The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury
Abstract
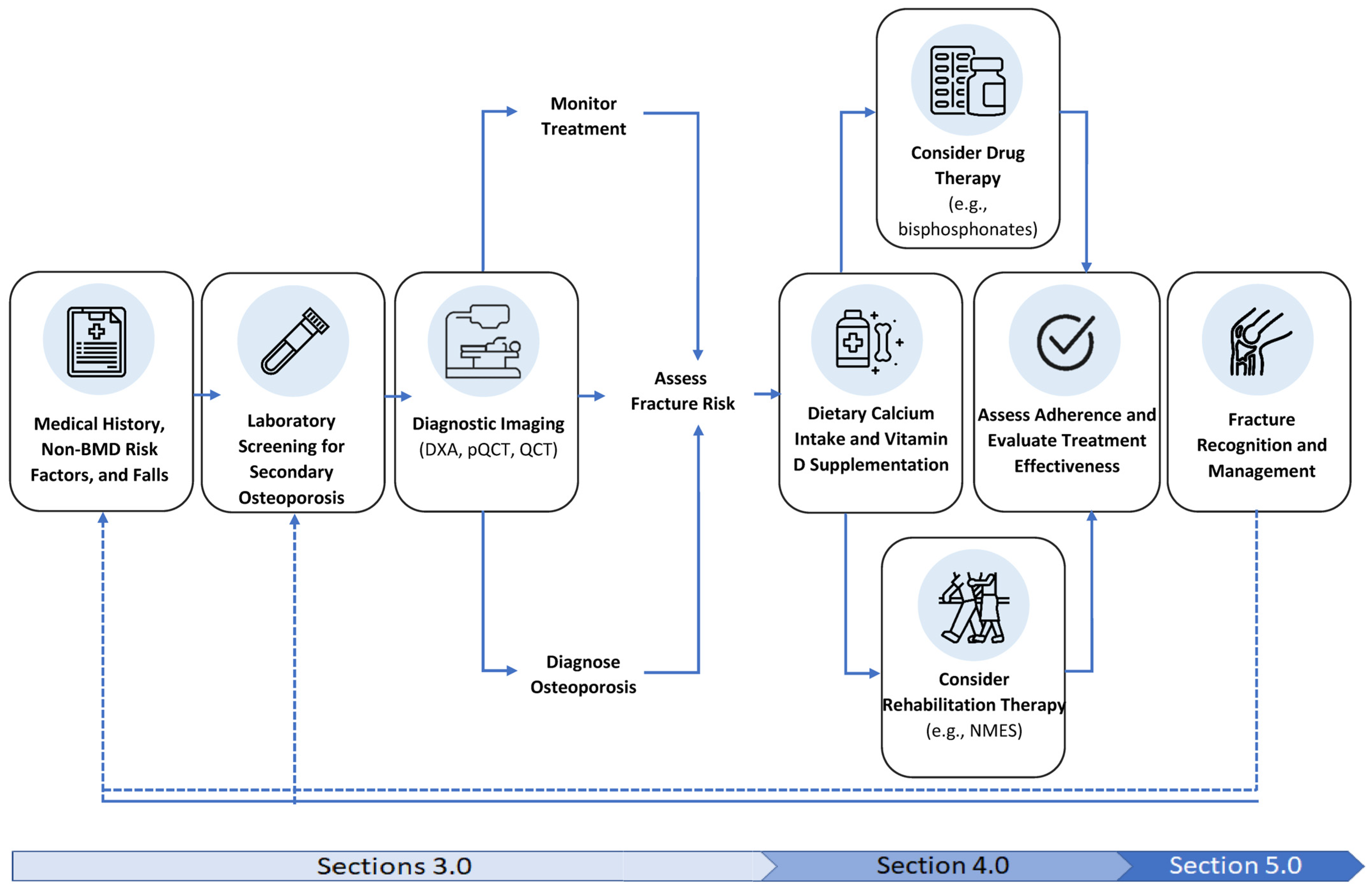
:1. Introduction
2. Overview of Bone Tissue and Remodeling
2.1. Pathophysiology of Osteoporosis in SCI
2.2. Indexes of Bone Turnover after SCI
2.3. Calcium Metabolism and Systemic Factors Regulating Bone Metabolism
3. Screening and Diagnosis of Sublesional Osteoporosis
3.1. Screening for Sublesional Osteoporosis
3.2. Laboratory Screening
3.3. Diagnostic Imaging for Sublesional Osteoporosis
| Age Range | Definition |
|---|---|
| Men ≥ 50 years or postmenopausal women | Hip or knee region T score ≤ −2.5 |
| Men < 49 years or premenopausal women | Hip or knee region Z score < −2.0 with ≥3 risk factors for fracture |
| Men or women age 16–90 | Prior fragility fracture and no identifiable etiology of osteoporosis other than SCI |
4. Treatment of Osteoporosis in SCI
4.1. Vitamin D Supplements
4.2. Dietary Calcium and Nutritional Considerations
4.3. Fracture Risk and Rehabilitation Therapy
4.4. Rehabilitation Therapy: Standing and Walking
4.5. Rehabilitation Therapy: Neuromuscular Electrical Stimulation (NMES) and Fucntional Electrical Stimulation (FES)
4.6. Drug Therapy: Alendronate, Zoledronic Acid and Densoumab
4.6.1. Safety Considerations, Absolute and Relative Contraindications to Therapy
4.6.2. Monitoring and Cessation of Ineffective Therapy
5. Fracture Management
5.1. Fracture Incidence
5.2. Operative or Non-Operative Management of Fractures
5.3. Rehabilitation Post Fracture
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acronym | Definition |
| AB | Able-bodied |
| AFF | Atypical Femoral Fracture |
| AIS | ASIA Impairment Scale |
| ASIA | American Spinal Cord Injury Association |
| BMD | Bone Mineral Density |
| CI | Confidence Interval |
| CPG | Clinical Practice Guideline |
| CTX-1 | C-terminal Cross-linking Telopeptide of Type I Collagen |
| DXA | Dual Energy X-ray Absorptiometry |
| DF | Distal Femur |
| FES | Functional Electrical Stimulation |
| FSH | Follicle-Stimulating Hormone |
| HR | Hazard Ratio |
| IGF-1 | Insulin-like Growth Factor 1 |
| IV | Intravenous |
| LH | Luteinizing Hormone |
| NMES | Neuromuscular Electrical Stimulation |
| NTX-1 | N-terminal Cross-linking Telopeptide of Type I Collagen |
| OC | Osteocalcin |
| ONJ | Osteonecrosis of the Jaw |
| OPG | Osteoprotegerin |
| ORIF | Open Reduction with Internal Fixation |
| PTH | Parathryroid Hormone |
| P1NP | Procollagen 1 Intact N-Terminal Propeptide |
| pQCT | Peripheral Quantitative Computed Tomography |
| PT | Proximal Tibia |
| PVA | Paralyzed Veterans of America |
| RANKL | Receptor Activator of Nuclear factor kappa-Β Ligand |
| SCI | Spinal Cord Injury |
| SCI/D | Spinal Cord Injury/Disease |
| ZA | Zolendronic Acid |
References
- Hou, S.; Rabchevsky, A.G. Autonomic consequences of spinal cord injury. Compr. Physiol. 2014, 4, 1419–1453. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, L.; Tong, B.; Geisler, F.; Schubert, M.; Röhrich, F.; Saur, M.; Weidner, N.; Rupp, R.; Kalke, Y.-B.B.; Abel, R.; et al. International surveillance study in acute spinal cord injury confirms viability of multinational clinical trials. BMC Med. 2022, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Cadotte, D.W.; Fehlings, M.G. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: A systematic review. J. Neurosurg. Spine 2012, 17 (Suppl. S1), 11–26. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.A.; Watzlawick, R.; Martus, P.; Failli, V.; Finkenstaedt, F.W.; Chen, Y.; DeVivo, M.J.; Dirnagl, U.; Schwab, J.M. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology 2017, 88, 892–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stampas, A.; Dominick, E.; Zhu, L. Evaluation of functional outcomes in traumatic spinal cord injury with rehabilitation-acquired urinary tract infections: A retrospective study. J. Spinal. Cord. Med. 2019, 42, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, C.A.; Hicks, A.L. Muscle characteristics and fatigue properties after spinal cord injury. Crit. Rev. Biomed. Eng. 2009, 37, 139–164. [Google Scholar] [CrossRef]
- Xu, X.; Talifu, Z.; Zhang, C.J.; Gao, F.; Ke, H.; Pan, Y.-Z.; Gong, H.; Du, H.-Y.; Yu, Y.; Jing, Y.-L.; et al. Mechanism of skeletal muscle atrophy after spinal cord injury: A narrative review. Front. Nutr. 2023, 10, 1099143. [Google Scholar] [CrossRef]
- Moore, C.D.; Craven, B.C.; Thabane, L.; Laing, A.; Frank-Wilson, A.; Kontulainen, S.; Papaioannou, A.; Adachi, J.; Giangregorio, L. Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J. Musculoskelet. Neuronal. Interact. 2015, 15, 32–41. [Google Scholar]
- Qin, W.; Bauman, W.A.; Cardozo, C. Bone and muscle loss after spinal cord injury: Organ interactions. Ann. N. Y. Acad. Sci. 2010, 1211, 66–84. [Google Scholar] [CrossRef]
- Craven, B.C.; Burns, A.S.; Carbone, L.; Cirnigliaro, C.M.; Cowley, K.; Eng, J.; Forrest, G.; Johnston, T.E.; Kiratli, B.J.; Morgan, S.; et al. Bone Health and Osteoporosis Management in Individuals with Spinal Cord Injury; Clinical Practice Guideline; Paralyzed Veterans of America: Washington, DC, USA, 2022; Available online: https://pva.org/wp-content/uploads/2022/05/CPG_Spinal-Cord-Medicine_2022_FINAL.pdf (accessed on 15 May 2023).
- Morse, L.R.; Biering-Soerensen, F.; Carbone, L.D.; Cervinka, T.; Cirnigliaro, C.M.; Johnston, T.E.; Liu, N.; Troy, K.; Weaver, F.M.; Shuhart, C.; et al. Bone Mineral Density Testing in Spinal Cord Injury: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 554–566. [Google Scholar] [CrossRef]
