Chronic Venous Disease during Pregnancy Is Related to Inflammation of the Umbilical Cord: Role of Allograft Inflammatory Factor 1 (AIF-1) and Interleukins 10 (IL-10), IL-12 and IL-18
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Inclusion/Exclusion Criteria and Clinical Assessment
2.3. Tissue Samples Management
2.4. Gene Expression Investigations Utilizing Quantitative PCR and Reverse Transcription (RT-qPCR)
2.5. Immunohistochemical Studies
2.6. Statistical and Microscopical Determination
3. Results
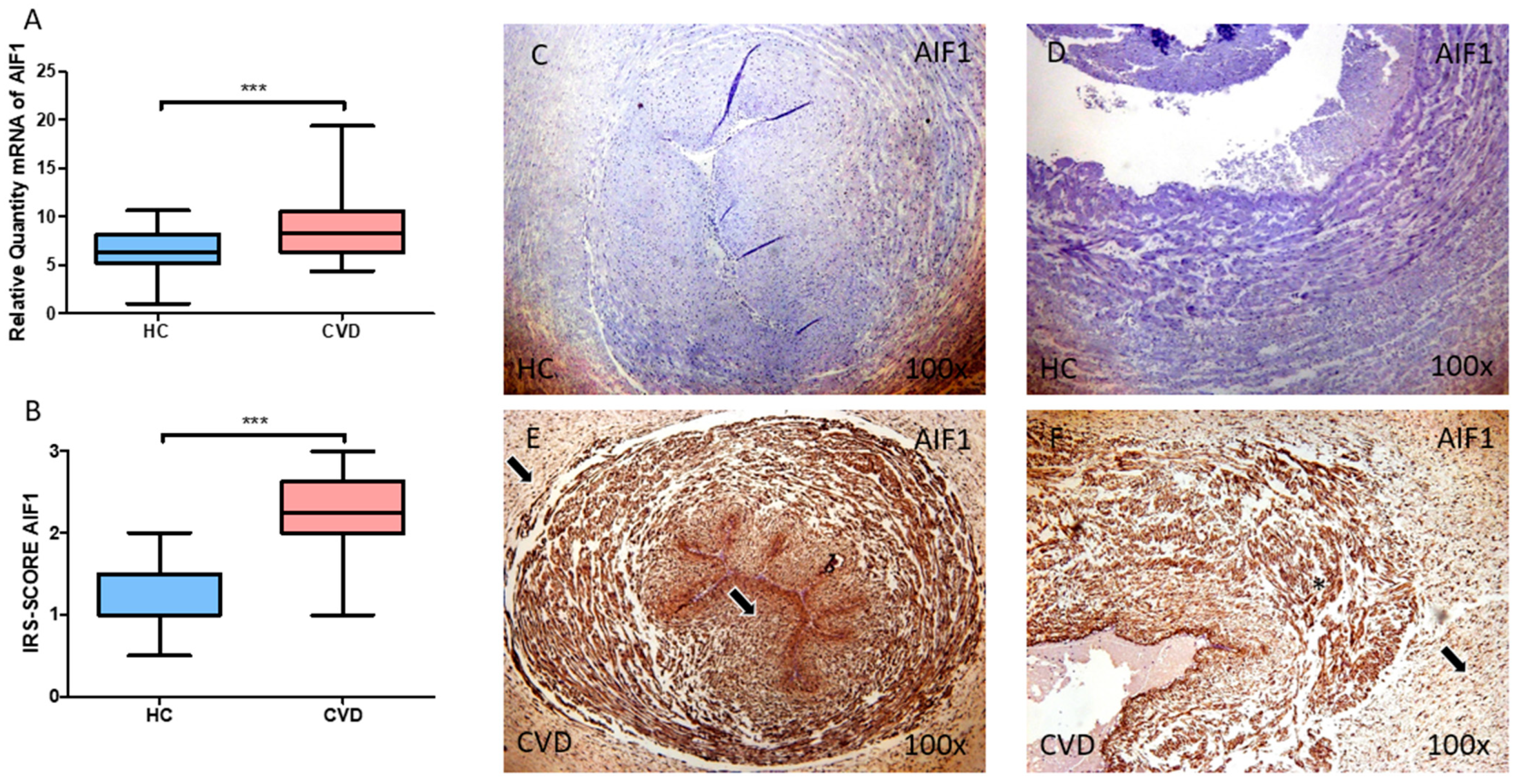
3.1. The Umbilical Cord of Women with Chronic Venous Disease during Pregnancy Exhibit Increased Allograft Inflammatory Factor (AIF-1) Expression
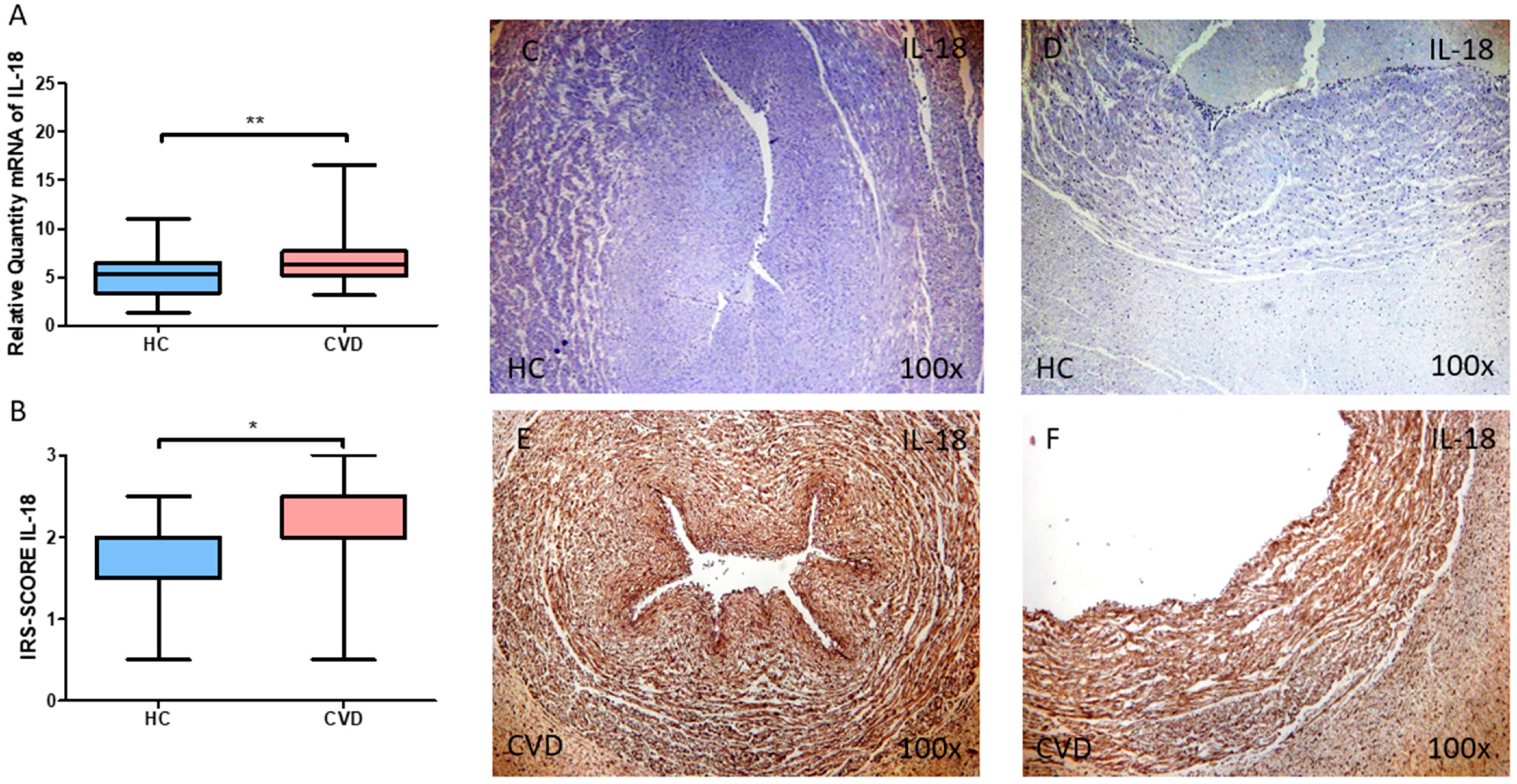
3.2. The Umbilical Cord of Women with Chronic Venous Disease during Pregnancy Display a Greater Expression of the Proinflammatory Cytokines IL-12A and IL-18
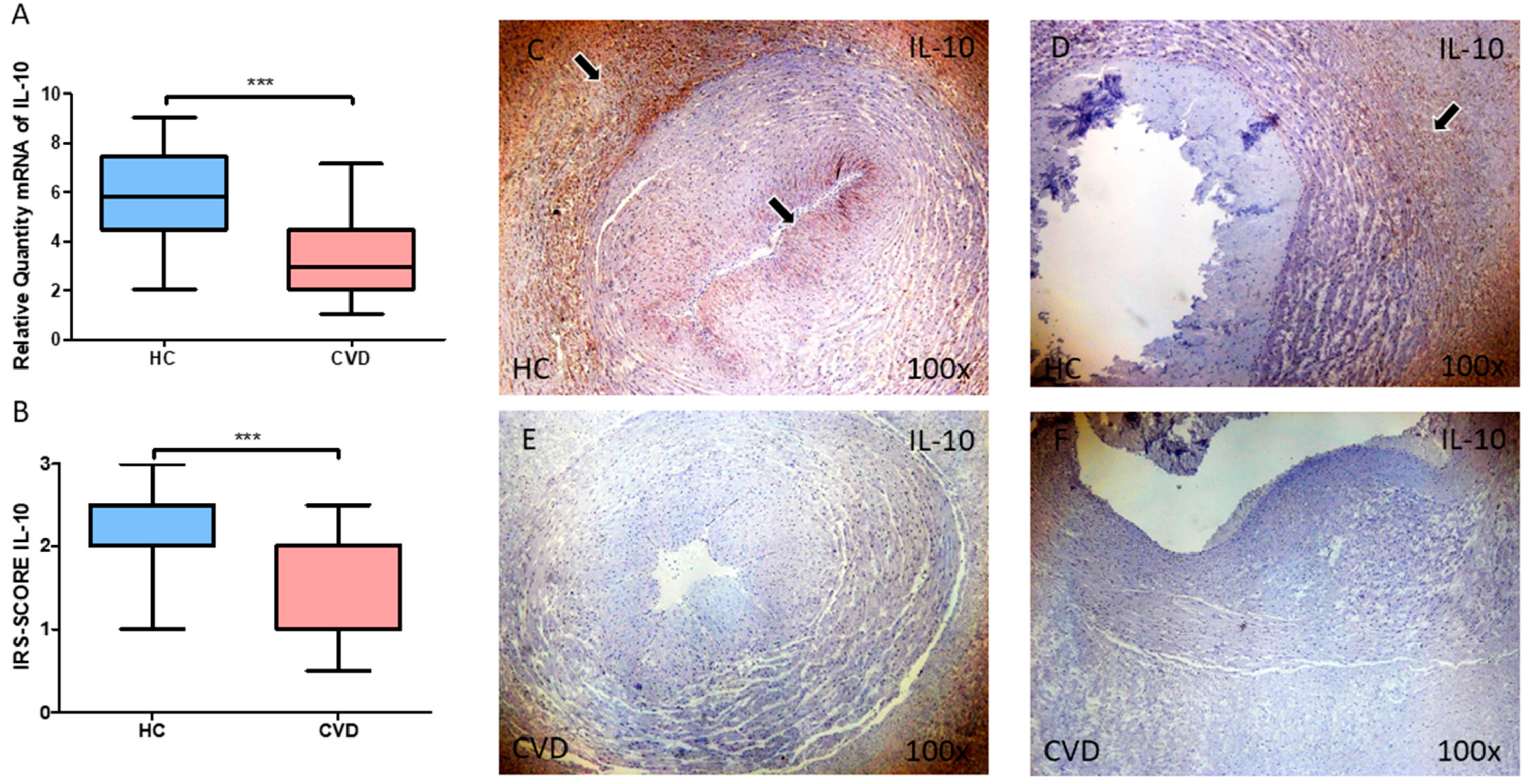
3.3. The Umbilical Cord of Women with Chronic Venous Disease during Pregnancy Show a Marked Reduction in the Expression of the Anti-Inflammatory Cytokine IL-10
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffetto, J.D.; Mannello, F. Pathophysiology of chronic venous disease. Int. Angiol. Ed. Minerva Med. 2014, 33, 212–221. [Google Scholar]
- Barron, G.S.; Jacob, S.E.; Kirsner, R.S. Dermatologic Complications of Chronic Venous Disease: Medical Management and Beyond. Ann. Vasc. Surg. 2007, 21, 652–662. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef] [PubMed]
- Vlajinac, H.D.; Radak, J.; Marinković, J.M.; Maksimović, M. Risk factors for chronic venous disease. Phlebology 2012, 27, 416–422. [Google Scholar] [CrossRef] [PubMed]
- de Barros, N.; Perez, M.D.C.J.; de Amorim, J.E.; Miranda, F. Pregnancy and lower limb varicose veins: Prevalence and risk factors. J. Vasc. Bras. Soc. Bras. Angiol. Cir. Vasc. (SBACV) 2010, 9, 29–35. [Google Scholar]
- Ropacka-Lesiak, M.; Jarosław, K.; Bręborowicz, G. Pregnancy-dependent blood flow velocity changes in lower extremities veins in venous insufficiency. Ginekol. Polska 2015, 86. [Google Scholar] [CrossRef]
- Troiano, N.H. Physiologic and hemodynamic changes during pregnancy. AACN Adv. Crit. Care Am. Assoc. Crit. Care Nurses 2018, 29, 273–283. [Google Scholar] [CrossRef]
