Shock Waves and Therapeutic Exercise in Greater Trochanteric Pain Syndrome: A Prospective Randomized Clinical Trial with Cross-Over
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
- -
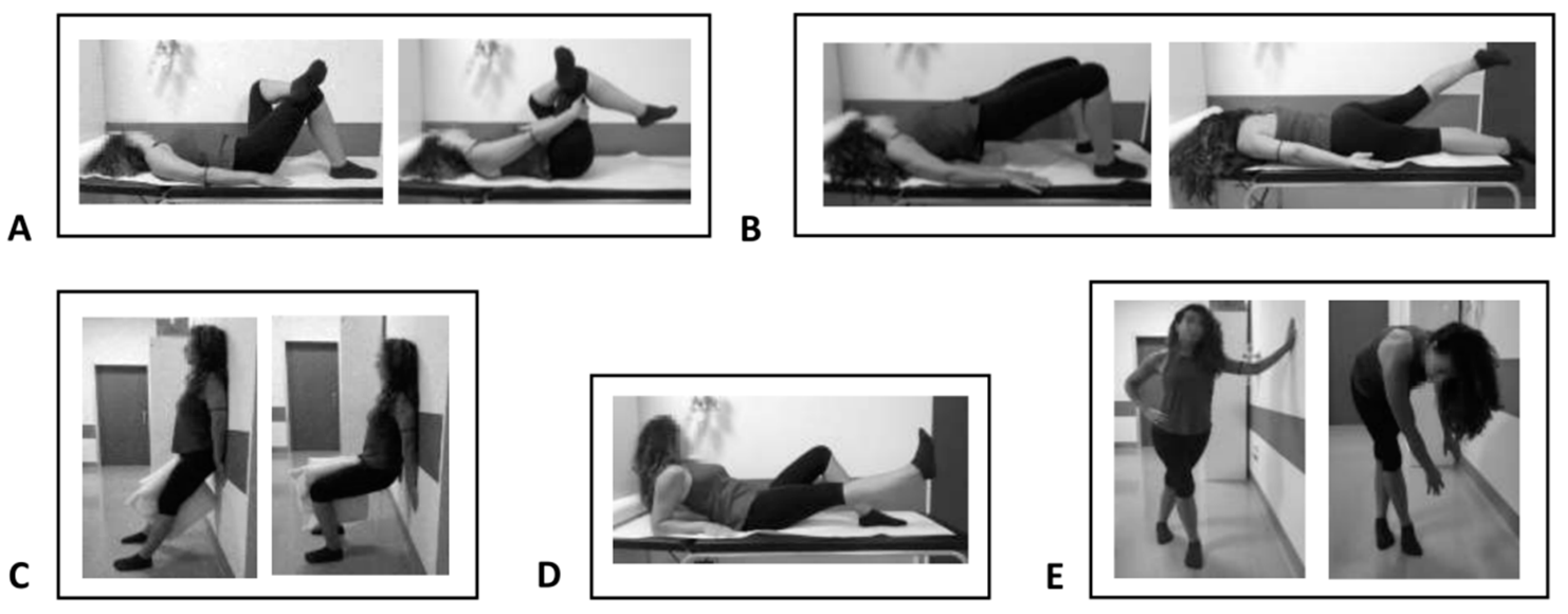
- Group A: Twenty-two patients undergoing eccentric therapeutic exercise according to the following protocol: 30 min per day, 5 days per week, for a total of 4 weeks of treatment and a total of 20 exercise sessions. Exercises included stretching for the pyriform muscle and the iliotibial tract, lifting the straight leg, squatting the band ll and strengthening the gluteus muscles. This rehabilitation protocol was carried out under the guidance of a therapist and exercises were adapted for each individual within a pain-free range.