- Carbone, L.D.; Ahn, J.; Adler, R.A.; Cervinka, T.; Craven, C.; Geerts, W.; Hsu, J.; Huang, D.; Karunakar, M.; Kiratli, B.; et al. Acute Lower Extremity Fracture Management in Chronic Spinal Cord Injury: 2022 Delphi Consensus Recommendations. JBJS Open Access 2022, 7, e21.00152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.D.; Dai, L.Y.; Jiang, L.S. Osteoporosis after spinal cord injury. Osteoporos. Int. 2006, 17, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The mechanostat: A proposed pathogenic mechanism of osteoporoses and the bone mass effects of mechanical and nonmechanical agents. Bone Miner. 1987, 2, 73–85. [Google Scholar] [PubMed]
- Ragnarsson, K.T.; Sell, G.H. Lower extremity fractures after spinal cord injury: A retrospective study. Arch. Phys. Med. Rehabil. 1981, 62, 418–423. [Google Scholar] [PubMed]
- Abderhalden, L.; Weaver, F.M.; Bethel, M.; Demirtas, H.; Burns, S.; Svircev, J.; Hoenig, H.; Lyles, K.; Miskevics, S.; Carbone, L.D. Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders. Osteoporos. Int. 2017, 28, 925–934. [Google Scholar] [CrossRef]
- Chantraine, A.; Nusgens, B.; Lapiere, C.M. Bone remodeling during the development of osteoporosis in paraplegia. Calcif. Tissue Int. 1986, 38, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Minaire, P.; Neunier, P.; Edouard, C.; Bernard, J.; Courpron, P.; Bourret, J. Quantitative histological data on disuse osteoporosis: Comparison with biological data. Calcif. Tissue Res. 1974, 17, 57–73. [Google Scholar] [CrossRef]
- Szollar, S.M.; Martin, E.M.; Sartoris, D.J.; Parthemore, J.G.; Deftos, L.J. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am. J. Phys. Med. Rehabil. 1998, 77, 28–35. [Google Scholar] [CrossRef]
- Zehnder, Y.; Luthi, M.; Michel, D.; Knecht, H.; Perrelet, R.; Neto, I.; Kraenzlin, M.; Lippuner, K. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: A cross-sectional observational study in 100 paraplegic men. Osteoporos. Int. 2004, 15, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Roberts, D.; Lee, W.; Cuneo, R.C.; Wittmann, J.; Ward, G.; Flatman, R.; McWhinney, B.; Hickman, P.E. Longitudinal study of bone turnover after acute spinal cord injury. J. Clin. Endocrinol. Metab. 1998, 83, 415–422. [Google Scholar] [CrossRef]
- Biering-Sorensen, F.; Bohr, H.H.; Schaadt, O.P. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur. J. Clin. Investig. 1990, 20, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Dauty, M.; Perrouin Verbe, B.; Maugars, Y.; Dubois, C.; Mathe, J.F. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 2000, 27, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Dudley-Javoroski, S.; Boaldin, K.M.; Corey, T.A.; Fog, D.B.; Ruen, J.M. Peripheral quantitative computed tomography: Measurement sensitivity in persons with and without spinal cord injury. Arch. Phys. Med. Rehabil. 2006, 87, 1376–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garland, D.; Adkins, R.; Stewar, C. Fracture Threshold and Risk for Osteoporosis and Pathologic Fractures in Individuals with Spinal Cord Injury. Top. Spinal Cord. Inj. 2005, 11, 61–69. [Google Scholar] [CrossRef]
- Cirnigliaro, C.M.; Myslinski, M.J.; Asselin, P.; Hobson, J.C.; Specht, A.; La Fountaine, M.F.; Kirshblum, S.C.; Forrest, G.F.; Dyson-Hudson, T.; Spungen, A.M.; et al. Progressive Sublesional Bone Loss Extends into the Second Decade after Spinal Cord Injury. J. Clin. Densitom. 2019, 22, 185–194. [Google Scholar] [CrossRef]
- Garland, D.E.; Adkins, R.H.; Stewart, C.A. Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. J. Spinal Cord. Med. 2008, 31, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Recker, R.; Lappe, J.; Davies, K.; Heaney, R. Characterization of perimenopausal bone loss: A prospective study. J. Bone Miner. Res. 2000, 15, 1965–1973. [Google Scholar] [CrossRef]
- Leblanc, A.D.; Schneider, V.S.; Evans, H.J.; Engelbretson, D.A.; Krebs, J.M. Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 1990, 5, 843–850. [Google Scholar] [CrossRef]
- Vico, L.; Collet, P.; Guignandon, A.; Lafage-Proust, M.-H.; Thomas, T.; Rehailia, M.; Alexandre, C. Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 2000, 355, 1607–1611. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Seeman, E. Quantifying the material and structural determinants of bone strength. Best. Pract. Res. Clin. Rheumatol. 2009, 23, 741–753. [Google Scholar] [CrossRef]
- Eser, P.; Frotzler, A.; Zehnder, Y.; Wick, L.; Knecht, H.; Denoth, J.; Schiessl, H. Relationship between the duration of paralysis and bone structure: A pQCT study of spinal cord injured individuals. Bone 2004, 34, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J.; Goosey-Tolfrey, V.L.; Cointry, G.; Ferretti, J.L. Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone 2010, 47, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. 2006, 65, 555–565. [Google Scholar] [CrossRef]
- Qin, W.; Sun, L.; Cao, J.; Peng, Y.; Collier, L.; Wu, Y.; Creasey, G.; Li, J.; Qin, Y.; Jarvis, J.; et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J. Biol. Chem. 2013, 288, 13511–13521. [Google Scholar] [CrossRef] [Green Version]
- Morse, L.R.; Nguyen, H.P.; Jain, N.; Williams, S.; Tun, C.G.; Battaglino, R.A.; Stashenko, P.; Garshick, E. Age and motor score predict osteoprotegerin level in chronic spinal cord injury. J. Musculoskelet. Neuronal. Interact. 2008, 8, 50–57. [Google Scholar]
- Gifre, L.; Ruiz-Gaspa, S.; Carrasco, J.L.; Portell, E.; Vidal, J.; Muxi, A.; Monegal, A.; Guañabens, N.; Peris, P. Effect of recent spinal cord injury on the OPG/RANKL system and its relationship with bone loss and the response to denosumab therapy. Osteoporos. Int. 2017, 28, 2707–2715. [Google Scholar] [CrossRef]