- Labropoulos, N. How Does Chronic Venous Disease Progress from the First Symptoms to the Advanced Stages? A Review. Adv. Ther. Springer Healthc. 2019, 36, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.; Hicks, C.; Heller, J.A. The hemodynamic effects of pregnancy on the lower extremity venous system. J. Vasc. Surgery Venous Lymphat. Disord. 2018, 6, 246–255. [Google Scholar] [CrossRef]
- Morton, A. Physiological Changes and Cardiovascular Investigations in Pregnancy. Heart Lung Circ. 2021, 30, e6–e15. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef] [PubMed]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 dichotomy of pregnancy and preterm labour. Mediat. Inflamm. 2012, 2012, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, M.A.; Gómez-Lahoz, A.M.; Sánchez-Trujillo, L.; Fraile-Martinez, O.; García-Montero, C.; Guijarro, L.G.; Bravo, C.; De Leon-Luis, J.A.; Saz, J.V.; Bujan, J.; et al. Chronic Venous Disease during Pregnancy Causes a Systematic Increase in Maternal and Fetal Proinflammatory Markers. Int. J. Mol. Sci. 2022, 23, 8976. [Google Scholar] [CrossRef] [PubMed]
- Hammad, I.A.; Blue, N.; Allshouse, A.A.; Silver, R.M.; Gibbins, K.J.; Page, J.M.; Goldenberg, R.L.; Reddy, U.M.; Saade, G.R.; Dudley, D.J.; et al. Umbilical Cord Abnormalities and Stillbirth. Obstet. Gynecol. 2020, 135, 644–652. [Google Scholar] [CrossRef]
- Fajersztajn, L.; Veras, M.M. Hypoxia: From Placental Development to Fetal Programming. Birth Defects Res. 2017, 109, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Konkel, L. Lasting Impact of an Ephemeral Organ: The Role of the Placenta in Fetal Programming. Environ. Health Perspect. 2016, 124, A124–A129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega, M.A.; Saez M, Á.; Asúnsolo, Á.; Romero, B.; Bravo, C.; Coca, S.; Sainz, F.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Upregulation of VEGF and PEDF in Placentas of Women with Lower Extremity Venous Insufficiency during Pregnancy and Its Implication in Villous Calcification. Biomed. Res. Int. 2019, 2019, 5320902. [Google Scholar] [CrossRef]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Martínez-Vivero, C.; Sainz, F.; Bravo, C.