- -
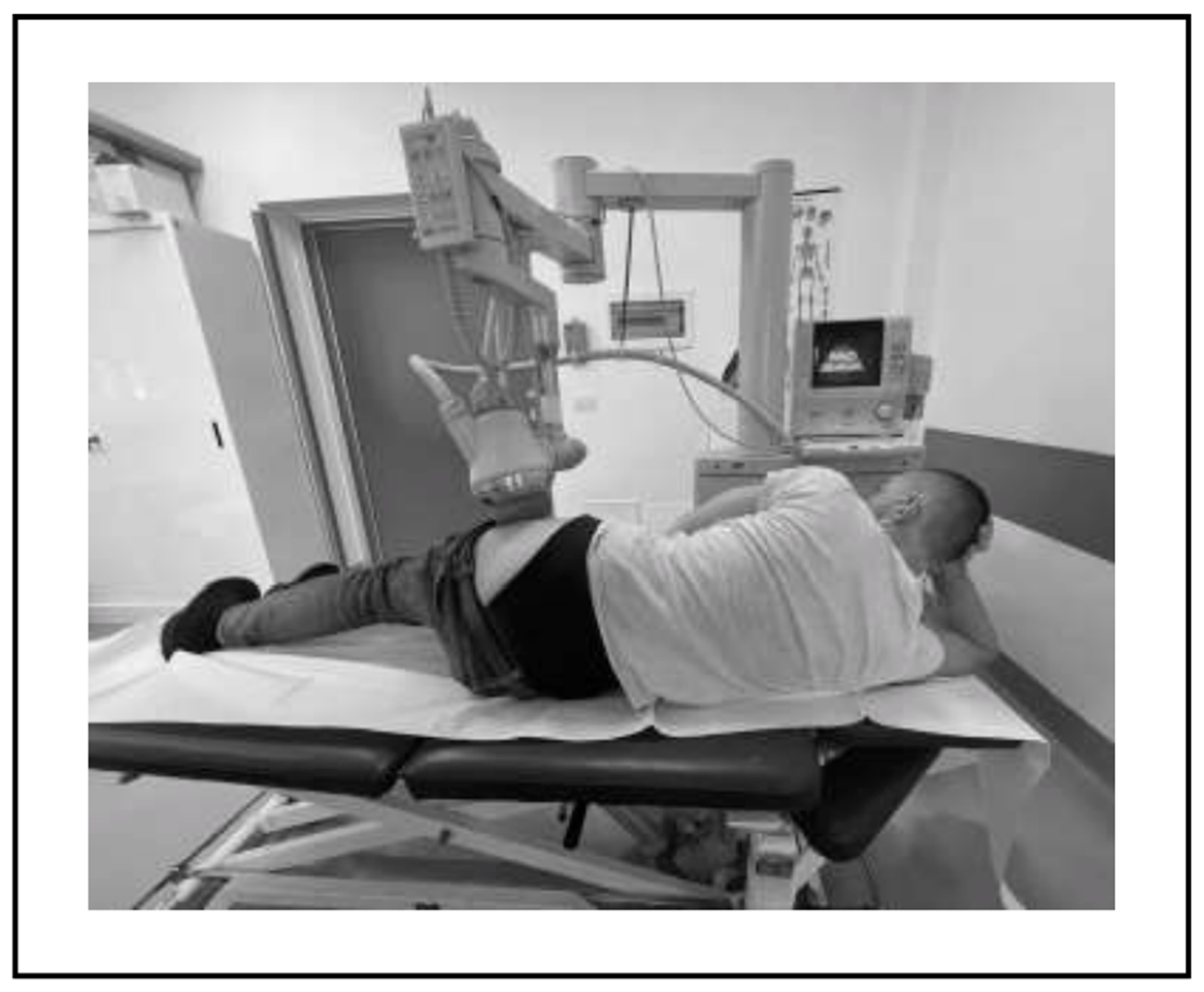
- Group B: Twenty-two patients undergoing treatment with focused ESWT, using an electromagnetic ESWT generator (MINILITH SL1-0G, Storz Medical).
- -
- Group C: Patients who belonged to Group A were subsequently treated with focused ESWT, according to the same protocol as Group B.
- -
- Group D: Patients previously in Group B were subsequently treated with therapeutic exercise, again according to the same type of sessions and duration as in Group A.
2.3. Intervention
2.4. Outcome Measures
- -
- Numeric Pain Rating Scale (NRS), from 0 (no pain) to 10 (unbearable pain) points [17].
- -
- Lower Extremity Functional Scale (LEFS), which is a valid patient-rated outcome measure for the measurement of lower extremity function; it quantifies the degree of difficulty in performing 20 types of activities of daily living, so patients are requested to select an answer for each activity (extreme difficulty or unable to perform activity = 0; no difficulty = 4), and the total amount ranges from 80 (very high function) to 0 (very low function) [18].
- -
- Roles and Maudsley Scale (RMS), which measures the patient’s perception of improvement, from 1 (excellent result) to 4 (identical or worse symptoms than before treatment). This score is a categorical classification scale that has three dimensions: pain, movement and activity. It has been widely used for reporting the results of shock wave treatments [19,20].