- Morse, L.R.; Sudhakar, S.; Lazzari, A.A.; Tun, C.; Garshick, E.; Zafonte, R.; Battaglino, R.A. Sclerostin: A candidate biomarker of SCI-induced osteoporosis. Osteoporos. Int. 2013, 24, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Battaglino, R.A.; Sudhakar, S.; Lazzari, A.A.; Garshick, E.; Zafonte, R.; Morse, L.R. Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 2012, 51, 600–605. [Google Scholar] [CrossRef] [Green Version]
- Gifre, L.; Vidal, J.; Carrasco, J.L.; Filella, X.; Ruiz-Gaspà, S.; Muxi, A.; Portell, E.; Monegal, A.; Guañabens, N.; Peris, P. Effect of recent spinal cord injury on wnt signaling antagonists (sclerostin and dkk-1) and their relationship with bone loss. A 12-month prospective study. J. Bone Miner. Res. 2015, 30, 1014–1021. [Google Scholar] [CrossRef]
- Pietschmann, P.; Pils, P.; Woloszczuk, W.; Maerk, R.; Lessan, D.; Stipicic, J. Increased serum osteocalcin levels in patients with paraplegia. Paraplegia 1992, 30, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Uebelhart, D.; Hartmann, D.; Vuagnat, H.; Castanier, M.; Hachen, H.J.; Chantraine, A. Early modifications of biochemical markers of bone metabolism in spinal cord injury patients. A preliminary study. Scand. J. Rehabil. Med. 1994, 26, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.F.; Adler, M.; Byers, C.M.; Segre, G.V.; Broadus, A.E. Calcium homeostasis in immobilization: An example of resorptive hypercalciuria. N. Engl. J. Med. 1982, 306, 1136–1140. [Google Scholar] [CrossRef]
- Heaney, R.P. Radiocalcium metabolism in disuse osteoporosis in man. Am. J. Med. 1962, 33, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Huang, T.S.; Lien, I.N. Hormone changes in men with spinal cord injuries. Am. J. Phys. Med. Rehabil. 1992, 71, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Tsitouras, P.D.; Zhong, Y.G.; Spungen, A.M.; Bauman, W.A. Serum testosterone and growth hormone/insulin-like growth factor-I in adults with spinal cord injury. Horm. Metab. Res. 1995, 27, 287–292. [Google Scholar] [CrossRef]
- Bauman, W.A.; La Fountaine, M.F.; Spungen, A.M. Age-related prevalence of low testosterone in men with spinal cord injury. J. Spinal Cord. Med. 2014, 37, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Bauman, W.A.; La Fountaine, M.F.; Cirnigliaro, C.M.; Kirshblum, S.C.; Spungen, A.M. Testicular responses to hCG stimulation at varying doses in men with spinal cord injury. Spinal Cord. 2017, 55, 659–663. [Google Scholar] [CrossRef] [Green Version]
- Bauman, W.A.; La Fountaine, M.F.; Cirnigliaro, C.M.; Kirshblum, S.C.; Spungen, A.M. Administration of increasing doses of gonadotropin-releasing hormone in men with spinal cord injury to investigate dysfunction of the hypothalamic-pituitary-gonadal axis. Spinal Cord. 2018, 56, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Song, R.X.; Santen, R.J. Apoptotic action of estrogen. Apoptosis 2003, 8, 55–60. [Google Scholar] [CrossRef]
- Tomkinson, A.; Reeve, J.; Shaw, R.W.; Noble, B.S. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 1997, 82, 3128–3135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerton, K.B.; Hu, B.; Woo, A.A.; Sinofsky, A.; Hernandez, C.; Majeska, R.; Jepsen, K.; Schaffler, M. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone 2010, 46, 577–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Ominsky, M.S.; Niu, Q.T.; Sun, N.; Daugherty, B.; D’Agostin, D.; Kurahara, C.; Gao, Y.; Cao, J.; Gong, J.; et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 2008, 23, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, R.C. Evaluation of 17-ketosteroid, estrogen and gonadotrophin excretion in patients with spinal cord injury. Am. J. Med. 1950, 8, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Slade, J.M.; Bickel, C.S.; Modlesky, C.M.; Majumdar, S.; Dudley, G.A. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporos. Int. 2005, 16, 263–272. [Google Scholar] [CrossRef]
- Battaglino, R.A.; Lazzari, A.A.; Garshick, E.; Morse, L.R. Spinal cord injury-induced osteoporosis: Pathogenesis and emerging therapies. Curr. Osteoporos. Rep. 2012, 10, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Ozan Tan, C. Spinal Cord Injury and Osteoporosis: Causes, Mechanisms, and Rehabilitation Strategies. Int. J. Phys. Rehabil. Med. 2013, 1. [Google Scholar] [CrossRef] [Green Version]
- Morse, L.R.; Battaglino, R.A.; Stolzmann, K.L.; Hallett, L.D.; Waddimba, A.; Gagnon, D.; Lazzari, A.A.; Garshick, E. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos. Int. 2009, 20, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Gifre, L.; Vidal, J.; Carrasco, J.; Portell, E.; Puig, J.; Monegal, A.; Guañabens, N.; Peris, P. Incidence of skeletal fractures after traumatic spinal cord injury: A 10-year follow-up study. Clin. Rehabil. 2014, 28, 361–369. [Google Scholar] [CrossRef]
- Lala, D.; Craven, B.C.; Thabane, L.; Papaioannou, A.; Adachi, J.D.; Popovic, M.R.; Giangregorio, L.M. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos. Int. 2014, 25, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelletier, C.A.; Dumont, F.S.; Leblond, J.; Noreau, L.; Giangregorio, L.; Craven, B.C. Self-report of one-year fracture incidence and osteoporosis prevalence in a community cohort of canadians with spinal cord injury. Top. Spinal Cord. Inj. Rehabil. 2014, 20, 302–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauman, W.A.; Zhong, Y.G.; Schwartz, E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism 1995, 44, 1612–1616. [Google Scholar] [CrossRef]