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Pregnancy-associated venous insufficiency course with placental and systemic oxidative stress. J. Cell. Mol. Med. 2020, 24, 4157–4170. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.A.; Saez, M.A.; Fraile-Martínez, O.; Asúnsolo, Á.; Pekarek, L.; Bravo, C.; Coca, S.; Sainz, F.; Mon, M.Á.; Buján, J.; et al. Increased Angiogenesis and Lymphangiogenesis in the Placental Villi of Women with Chronic Venous Disease during Pregnancy. Int. J. Mol. Sci. 2020, 21, 2487. [Google Scholar] [CrossRef] [Green Version]
- Honduvilla, N.G.; A Ortega, M.; Asúnsolo, Á.; Álvarez-Rocha, M.J.; Romero, B.; De León-Luis, J.; Álvarez-Mon, M.; Buján, J. Placentas from women with pregnancy-associated venous insufficiency show villi damage with evidence of hypoxic cellular stress. Hum. Pathol. 2018, 77, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Sánchez-Trujillo, L.; Bravo, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Alvarez-Mon, M.A.; Sainz, F.; Alvarez-Mon, M.; Bujan, J.; et al. Newborns of Mothers with Venous Disease during Pregnancy Show Increased Levels of Lipid Peroxidation and Markers of Oxidative Stress and Hypoxia in the Umbilical Cord. Antioxidants 2021, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Fragoso, M.B.T.; Ferreira, R.C.; Tenório, M.C.D.S.; Moura, F.A.; de Araújo, O.R.P.; Bueno, N.B.; Goulart, M.O.F.; de Oliveira, A.C.M. Biomarkers of Inflammation and Redox Imbalance in Umbilical Cord in Pregnancies with and without Preeclampsia and Consequent Perinatal Outcomes. Oxidative Med. Cell. Longev. 2021, 2021, 9970627. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Park, C.-W.; Moon, K.C.; Park, J.S.; Jun, J.K. The relationship among the progression of inflammation in umbilical cord, fetal inflammatory response, early-onset neonatal sepsis, and chorioamnionitis. PLoS ONE 2019, 14, e0225328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redline, R.W. Inflammatory responses in the placenta and umbilical cord. Semin. Fetal Neonatal Med. 2006, 11, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.H.; Meyermann, R.; Schluesener, H.J. The allograft inflammatory factor-1 family of proteins. FEBS Lett. 2002, 514, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, M.; Kopeć, B.; Piotrowska, K.; Pawlik, A. Role of allograft inflammatory factor-1 in pathogenesis of diseases. Immunol. Lett. 2019, 218, 1–4. [Google Scholar] [CrossRef]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef]
