic risk score and 20-year course of illness in psychotic disorders. Transl. Psychiatry 2019, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Arehart, C.H.; Sterrett, J.D.; Garris, R.L.; Quispe-Pilco, R.E.; Gignoux, C.R.; Evans, L.M.; Stanislawski, M.A. Poly-omic risk scores predict inflammatory bowel disease diagnosis. bioRxiv 2022, 508056. [Google Scholar]
- Wheeler, H.E.; Aquino-Michaels, K.; Gamazon, E.R.; Trubetskoy, V.V.; Dolan, M.E.; Huang, R.S.; Cox, N.J.; Im, H.K. Poly-Omic Prediction of Complex Traits: OmicKriging. Genet. Epidemiol. 2014, 38, 402–415. [Google Scholar] [CrossRef]
- Habtewold, T.D.; Islam, A.; Liemburg, E.J.; Bruggeman, R.; Alizadeh, B.Z.; Bartels-Velthuis, A.A.; van Beveren, N.J.; Cahn, W.; de Haan, L.; Delespaul, P.; et al. Polygenic risk score for schizophrenia was not associated with glycemic level (HbA1c) in patients with non-affective psychosis: Genetic Risk and Outcome of Psychosis (GROUP) cohort study. J. Psychosom. Res. 2020, 132, 109968. [Google Scholar] [CrossRef]
- McElreath, R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Li, Y.; Lord-Bessen, J.; Shiyko, M.; Loeb, R. Bayesian Latent Class Analysis Tutorial. Multivar. Behav. Res. 2018, 53, 430–451. [Google Scholar] [CrossRef]
- Asparouhov, T.; Muthén, B. Using Bayesian priors for more flexible latent class analysis. In Proceedings of the 2011 Joint Statistical Meeting, Miami Beach, FL, USA, 4 August 2011; American Statistical Association: Alexandria, VA, USA, 2011. [Google Scholar]
- Sarker, I.H. Machine Learning: Algorithms, Real-World Applications and Research Directions. SN Comput. Sci. 2021, 2, 160. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habtewold, T.D.; Hao, J.; Liemburg, E.J.; Baştürk, N.; Bruggeman, R.; Alizadeh, B.Z. Deep Clinical Phenotyping of Schizophrenia Spectrum Disorders Using Data-Driven Methods: Marching towards Precision Psychiatry. J. Pers. Med. 2023, 13, 954. https://doi.org/10.3390/jpm13060954
Habtewold TD, Hao J, Liemburg EJ, Baştürk N, Bruggeman R, Alizadeh BZ. Deep Clinical Phenotyping of Schizophrenia Spectrum Disorders Using Data-Driven Methods: Marching towards Precision Psychiatry. Journal of Personalized Medicine. 2023; 13(6):954. https://doi.org/10.3390/jpm13060954
Chicago/Turabian StyleHabtewold, Tesfa Dejenie, Jiasi Hao, Edith J. Liemburg, Nalan Baştürk, Richard Bruggeman, and Behrooz Z. Alizadeh. 2023. "Deep Clinical Phenotyping of Schizophrenia Spectrum Disorders Using Data-Driven Methods: Marching towards Precision Psychiatry" Journal of Personalized Medicine 13, no. 6: 954. https://doi.org/10.3390/jpm13060954
APA StyleHabtewold, T. D., Hao, J., Liemburg, E. J., Baştürk, N., Bruggeman, R., & Alizadeh, B. Z. (2023). Deep Clinical Phenotyping of Schizophrenia Spectrum Disorders Using Data-Driven Methods: Marching towards Precision Psychiatry. Journal of Personalized Medicine, 13(6), 954. https://doi.org/10.3390/jpm13060954