- Vestergaard, P.; Mosekilde, L. Hyperthyroidism, bone mineral, and fracture risk—A meta-analysis. Thyroid 2003, 13, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Thomson, C.A.; Aickin, M.; Nicholas, J.S.; Van Wyck, D.; Lewis, C.E.; Cauley, J.A.; Bassford, T. The relationship between incidence of fractures and anemia in older multiethnic women. J. Am. Geriatr. Soc. 2010, 58, 2337–2344. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.D. Chronic kidney disease and the skeleton. Bone Res. 2014, 2, 14044. [Google Scholar] [CrossRef] [Green Version]
- Upala, S.; Jaruvongvanich, V.; Wijarnpreecha, K.; Sanguankeo, A. Nonalcoholic fatty liver disease and osteoporosis: A systematic review and meta-analysis. J. Bone Miner. Metab. 2017, 35, 685–693. [Google Scholar] [CrossRef]
- Bang, C.S.; Shin, I.S.; Lee, S.W.; Kim, J.B.; Baik, G.H.; Suk, K.T.; Yoon, J.H.; Kim, Y.S.; Kim, D.J. Osteoporosis and bone fractures in alcoholic liver disease: A meta-analysis. World J. Gastroenterol. 2015, 21, 4038–4047. [Google Scholar] [CrossRef]
- Valderrabano, R.J.; Linares, M.I. Diabetes mellitus and bone health: Epidemiology, etiology and implications for fracture risk stratification. Clin. Diabetes Endocrinol. 2018, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Arnaldi, G.; Angeli, A.; Atkinson, A.B.; Bertagna, X.; Cavagnini, F.; Chrousos, G.P.; Fava, G.A.; Findling, J.W.; Gaillard, R.C.; Grossman, A.B.; et al. Diagnosis and complications of Cushing’s syndrome: A consensus statement. J. Clin. Endocrinol. Metab. 2003, 88, 5593–5602. [Google Scholar] [CrossRef] [Green Version]
- Bauman, W.A.; Kirshblum, S.; Cirnigliaro, C.; Forrest, G.F.; Spungen, A.M. Underestimation of bone loss of the spine with posterior-anterior dual-energy X-ray absorptiometry in patients with spinal cord injury. J. Spinal Cord. Med. 2010, 33, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangregorio, L.M.; Gibbs, J.C.; Craven, B.C. Measuring muscle and bone in individuals with neurologic impairment; lessons learned about participant selection and pQCT scan acquisition and analysis. Osteoporos. Int. 2016, 27, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Craven, B.C.; Moreno, J.C.; Brown, A.; Raja, N.; Wong, L. Acquisition and Analysis of Bone Mineral Density of the Distal Femur and Proximal Tibia; 2019. Available online: https://kite-uhn-contents.s3.ca-central-1.amazonaws.com/resourceMaterial/KneeDXAProtocol2019_+06_27_V5.0.pdf (accessed on 19 April 2023).
- Craven, B.C.; Robertson, L.A.; McGillivray, C.F.; Adachi, J.D. Detection and Treatment of Sublesional Osteoporosis among Patients with Chronic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2009, 14, 1–22. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef]
- Pappa, H.M.; Bern, E.; Kamin, D.; Grand, R.J. Vitamin D status in gastrointestinal and liver disease. Curr. Opin. Gastroenterol. 2008, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health: Office of Dietary Supplements. Vitamin D Fact Sheet for Consumers. Internet. Available online: https://ods.od.nih.gov/factsheets/VitaminD-Consumer/ (accessed on 19 April 2023).
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2016—Executive Summary. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Farkas, G.J.; Pitot, M.A.; Berg, A.S.; Gater, D.R. Nutritional status in chronic spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2019, 57, 3–17. [Google Scholar] [CrossRef]
- Javidan, A.N.; Sabour, H.; Latifi, S.; Vafa, M.; Shidfar, F.; Khazaeipour, Z.; Shahbazi, F.; Rahimi, A.; Razavi, S.-H.E. Calcium and vitamin D plasma concentration and nutritional intake status in patients with chronic spinal cord injury: A referral center report. J. Res. Med. Sci. 2014, 19, 881–884. [Google Scholar]
- Koutrakis, N.E.; Goldstein, R.L.; Walia, P.; Polak, M.M.; Lazzari, A.A.; Tun, C.G.; Hart, J.E.; Garshick, E. Vitamin D, diet, and lifestyle in a chronic SCI population. Spinal Cord. 2019, 57, 117–127. [Google Scholar] [CrossRef]
- Walters, J.L.; Buchholz, A.C.; Martin Ginis, K.A. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord. 2009, 47, 318–322. [Google Scholar] [CrossRef] [Green Version]
- Flueck, J.L.; Perret, C. Vitamin D deficiency in individuals with a spinal cord injury: A literature review. Spinal Cord. 2017, 55, 428–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbonetti, A.; Vassallo, M.R.; Felzani, G.; Francavilla, S.; Francavilla, F. Association between 25(OH)-vitamin D and testosterone levels: Evidence from men with chronic spinal cord injury. J. Spinal Cord. Med. 2016, 39, 246–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbonetti, A.; D’Andrea, S.; Martorella, A.; Felzani, G.; Francavilla, S.; Francavilla, F. Low vitamin D levels are independent predictors of 1-year worsening in physical function in people with chronic spinal cord injury: A longitudinal study. Spinal Cord. 2018, 56, 494–501. [Google Scholar] [CrossRef]
- Binkley, N.; Carter, G.D. Toward Clarity in Clinical Vitamin D Status Assessment: 25(OH)D Assay Standardization. Endocrinol. Metab. Clin. N. Am. 2017, 46, 885–899. [Google Scholar] [CrossRef] [PubMed]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef]
- Sempos, C.T.; Betz, J.M.; Camara, J.E.; Carter, G.D.; Cavalier, E.; Clarke, M.; Dowling, K.G.; Durazo-Arvizu, R.A.; Hoofnagle, A.N.; Liu, A.; et al. General Steps to Standardize the Laboratory Measurement of Serum Total 25-Hydroxyvitamin, D.J. AOAC Int. 2017, 100, 1230–1233. [Google Scholar] [CrossRef]
- Binkley, N.; Dawson-Hughes, B.; Durazo-Arvizu, R.; Thamm, M.; Tian, L.; Merkel, J.; Jones, J.; Carter, G.; Sempos, C. Vitamin D measurement standardization: The way out of the chaos. J. Steroid Biochem. Mol. Biol. 2017, 173, 117–121. [Google Scholar] [CrossRef]