- Ortega, M.A.; Chaowen, C.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cruza, I.; Pereda-Cerquella, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Fatych, Y.; et al. Chronic Venous Disease in Pregnant Women Causes an Increase in ILK in the Placental Villi Associated with a Decrease in E-Cadherin. J. Pers. Med. 2022, 12, 277. [Google Scholar] [CrossRef]
- Guss, L.G.; Javvaji, S.; Case, J.; Bs, B.B.; Schaefer, K.N.; Bs, R.G.; Waalen, J.; Greenway, H.T.; Housman, L.B. Differences in Inflammatory Cytokine Levels between Patients with Varying Severity of Chronic Venous Insufficiency. J. Vasc. Med. Surg. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Saez, M.A.; Álvarez-Mon, M.A.; Gómez-Lahoz, A.M.; Bravo, C.; Luis, J.A.L.; Sainz, F.; Coca, S.; Asúnsolo, Á.; et al. Abnormal proinflammatory and stressor environmental with increased the regulatory cellular IGF-1/PAPP-A/STC and Wnt-1/β-Catenin canonical pathway in placenta of women with Chronic venous Disease during Pregnancy. Int. J. Med. Sci. 2021, 18, 2814–2827. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Sainz, F.; Martinez-Vivero, C.; Álvarez-Mon, M.; Buján, J.; García-Honduvilla, N. Behavior of Smooth Muscle Cells under Hypoxic Conditions: Possible Implications on the Varicose Vein Endothelium. BioMed Res. Int. 2018, 2018, 7156150. [Google Scholar] [CrossRef] [Green Version]
- Lohr, J.M.; Bush, R.L. Venous disease in women: Epidemiology, manifestations, and treatment. J. Vasc. Surg. 2013, 57, 37S–45S. [Google Scholar] [CrossRef] [Green Version]
- Asúnsolo, Á.; Chaowen, C.; Ortega, M.A.; Coca, S.; Borrell, L.N.; De León-Luis, J.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J. Association Between Lower Extremity Venous Insufficiency and Intrapartum Fetal Compromise: A Nationwide Cross-Sectional Study. Front. Med. 2021, 8, 577096. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Oliva, D.; Garcia-Montero, C.; Fraile-Martinez, O.; Boaru, D.L.; García-Puente, L.; Rios-Parra, A.; Garrido-Gil, M.J.; Casanova-Martín, C.; García-Honduvilla, N.; Bujan, J. AIF1: Function and Connection with Inflammatory Diseases. Biology 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Yan, D.-J.; Chen, Z.-W. Role of AIF-1 in the regulation of inflammatory activation and diverse disease processes. Cell. Immunol. 2013, 284, 75–83. [Google Scholar] [CrossRef]
- Jia, J.; Cai, Y.; Wang, R.; Fu, K.; Zhao, Y.-F. Overexpression of Allograft Inflammatory Factor-1 Promotes the Proliferation and Migration of Human Endothelial Cells (HUV-EC-C) Probably by Up-Regulation of Basic Fibroblast Growth Factor. Pediatr. Res. 2010, 67, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.A.; Romero, B.; Asúnsolo, Á.; Sola, M.; Álavrez-Rocha, M.J.; Sainz, F.; Álavrez-Mon, M.; Buján, J.; García-Honduvilla, N. Patients with Incompetent Valves in Chronic Venous Insufficiency Show Increased Systematic Lipid Peroxidation and Cellular Oxidative Stress Markers. Oxid. Med. Cell. Longev. 2019, 2019, 5164576. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ho, D.W.; Lau, C.K.; Lam, C.T.; Lum, C.T.; Poon, R.T.P.; Fan, S.T. Allograft inflammatory factor-1 (AIF-1) is crucial for the survival and pro-inflammatory activity of macrophages. Int. Immunol. 2005, 17, 1391–1397. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ma, H.; Jiang, L.; Zhao, Y. Allograft inflammatory factor-1 and its immune regulation. Autoimmunity 2007, 40, 95–102. [Google Scholar] [CrossRef]
- Kadoya, M.; Yamamoto, A.; Hamaguchi, M.; Obayashi, H.; Mizushima, K.; Ohta, M.; Seno, T.; Oda, R.; Fujiwara, H.; Kohno, M.; et al. Allograft inflammatory factor-1 stimulates chemokine production and induces chemotaxis in human peripheral blood mononuclear cells. Biochem. Biophys. Res. Commun. 2014, 448, 287–291. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. IL-12 Family Cytokines: General Characteristics, Pathogenic Microorganisms, Receptors, and Signalling Pathways. Acta Microbiol. Immunol. Hung. 2016, 63, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vignali, D.A.A.; Kuchroo, V.K. IL-12 family cytokines: Immunological playmakers. Nat. Immunol. 