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.S.; Cohen, S.P. Greater trochanteric pain syndrome: A review of anatomy, diagnosis and treatment. Anesth. Analg. 2009, 108, 1662–1670. [Google Scholar] [CrossRef]
- Reid, D. The management of greater trochanteric pain syndrome: A systematic literature review. J. Orthop. 2016, 13, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Baker, C.L.; Massie, R.V.; Hurt, W.G.; Savory, C.G. Arthroscopic bursectomy for recalcitrant trochanteric bursitis. Arthroscopy 2007, 23, 827–832. [Google Scholar] [CrossRef]
- Agostino, M.C.D.; Frairia, R.; Romeo, P.; Amelio, E.; Berta, L.; Bosco, V.; Gigliotti, S.; Guerra, C.; Messina, S.; Messuri, L.; et al. Extracorporeal shockwaves as regenerative therapy in orthopaedic traumatology: A narrative review from basic research to clinical practice. J. Biol. Regul. Homeost. Agents 2016, 30, 323–332. [Google Scholar]
- Notarnicola, A.; Moretti, L.; Maccagnano, G.; Tafuri, S.; Moretti, B. Tendonitis of the rotator cuff treated with extracorporeal shock wave therapy: Radiographic monitoring to identify prognostic factors for disintegration. J. Biol. Regul. Homeost. Agents. 2016, 30, 1195–1202. [Google Scholar] [PubMed]
- Notarnicola, A.; Moretti, B. The biological effects of extracorporeal shock wave therapy (ESWT) on tendon tissue. Muscles Ligaments Tendons J. 2012, 2, 33–37. [Google Scholar]
- Abo Al-Khair, M.A.; El Khouly, R.M.; Khodair, S.A.; Al Sattar Elsergany, M.A.; Hussein, M.I.; Eldin Mowafy, M.E. Focused, radial and combined shock wave therapy in the treatment of calcific shoulder tendinopathy. Physician Sportsmed. 2021, 49, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Notarnicola, A.; Maccagnano, G.; Moretti, L.; Farì, G.; Bianchi, F.P.; Working Group SWCB; Covelli, I.; Moretti, B. Could the presence of heel spur be a prognostic factor for the outcome of extracorporeal shock wave therapy for plantar fasciitis? J. Biol. Regul. Homeost. Agents. 2019, 33, 1949–1954. [Google Scholar] [PubMed]
- Megna, M.; Marvulli, R.; Farì, G.; Gallo, G.; Dicuonzo, F.; Fiore, P.; Ianieri, G. Pain and Muscles Properties Modifications After Botulinum Toxin Type A (BTX-A) and Radial Extracorporeal Shock Wave (rESWT) Combined Treatment. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1127–1133. [Google Scholar]
- Häußer, J.; Wieber, J.; Catalá-Lehnen, P. The use of extracorporeal shock wave therapy for the treatment of bone marrow oedema—A systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 369. [Google Scholar] [CrossRef]
- Rompe, J.D.; Segal, N.A.; Cacchio, A.; Furia, J.P.; Morral, A.; Maffulli, N. Home training, local corticosteroid injection, or radial shock wave therapy for greater trochanter pain syndrome. Am. J. Sport. Med. 2009, 37, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Furia, J.P.; Rompe, J.D.; Maffulli, N. Low-energy extracorporeal shock wave therapy as a treatment for greater trochanteric pain syndrome. Am. J. Sport. Med. 2009, 37, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Lee, J.Y.; Yoon, K.; Do, J.G.; Park, H.J.; Lee, S.Y.; Park, Y.S.; Lee, Y.T. Long-term outcome of low-energy extracorporeal shockwave therapy on gluteal tendinopathy documented by magnetic resonance imaging. PLoS ONE 2018, 13, e0197460. [Google Scholar] [CrossRef] [PubMed]
- Stanish, W.D.; Rubinovich, R.M.; Curwin, S. Eccentric exercise in chronic tendinitis. Clin. Orthop. Relat. Res. 1986, 208, 65–68. [Google Scholar] [CrossRef]
- Mellor, R.; Bennell, K.; Grimaldi, A.; Nicolson, P.; Kasza, J.; Hodges, P.; Wajswelner, H.; Vicenzino, B. Education plus exercise versus corticosteroid injection use versus a wait and see approach on global outcome and pain from gluteal tendinopathy: Prospective, single blinded, randomised clinical trial. BMJ 2018, 361, k1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiele, R. New guidelines for ESWT. Newslett ISMST 2009, 5, 20. [Google Scholar]