- Binkley, N.; Sempos, C.T. Vitamin D Standardization Program. Standardizing vitamin D assays: The way forward. J. Bone Miner. Res. 2014, 29, 1709–1714. [Google Scholar] [CrossRef] [Green Version]
- Carter, G.D.; Berry, J.; Durazo-Arvizu, R.; Gunter, E.; Jones, G.; Jones, J.; Makin, H.; Pattni, P.; Phinney, K.; Sempos, C.; et al. Quality assessment of vitamin D metabolite assays used by clinical and research laboratories. J. Steroid Biochem. Mol. Biol. 2017, 173, 100–104. [Google Scholar] [CrossRef]
- Durazo-Arvizu, R.A.; Tian, L.; Brooks, S.P.J.; Sarafin, K.; Cashman, K.D.; Kiely, M.; Merkel, J.; Myers, G.L.; Coates, P.M.; Sempos, C.T. The Vitamin D Standardization Program (VDSP) Manual for Retrospective Laboratory Standardization of Serum 25-Hydroxyvitamin D Data. J. AOAC Int. 2017, 100, 1234–1243. [Google Scholar] [CrossRef]
- Wise, S.A.; Phinney, K.W.; Tai, S.S.; Camara, J.E.; Myers, G.L.; Durazo-Arvizu, R.; Tian, L.; Hoofnagle, A.N.; Bachmann, L.M.; Young, I.; et al. Baseline Assessment of 25-Hydroxyvitamin D Assay Performance: A Vitamin D Standardization Program (VDSP) Interlaboratory Comparison Study. J. AOAC Int. 2017, 100, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Tai, S.S.; Burdette, C.Q.; Camara, J.E.; Bedner, M.; Lippa, K.A.; Nelson, M.A.; Nalin, F.; Phinney, K.W.; Sander, L.C.; et al. Role of the National Institute of Standards and Technology (NIST) in Support of the Vitamin D Initiative of the National Institutes of Health, Office of Dietary Supplements. J. AOAC Int. 2017, 100, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Toward a physiological referent for the vitamin D requirement. J. Endocrinol. Investig. 2014, 37, 1127–1130. [Google Scholar] [CrossRef]
- Binkley, N.; Lewiecki, E.M. Vitamin D and common sense. J. Clin. Densitom. 2011, 14, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Emmons, R.R.; Cirnigliaro, C.M.; Kirshblum, S.C.; Spungen, A.M. An effective oral vitamin D replacement therapy in persons with spinal cord injury. J. Spinal Cord. Med. 2011, 34, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyatani, M.; Craven, B.; Loewenberger, E.; McGillivray, C.; Adachi, J. The Dietary Intakes of Calcium and Bone Health Related Nutrients among Individuals with and without Spinal Cord Injury. J. Nutr. Ther. 2014, 3, 103–113. [Google Scholar] [CrossRef]
- University Health Network Osteoporosis Program. Calcium Assessment Tool. Internet. Available online: https://osteoconnection.files.wordpress.com/2014/04/cat_patient-version-apr2014.pdf (accessed on 19 April 2023).
- National Institutes of Health: Office of Dietary Supplements. Calcium Fact Sheet for Health Professionals. Internet. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/#:~:text=Calcium%20Intakes%20and%20Status,-A%20substantial%20proportion&text=Average%20daily%20intakes%20of%20calcium,to%201%2C015%20mg%20%5B18%5D (accessed on 19 April 2023).
- Welk, B.; Fuller, A.; Razvi, H.; Denstedt, J. Renal stone disease in spinal-cord-injured patients. J. Endourol. 2012, 26, 954–959. [Google Scholar] [CrossRef]
- Hansen, R.B.; Biering-Sorensen, F.; Kristensen, J.K. Urinary calculi following traumatic spinal cord injury. Scand. J. Urol. Nephrol. 2007, 41, 115–119. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, M.; Carter, R.E.; Leng, S.; Ramirez-Giraldo, J.C.; Jaramillo, G.; Krambeck, A.E.; Lieske, J.C.; Vrtiska, T.J.; McCollough, C.H. Differentiating calcium oxalate and hydroxyapatite stones in vivo using dual-energy CT and urine supersaturation and pH values. Acad. Radiol. 2013, 20, 1521–1525. [Google Scholar] [CrossRef] [Green Version]
- El-Kotob, R.; Pagcanlungan, J.R.; Craven, B.C.; Sherrington, C.; Mourtzakis, M.; Giangregorio, L.M. Researchers’ perspectives on adverse event reporting in resistance training trials: A qualitative study. Appl. Physiol. Nutr. Metab. 2022, 47, 893–902. [Google Scholar] [CrossRef]
- Schenkman, M.; Deutsch, J.E.; Gill-Body, K.M. An integrated framework for decision making in neurologic physical therapist practice. Phys. Ther. 2006, 86, 1681–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alekna, V.; Tamulaitiene, M.; Sinevicius, T.; Juocevicius, A. Effect of weight-bearing activities on bone mineral density in spinal cord injured patients during the period of the first two years. Spinal Cord. 2008, 46, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Karimi, T.M. The Physiological Benefits and Problems Associated with using Standing and Walking Orthoses in Individuals with Spinal Cord Injury—A Meta-analytic Review. J. Orthop. Trauma. Rehabil. 2012, 16, 37–40. [Google Scholar] [CrossRef]
- Esquenazi, A.; Talaty, M.; Jayaraman, A. Powered Exoskeletons for Walking Assistance in Persons with Central Nervous System Injuries: A Narrative Review. PM R. 2017, 9, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.; Apple, D.; Barbeau, H.; Basso, M.; Behrman, A.; Deforge, D.; Ditunno, J.; Dudley, G.; Elashoff, R.; Fugate, L.; et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 2006, 66, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Worthen, L.C.; Kim, C.M.; Kautz, S.A.; Lew, H.L.; Kiratli, B.J.; Beaupre, G.S. Key characteristics of walking correlate with bone density in individuals with chronic stroke. J. Rehabil. Res. Dev. 2005, 42, 761–768. [Google Scholar] [CrossRef]
- National Institutes of Health: Osteoporosis and Related Bone Diseases National Resource Centre. Exercise for your Bone Health. Internet. Available online: https://www.niams.nih.gov/health-topics/exercise-your-bone-health (accessed on 19 April 2023).