2012, 13, 722–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.; Wirtz, S.; Neurath, M.F. Stepwise Regulation of T H 1 Responses in Autoimmunity: IL-12-related Cytokines and Their Receptors IL-12 Receptor Signaling. Inflamm. Bowel Dis. 2005, 11, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; De Carolis, C.; Perricone, R. Pregnancy and autoimmunity: A common problem. Best. Pract. Res. Clin. Rheumatol. 2012, 26, 47–60. [Google Scholar] [CrossRef] [PubMed]
- El-Kabarity, R.H.; Naguib, A.H. Serum levels of IL-18, IL-12 and TH-1/TH-2 ratio in patients with pre-eclampsia. Egypt. J. Immunol. 2011, 18, 1–8. [Google Scholar]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef] [Green Version]
- Tsutsui, H.; Nakanishi, K. Immunotherapeutic applications of IL-18. Immunotherapy 2012, 4, 1883–1894. [Google Scholar] [CrossRef]
- Wawrocki, S.; Druszczynska, M.; Kowalewicz-Kulbat, M.; Rudnicka, W. Interleukin 18 (IL-18) as a target for immune intervention. Acta Biochim. Pol. 2016, 63, 59–63. [Google Scholar] [CrossRef] [Green Version]
- van de Veerdonk, F.L.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A. Inflammasome activation and IL-1&β; and IL-18 processing during infection. Trends Immunol. 2011, 32, 110–116. [Google Scholar] [CrossRef]
- Menon, R.; Lombardi, S.J.; Fortunato, S.J. IL-18, a Product of Choriodecidual Cells, Increases During Premature Rupture of Membranes but Fails to Turn on the Fas-FasL-Mediated Apoptosis Pathway. J. Assist. Reprod. Genet. 2001, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, S.; Ghiam, A.F.; Mojtahedi, Z.; Dehaghani, A.S.; Amani, D.; Ghaderi, A. Interleukin-18 gene promoter polymorphisms and recurrent spontaneous abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 5–9. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Huang, H.; Dong, M.; Yao, Q.; Wang, H. Serum and placental interleukin-18 are elevated in preeclampsia. J. Reprod. Immunol. 2005, 65, 77–87. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wong, K.; Ouyang, W.; Rutz, S. Targeting IL-10 Family Cytokines for the Treatment of Human Diseases. Cold Spring Harb. Perspect. Biol. 2017, 11, a028548. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.A.; Qurie, A. Interleukin. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Brogin Moreli, J.; Cirino Ruocco, A.M.; Vernini, J.M.; Rudge, M.V.C.; Calderon, I.M.P. Interleukin 10 and Tumor Necrosis Factor-Alpha in Pregnancy: Aspects of Interest in Clinical Obstetrics. ISRN Obstet. Gynecol. 2012, 2012, 230742. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the anti-inflammatory cytokines interleukin-4 and interleukin-10 during pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-García, L.A.; Minor-Borrego, H.; Sánchez-Del Real, A.L.; Aguayo-Guerrero, J.A.; Alvarado-Monroy, T.; Trejo-Millán, F.; Rosas-Salinas, J.; Rizo-Tellez, S.A.; Islas-Andrade, S.; Briones-Garduño, J.C.; et al. Cord blood levels of interleukin-10 decrease in neonates with increased birth weight: Novel implications of the cytokine network in early obesity. Eur. J. Pediatr. 2021, 180, 2529–2537. [Google Scholar] [CrossRef]
- Rocha, G.; Proença, E.; Guedes, A.; Carvalho, C.; Areias, A.; Ramos, J.P.; Rodrigues, T.; Guimarães, H. Cord blood levels of IL-6, IL-8 and IL-10 may be early predictors of bronchopulmonary dysplasia in preterm newborns small for gestational age. Dis. Markers 2012, 33, 51–60. [Google Scholar] [CrossRef]

| CVD (n = 62) | HC (n = 52) | |
|---|---|---|
| Median age (IQR), years | 33 (22–40) | 34 (27–41) |
| Median gestational age (IQR), weeks | 40.5 (39–41.5) | 41 (39–42) |
| C-section delivery, n (%) | 12 (19.4) | 9 (17.3) |
| Vaginal delivery, n (%) | 50 (80.6) | 43 (82.7) |
| CVD (CEAP), n (%) | ||
| CEAP 1 | 37 (59.7) | 0 (0) |
| CEAP 2 | 21 (33.8) | 0 (0) |
| CEAP 3 | 4 (6.5) | 0 (0) |
| Previous pregnancies, n (%) | 33 (53.2) | 19 (36.5) |
| Previous abortions, n (%) | 14 (22.6) | 9 (17.3) |
| Regular menstrual cycles, n (%) | 50 (80.6) | 42 (80.7) |
| Sedentary profession, n (%) | 41 (66.1) | 40 (76.9) |
| Gene | Sequence Fwd (5′→3′) | Sequence Rev (5′→3′) | Temp |
|---|---|---|---|
| TBP | TGCACAGGAGCCAAGAGTGAA | CACATCACAGCTCCCCACCA | 60 °C |
| AIF-1 | TGAAAACCCTCCAGTCAGCG | GTCAGGGTAGCTGAACGTCT | 60 °C |
| IL-12A | GCACAGTGGAGGCCTGTTTA | GCCAGGCAACTCCCATTAGT | 60.2 °C |
| IL-18 | GCTGAAGATGATGAAAACCTGGA | GAGGCCGATTTCCTTGGTCA | 59.5 °C |
| IL-10 | TGCTCTTGCAAAACCAAACCA | GGGAGGTCAGGGAAAACAGC | 60 °C |