- Giangregorio, L.M.; Webber, C.E.; Phillips, S.M.; Hicks, A.L.; Craven, B.C.; Bugaresti, J.M.; McCartney, N. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl. Physiol. Nutr. Metab. 2006, 31, 283–291. [Google Scholar] [CrossRef]
- Ogilvie, C.; Bowker, P.; Rowley, D.I. The physiological benefits of paraplegic orthotically aided walking. Paraplegia 1993, 31, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Thoumie, P.; Le Claire, G.; Beillot, J.; Dassonville, J.; Chevalier, T.; Perrouin-Verbe, B.; Bedoiseau, M.; Busnel, M.; Cormerais, A.; Courtillon, A.; et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. II: Physiological evaluation. Paraplegia 1995, 33, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Belanger, M.; Stein, R.B.; Wheeler, G.D.; Gordon, T.; Leduc, B. Electrical stimulation: Can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch. Phys. Med. Rehabil. 2000, 81, 1090–1098. [Google Scholar] [CrossRef]
- Shields, R.K.; Dudley-Javoroski, S. Musculoskeletal adaptations in chronic spinal cord injury: Effects of long-term soleus electrical stimulation training. Neurorehabil. Neural Repair 2007, 21, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Dudley-Javoroski, S.; Saha, P.K.; Liang, G.; Li, C.; Gao, Z.; Shields, R.K. High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos. Int. 2012, 23, 2335–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frotzler, A.; Coupaud, S.; Perret, C.; Kakebeeke, T.H.; Hunt, K.J.; Donaldson, N.D.N.; Eser, P. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 2008, 43, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Lai, C.H.; Chan, W.P.; Huang, M.H.; Tsai, H.W.; Chen, J.J. Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil. Rehabil. 2005, 27, 1337–1341. [Google Scholar] [CrossRef]
- Mohr, T.; Podenphant, J.; Biering-Sorensen, F.; Galbo, H.; Thamsborg, G.; Kjaer, M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif. Tissue Int. 1997, 61, 22–25. [Google Scholar] [CrossRef]
- Coupaud, S.; McLean, A.N.; Allan, D.B. Role of peripheral quantitative computed tomography in identifying disuse osteoporosis in paraplegia. Skelet. Radiol. 2009, 38, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Coupaud, S.; McLean, A.N.; Lloyd, S.; Allan, D.B. Predicting patient-specific rates of bone loss at fracture-prone sites after spinal cord injury. Disabil. Rehabil. 2012, 34, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Edwards, W.B.; Schnitzer, T.J.; Troy, K.L. Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporos. Int. 2014, 25, 1005–1015. [Google Scholar] [CrossRef]
- Zehnder, Y.; Risi, S.; Michel, D.; Knecht, H.; Perrelet, R.; Kraenzlin, M.; Zäch, G.A.; Lippuner, K. Prevention of bone loss in paraplegics over 2 years with alendronate. J. Bone Miner. Res. 2004, 19, 1067–1074. [Google Scholar] [CrossRef]
- Haider, I.T.; Simonian, N.; Saini, A.S.; Leung, F.M.; Edwards, W.B.; Schnitzer, T.J. Open-label clinical trial of alendronate after teriparatide therapy in people with spinal cord injury and low bone mineral density. Spinal Cord. 2019, 57, 832–842. [Google Scholar] [CrossRef]
- Morse, L.R.; Troy, K.L.; Fang, Y.; Nguyen, N.; Battaglino, R.; Goldstein, R.F.; Gupta, R.; Taylor, J.A. Combination Therapy with Zoledronic Acid and FES-Row Training Mitigates Bone Loss in Paralyzed Legs: Results of a Randomized Comparative Clinical Trial. JBMR Plus 2019, 3, e10167. [Google Scholar] [CrossRef] [Green Version]
- Gifre, L.; Vidal, J.; Carrasco, J.L.; Muxi, A.; Portell, E.; Monegal, A.; Guanabens, N.; Peris, P. Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporos. Int. 2016, 27, 405–410. [Google Scholar] [CrossRef]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Watts, N.B.; Roux, C.; Modlin, J.F.; Brown, J.P.; Daniels, A.; Jackson, S.; Smith, S.; Zack, D.J.; Zhou, L.; Grauer, A.; et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: Coincidence or causal association? Osteoporos. Int. 2012, 23, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Kajizono, M.; Sada, H.; Sugiura, Y.; Soga, Y.; Kitamura, Y.; Matsuoka, J.; Sendo, T. Incidence and Risk Factors of Osteonecrosis of the Jaw in Advanced Cancer Patients after Treatment with Zoledronic Acid or Denosumab: A Retrospective Cohort Study. Biol. Pharm. Bull. 2015, 38, 1850–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Morrison, A.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and management of osteonecrosis of the jaw: A systematic review and international consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, R.M.; Adams, A.L.; Greene, D.F.; Funahashi, T.T.; Silverman, S.L.; Eisemon, E.O.; Zhou, H.; Burchette, R.J.; Ott, S.M. Incidence of atypical nontraumatic diaphyseal fractures of the femur. J. Bone Miner. Res. 2012, 27, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.A.; El-Hajj Fuleihan, G.; Bauer, D.C.; Camacho, P.M.; Clarke, B.L.; Clines, G.A.; Compston, J.E.; Drake, M.T.; Edwards, B.J.; Favus, M.J.; et al. Managing Osteoporosis in Patients on Long-Term Bisphosphonate Treatment: Report of a Task Force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2016, 31, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Begin, M.J.; Audet, M.C.; Chevalley, T.; Portela, M.; Padlina, I.; Hannouche, D.; Lorenzini, K.I.; Meier, R.; Peter, R.; Uebelhart, B.; et al. Fracture Risk Following an Atypical Femoral Fracture. J. Bone Miner. Res. 2022, 37, 87–94. [Google Scholar] [CrossRef]
- Goenka, S.; Sethi, S.; Pandey, N.; Joshi, M.; Jindal, R. Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: A randomized controlled trial. Spinal Cord. 2018, 56, 1207–1211. [Google Scholar] [CrossRef]
- Bauman, W.A.; Cirnigliaro, C.M.; La Fountaine, M.F.; Martinez, L.; Kirshblum, S.C.; Spungen, A.M. Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: An observational cohort study. J. Bone Miner. Metab. 2015, 33, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Bauman, W.A.; Wecht, J.M.; Kirshblum, S.; Spungen, A.M.; Morrison, N.; Cirnigliaro, C.; Schwartz, E. Effect of pamidronate administration on bone in patients with acute spinal cord injury. J. Rehabil. Res. Dev. 2005, 42, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Bubbear, J.S.; Gall, A.; Middleton, F.R.; Ferguson-Pell, M.; Swaminathan, R.; Keen, R.W. Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos. Int. 2011, 22, 271–279. [Google Scholar] [CrossRef]
- Nance, P.W.; Schryvers, O.; Leslie, W.; Ludwig, S.J.; Uebelhart, D. Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch. Phys. Med. Rehabil. 1999, 80, 243–251. [Google Scholar] [CrossRef]
- Shapiro, J.; Smith, B.; Beck, T.; Ballard, P.; Dapthary, M.; BrintzenhofeSzoc, K.; Caminis, J. Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif. Tissue Int. 2007, 80, 316–322. [Google Scholar] [CrossRef]
- Gilchrist, N.L.; Frampton, C.M.; Acland, R.H.; Nicholls, M.G.; March, R.L.; Maguire, P.; Heard, A.; Reilly, P.; Marshall, K. Alendronate prevents bone loss in patients with acute spinal cord injury: A randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007, 92, 1385–1390. [Google Scholar] [CrossRef]
- Schnitzer, T.J.; Kim, K.; Marks, J.; Yeasted, R.; Simonian, N.; Chen, D. Zoledronic Acid Treatment after Acute Spinal Cord Injury: Results of a Randomized, Placebo-Controlled Pilot Trial. PM R. 2016, 8, 833–843. [Google Scholar] [CrossRef]
- Guilcher, S.J.T.; Hogan, M.E.; Calzavara, A.; Nicholls, M.G.; March, R.L.; Maguire, P.; Heard, A.; Reilly, P.; Marshall, K. Prescription drug claims following a traumatic spinal cord injury for older adults: A retrospective population-based study in Ontario, Canada. Spinal Cord. 2018, 56, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Ray, C.; Gonzalez, B.; Miskevics, S.; Weaver, F.M.; Priebe, M.; Carbone, L.D. Reasons for Initiation and Discontinuation of Pharmacological Therapies for Osteoporosis in Veterans with Spinal Cord Injury and Disorders. J. Clin. Densitom. 2021, 24, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ 2010, 182, 1864–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, D.L. Long-term bisphosphonate use: When to stop? when to restart. TodayLs Geriatr. Med. 2015, 8, 10–12. [Google Scholar]
- Ott, S.M. Osteoporosis in Women with Spinal Cord Injuries. Phys. Med. Rehabil. Clin. N. Am. 2001, 12, 111–131. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Patil, A.A.; Chamczuk, A.J.; Agrawal, D.K. Hormonal therapy in traumatic spinal cord injury. Am. J. Transl. Res. 2017, 9, 3881–3895. [Google Scholar]
- Vestergaard, P.; Krogh, K.; Rejnmark, L.; Mosekilde, L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998, 36, 790–796. [Google Scholar] [CrossRef]
- Frotzler, A.; Cheikh-Sarraf, B.; Pourtehrani, M.; Krebs, J.; Lippuner, K. Long-bone fractures in persons with spinal cord injury. Spinal Cord. 2015, 53, 701–704. [Google Scholar] [CrossRef] [Green Version]
- Grassner, L.; Klein, B.; Maier, D.; Buhren, V.; Vogel, M. Lower extremity fractures in patients with spinal cord injury characteristics, outcome and risk factors for non-unions. J. Spinal Cord. Med. 2018, 41, 676–683. [Google Scholar] [CrossRef]
- Akhigbe, T.; Chin, A.S.; Svircev, J.N.; Hoenig, H.; Burns, S.P.; Weaver, F.M.; Bailey, L.; Carbone, L. A retrospective review of lower extremity fracture care in patients with spinal cord injury. J. Spinal Cord. Med. 2015, 38, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Benson, I.; Hart, K.; Tussler, D.; van Middendorp, J.J. Lower-limb exoskeletons for individuals with chronic spinal cord injury: Findings from a feasibility study. Clin. Rehabil. 2016, 30, 73–84. [Google Scholar] [CrossRef]
- Bass, A.; Morin, S.N.; Vermette, M.; Aubertin-Leheudre, M.; Gagnon, D.H. Incidental bilateral calcaneal fractures following overground walking with a wearable robotic exoskeleton in a wheelchair user with a chronic spinal cord injury: Is zero risk possible? Osteoporos. Int. 2020, 31, 1007–1011. [Google Scholar] [CrossRef]
- Miller, L.E.; Zimmermann, A.K.; Herbert, W.G. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: Systematic review with meta-analysis. Med. Devices 2016, 9, 455–466. [Google Scholar] [CrossRef] [Green Version]
- Bethel, M.; Bailey, L.; Weaver, F.; Harmon, R.L.; Priebe, M.M.; Le, B.; Aslam, H.; Fausel, Z.; Hoenig, H.; Carbone, L.D. A historical study of appendicular fractures in veterans with traumatic chronic spinal cord injury: 2002–2007. J. Spinal Cord. Med. 2016, 39, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Carbone, L.D.; Chin, A.S.; Burns, S.P.; Svircev, J.N.; Hoenig, H.; Heggeness, M.; Bailey, L.; Weaver, F. Mortality after lower extremity fractures in men with spinal cord injury. J. Bone Miner. Res. 2014, 29, 432–439. [Google Scholar] [CrossRef]
- Lems, W.F.; Dreinhofer, K.E.; Bischoff-Ferrari, H.; Blauth, M.; Czerwinski, E.; Da Silva, J.; Herrera, A.; Hoffmeyer, P.; Kvien, T.; Maalouf, G.; et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann. Rheum. Dis. 2017, 76, 802–810. [Google Scholar] [CrossRef]
- Barlehner, C.; Bohm, V.; Flieger, R.; Meiners, T. Surgery for fractures of the lower extremities in cases of chronic spinal cord injury. Orthopade 2005, 34, 137–143. [Google Scholar] [CrossRef]
- Asselin, P.; Knezevic, S.; Kornfeld, S.; Cirnigliaro, C.; Agranova-Breyter, I.; Bauman, W.A.; Spungen, A.M. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J. Rehabil. Res. Dev. 2015, 52, 147–158. [Google Scholar] [CrossRef]
- Bach Baunsgaard, C.; Vig Nissen, U.; Katrin Brust, A.; Frotzler, A.; Ribeill, C.; Kalke, Y.-B.; León, N.; Gómez, B.; Samuelsson, K.; Antepohl, W.; et al. Gait training after spinal cord injury: Safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018, 56, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Evans, N.; Hartigan, C.; Kandilakis, C.; Pharo, E.; Clesson, I. Acute Cardiorespiratory and Metabolic Responses During Exoskeleton-Assisted Walking Overground Among Persons with Chronic Spinal Cord Injury. Top. Spinal Cord. Inj. Rehabil. 2015, 21, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Federici, S.; Meloni, F.; Bracalenti, M.; De Filippis, M.L. The effectiveness of powered, active lower limb exoskeletons in neurorehabilitation: A systematic review. NeuroRehabilitation 2015, 37, 321–340. [Google Scholar] [CrossRef]
- Gorgey, A.S.; Sumrell, R.; Goetz, L.L. 44—Exoskeletal Assisted Rehabilitation after Spinal Cord Injury. In Atlas of Orthoses and Assistive Devices, 5th ed.; Webster, J.B., Murphy, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 440–447. [Google Scholar]
- Gorgey, A.S. Robotic exoskeletons: The current pros and cons. World J. Orthop. 2018, 9, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Wade, R.; Sumrell, R.; Villadelgado, L.; Khalil, R.E.; Lavis, T. Exoskeleton Training May Improve Level of Physical Activity after Spinal Cord Injury: A Case Series. Top. Spinal Cord. Inj. Rehabil. 2017, 23, 245–255. [Google Scholar] [CrossRef]
- Kressler, J.; Thomas, C.K.; Field-Fote, E.C.; Sanchez, J.; Widerstrom-Noga, E.; Cilien, D.C.; Gant, K.; Ginnety, K.; González, H.; Martinez, A.; et al. Understanding therapeutic benefits of overground bionic ambulation: Exploratory case series in persons with chronic, complete spinal cord injury. Arch. Phys. Med. Rehabil. 2014, 95, 1878–1887.e4. [Google Scholar] [CrossRef]
- Louie, D.R.; Eng, J.J.; Lam, T. Spinal Cord Injury Research Evidence Research, T. Gait speed using powered robotic exoskeletons after spinal cord injury: A systematic review and correlational study. J. Neuroeng. Rehabil. 2015, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Etingen, B.; Carbone, L.D.; Guihan, M.; Ray, C.; Aslam, H.; Elam, R.; Weaver, F.M. Lower extremity fracture prevention and management in persons with spinal cord injuries and disorders: The patient perspective. J. Spinal Cord. Med. 2022, 45, 946–956. [Google Scholar] [CrossRef]
- Comarr, A.E.; Hutchinson, R.H.; Bors, E. Extremity fractures of patients with spinal cord injuries. Am. J. Surg. 1962, 103, 732–739. [Google Scholar] [CrossRef]
- Ingram, R.R.; Suman, R.K.; Freeman, P.A. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 1989, 27, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Baird, R.A.; Kreitenberg, A.; Eltorai, I. External fixation of femoral shaft fractures in spinal cord injury patients. Paraplegia 1986, 24, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Ochoa, S.; Haddad, S.; Rodriguez-Alabau, S.; Teixidor, J.; Tomas, J.; Molero, V. Should lower limb fractures be treated surgically in patients with chronic spinal injuries? Experience in a reference centre. Rev. Esp. Cir. Ortop. Traumatol. 2017, 61, 19–27. [Google Scholar] [CrossRef]