| Antigen | Species | Dilution | Provider | Protocol Specifications |
|---|---|---|---|---|
| AIF-1 | Goat polyclonal | 1: 500 | Abcam (ab5076) | EDTA pH = 9 before incubation with blocking solution |
| IL-12A | Rabbit monoclonal | 1:100 | Abcam (ab131039) | EDTA pH = 9 before incubation with blocking solution |
| IL-18 | Rabbit monoclonal | 1:250 | Abcam (ab243091) | 10 mM Sodium citrate pH = 6 before incubation with blocking solution |
| IL-10 | Rabbit Polyclonal | 1:100 | Abcam (ab217941) | 100% Triton 0.1% in PBS, 10 min, before incubation with blocking solution |
| IgG (Rabbit) | Mouse | 1:1000 | Sigma-Aldrich (RG-96/B5283) | ------ |
| IgG (Goat) | Mouse | 1:100 | Sigma-Aldrich [GT- 4/B3148] | ------ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Trujillo, L.; Fraile-Martinez, O.; García-Montero, C.; García-Puente, L.M.; Guijarro, L.G.; De Leon-Oliva, D.; Boaru, D.L.; Gardón-Alburquerque, D.; del Val Toledo Lobo, M.; Royuela, M.; et al. Chronic Venous Disease during Pregnancy Is Related to Inflammation of the Umbilical Cord: Role of Allograft Inflammatory Factor 1 (AIF-1) and Interleukins 10 (IL-10), IL-12 and IL-18. J. Pers. Med. 2023, 13, 956. https://doi.org/10.3390/jpm13060956
Sánchez-Trujillo L, Fraile-Martinez O, García-Montero C, García-Puente LM, Guijarro LG, De Leon-Oliva D, Boaru DL, Gardón-Alburquerque D, del Val Toledo Lobo M, Royuela M, et al. Chronic Venous Disease during Pregnancy Is Related to Inflammation of the Umbilical Cord: Role of Allograft Inflammatory Factor 1 (AIF-1) and Interleukins 10 (IL-10), IL-12 and IL-18. Journal of Personalized Medicine. 2023; 13(6):956. https://doi.org/10.3390/jpm13060956
Chicago/Turabian StyleSánchez-Trujillo, Lara, Oscar Fraile-Martinez, Cielo García-Montero, Luis M. García-Puente, Luis G. Guijarro, Diego De Leon-Oliva, Diego Liviu Boaru, David Gardón-Alburquerque, María del Val Toledo Lobo, Mar Royuela, and et al. 2023. "Chronic Venous Disease during Pregnancy Is Related to Inflammation of the Umbilical Cord: Role of Allograft Inflammatory Factor 1 (AIF-1) and Interleukins 10 (IL-10), IL-12 and IL-18" Journal of Personalized Medicine 13, no. 6: 956. https://doi.org/10.3390/jpm13060956