- Eichenholtz, S.N. Management of Long-Bone Fractures in Paraplegic Patients. J. Bone Jt. Surg. 1963, 45, 299–310. [Google Scholar] [CrossRef]
- Freehafer, A.A.; Mast, W.A. Lower Extremity Fractures in Patients with Spinal-Cord Injury. J. Bone Jt. Surg. 1965, 47, 683–694. [Google Scholar] [CrossRef]
- McMaster, W.C.; Stauffer, E.S. The Management of Long Bone Fracture in the Spinal Cord Injured Patient. Clin. Orthop. Relat. Res. 1975, 112, 44–52. [Google Scholar]
- Nottage, W.M. A Review of Long-Bone Fractures in Patients with Spinal Cord Injuries. Clin. Orthop. Relat. Res. 1981, 155, 65–70. [Google Scholar] [CrossRef]
- Perkins, C.; Buck, J.S.; Karunakar, M.A. Outcomes in the treatment of femur fractures in patients with pre-existing spinal cord injury. Bull. NYU Hosp. Jt. Dis. 2019, 77, 211–216. [Google Scholar]
- Bethel, M.; Bailey, L.; Weaver, F.; Le, B.; Burns, S.P.; Svircev, J.N.; Heggeness, M.H.; Carbone, L.D. Surgical compared with nonsurgical management of fractures in male veterans with chronic spinal cord injury. Spinal Cord. 2015, 53, 402–407. [Google Scholar] [CrossRef] [Green Version]
- Freehafer, A.A. Limb fractures in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 1995, 76, 823–827. [Google Scholar] [CrossRef]
- Bishop, J.A.; Suarez, P.; DiPonio, L.; Ota, D.; Curtin, C.M. Surgical versus nonsurgical treatment of femur fractures in people with spinal cord injury: An administrative analysis of risks. Arch. Phys. Med. Rehabil. 2013, 94, 2357–2364. [Google Scholar] [CrossRef]
- Nelson, A.; Ahmed, S.; Harrow, J.; Fitzgerald, S.; Sanchez-Anguiano, A.; Gavin-Dreschnack, D. Fall-related fractures in persons with spinal cord impairment: A descriptive analysis. SCI Nurs. 2003, 20, 30–37. [Google Scholar]
- Cochran, T.P.; Bayley, J.C.; Smith, M. Lower Extremity Fractures in Paraplegics: Pattern, Treatment, and Functional Results. Clin. Spine Surg. 1988, 1, 219–223. [Google Scholar] [CrossRef]
- Fouasson-Chailloux, A.; Gross, R.; Dauty, M.; Gadbled, G.; Touchais, S.; Le Fort, M.; Perrouin-Verbe, B. Surgical management of lower limb fractures in patients with spinal cord injury less associated with complications than non-operative management: A retrospective series of cases. J. Spinal Cord. Med. 2019, 42, 39–44. [Google Scholar] [CrossRef]
- Sugi, M.T.; Davidovitch, R.; Montero, N.; Nobel, T.; Egol, K.A. Treatment of lower-extremity long-bone fractures in active, nonambulatory, wheelchair-bound patients. Orthopedics 2012, 35, e1376–e1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freehafer, A.A.; Hazel, C.M.; Becker, C.L. Lower extremity fractures in patients with spinal cord injury. Paraplegia 1981, 19, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elam, R.E.; Ray, C.E.; Miskevics, S.; Weaver, F.M.; Gonzalez, B.; Obremskey, W.; Carbone, L.D. Predictors of lower extremity fracture-related amputation in persons with traumatic spinal cord injury: A case-control study. Spinal Cord. 2023, 61, 260–268. [Google Scholar] [CrossRef]
- Garland, D.E.; Saucedo, T.; Reiser, T.V. The Management of Tibial Fractures in Acute Spinal Cord Injury Patients. Clin. Orthop. Relat. Res. 1986, 213, 237–240. [Google Scholar] [CrossRef]
- Sinnott, B.; Ray, C.; Weaver, F.; Gonzalez, B.; Chu, E.; Premji, S.; Raiford, M.; Elam, R.; Miskevics, S.; Parada, S.; et al. Risk Factors and Consequences of Lower Extremity Fracture Nonunions in Veterans with Spinal Cord Injury. JBMR Plus 2022, 6, e10595. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Gonzalez, B.; Weaver, F.; Sinnott, B.; Ray, C.; Chu, E.; Premji, S.; Raiford, M.; Mayur, O.; Carbone, L. Malunions following lower extremity fractures in veterans with a spinal cord injury/disorder. J. Spinal Cord. Med. 2023, 1–7. [Google Scholar] [CrossRef]
- Svircev, J.; Tan, D.; Garrison, A.; Pennelly, B.; Burns, S.P. Limb loss in individuals with chronic spinal cord injury. J. Spinal Cord. Med. 2022, 45, 420–425. [Google Scholar] [CrossRef]
- Guihan, M.; Roddick, K.; Cervinka, T.; Ray, C.; Sutton, C.; Carbone, L.; Weaver, F.M. Physical and occupational therapist rehabilitation of lower extremity fractures in veterans with spinal cord injuries and disorders. J. Spinal Cord. Med. 2022, 45, 33–41. [Google Scholar] [CrossRef]
- Halanski, M.; Noonan, K.J. Cast and splint immobilization: Complications. J. Am. Acad. Orthop. Surg. 2008, 16, 30–40. [Google Scholar] [CrossRef]
- Ung, L.; Ohlmeier, M.; Jettkant, B.; Grasmücke, D.; Aach, M.; Meindl, R.; Nicolas, V.; Schildhauer, T.; Citak, M. Clinical and Radiological Outcomes after Surgical Treatment of Lower Limb Fractures in Patients with Spinal Cord Injury. Glob. Spine J. 2020, 10, 715–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, L.D.; Chin, A.S.; Burns, S.P.; Svircev, J.N.; Hoenig, H.; Heggeness, M.; Weaver, F. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos. Int. 2013, 24, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Abukhder, M.; Dobbs, T.; Shaw, J.; Whelan, R.; Jones, E. A systematic literature review and narrative synthesis on the risk factors for developing affective disorders in open lower-limb fracture patients. Ann. Med. Surg. 2022, 80, 104190. [Google Scholar] [CrossRef] [PubMed]
| Group and Age | Calcium Recommendation |
|---|---|
| Men & premenopausal women age 19–50 years | 1000 mg/day |
| Men 50–70 years | 1000 mg/day |
| Women 50–70 years | 1000–1200 mg/day |
| Men and women 71+ years | 1000–1200 mg/day |
| Established Fracture Risk Factors | |
|---|---|
| □ | Alcohol intake > 5 servings per day |
| □ | Paraplegia |
| □ | Duration of SCI ≥ 10 years |
| □ | Motor complete injury (AIS A–B) |
| □ | Family history of fracture |
| □ | Hip fracture in the last year |
| □ | Routine use of benzodiazepines, anticonvulsants (i.e., carbamazepine, phenytoin), heparin, opioid analgesia (≥28 mg morphine for a 3-month period) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craven, B.C.; Cirnigliaro, C.M.; Carbone, L.D.; Tsang, P.; Morse, L.R. The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury. J. Pers. Med. 2023, 13, 966. https://doi.org/10.3390/jpm13060966
Craven BC, Cirnigliaro CM, Carbone LD, Tsang P, Morse LR. The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury. Journal of Personalized Medicine. 2023; 13(6):966. https://doi.org/10.3390/jpm13060966
Chicago/Turabian StyleCraven, Beverley Catharine, Christopher M. Cirnigliaro, Laura D. Carbone, Philemon Tsang, and Leslie R. Morse. 2023. "The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury" Journal of Personalized Medicine 13, no. 6: 966. https://doi.org/10.3390/jpm13060966
APA StyleCraven, B. C., Cirnigliaro, C. M., Carbone, L. D., Tsang, P., & Morse, L. R. (2023). The Pathophysiology, Identification and Management of Fracture Risk, Sublesional Osteoporosis and Fracture among Adults with Spinal Cord Injury. Journal of Personalized Medicine, 13(6), 966. https://doi.org/10.3390/jpm13060966