- Euasobhon, P.; Atisook, R.; Bumrungchatudom, K.; Zinboonyahgoon, N.; Saisavoey, N.; Jensen, M.P. Reliability and responsivity of pain intensity scales in individuals with chronic pain. Pain 2022, 163, e1184–e1191. [Google Scholar] [CrossRef]
- Cacchio, A.; De Blasis, E.; Necozione, S.; Rosa, F.; Riddle, D.L.; di Orio, F.; De Blasis, D.; Santilli, V. The Italian version of the lower extremity functional scale was reliable, valid, and responsive. J. Clin. Epidemiol. 2010, 63, 550–557. [Google Scholar]
- Hwang, J.T.; Yoon, K.J.; Park, C.H.; Choi, J.H.; Park, H.J.; Park, Y.S.; Lee, Y.T. Follow-up of clinical and sonographic features after extracorporeal shock wave therapy in painful plantar fibromatosis. PLoS ONE 2020, 15, e0237447. [Google Scholar] [CrossRef]
- Beyer, R.; Kongsgaard, M.; Hougs Kjær, B.; Øhlenschlæger, T.; Kjær, M.; Magnusson, S.P. Heavy Slow Resistance Versus Eccentric Training as Treatment for Achilles Tendinopathy A Randomized Controlled Trial. Am. J. Sport. Med. 2015, 7, 43. [Google Scholar]
- French, H.P.; Woodley, S.J.; Fearon, A.; O’Connor, L.; Grimaldi, A. Physiotherapy management of greater trochanteric pain syndrome (GTPS): An international survey of current physiotherapy practice. Physiotherapy 2019, 109, 111–120. [Google Scholar] [CrossRef] [PubMed]
- LaStayo, P.C.; Woolf, J.M.; Lewek, M.D.; Snyder-Mackler, L.; Reich, T.; Lindstedt, S.L. Eccentric muscle contractions: Their contribution to injury, prevention, rehabilitation, and sport. J. Orthop. Sport. Phys. Ther. 2003, 33, 557–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwerver, J.; Dekker, F.; Pepping, G.J. Patient guided Piezo-electric Extracorporeal Shockwave Therapy as treatment for chronic severe patellar tendinopathy: A pilot study. J. Back Musculoskelet. Rehabil. 2010, 23, 111–115. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Farì, G.; Bianchi, F.P.; Moretti, L.; Covelli, I.; Ribatti, P.; Mennuni, C.; Tafuri, S.; Pesce, V.; et al. Extracorporeal shockwave therapy for plantar fasciitis and gastrocnemius muscle: Effectiveness of a combined treatment. J. Biol. Regul. Homeostatic. Agents 2020, 34, 285–290. [Google Scholar]
- Heaver, C.; Pinches, M.; Kuiper, J.H.; Thomas, G.; Lewthwaite, S.; Burston, B.J.; Banerjee, R.D. Greater trochanteric pain syndrome: Focused shockwave therapy versus an ultrasound guided injection: A randomised control trial. Hip Int. 2021, 33, 490–499. [Google Scholar] [PubMed]
- Sorg, H.; Zwetzich, I.; Tilkorn, D.J.; Kolbenschlag, J.; Hauser, J.; Goertz, O.; Spindler, N.; Langer, S.; Ring, A. Effects of Extracorporeal Shock Waves on Microcirculation and Angiogenesis in the in vivo Wound Model of the Diver Box. Eur. Surg. Res. 2021, 62, 134–143. [Google Scholar] [CrossRef]
- Moretti, B.; Iannone, F.; Notarnicola, A.; Lapadula, G.; Moretti, L.; Patella, V.; Garofalo, R. Extracorporeal shock waves down-regulate the expression of interleukin-10 and tumor necrosis factor-alpha in osteoarthritic chondrocytes. BMC Musculoskelet. Disord. 2008, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- McClure, S.R.; Sonea, I.M.; Evans, R.B.; Yaeger, M.J. Evaluation of analgesia resulting from extracorporeal shock wave therapy and radial pressure wave therapy in the limbs of horses and sheep. Am. J. Vet. Res. 2005, 66, 1702–1708. [Google Scholar] [CrossRef]
- Ramon, S.; Russo, S.; Santoboni, F.; Lucenteforte, G.; Di Luise, C.; de Unzurrunzaga, R.; Vetrano, M.; Albano, M.; Baldini, R.; Cugat, R.; et al. Focused Shockwave Treatment for Greater Trochanteric Pain Syndrome: A Multicenter, Randomized, Controlled Clinical Trial. J. Bone Jt. Surg. Am. 2020, 102, 1305–1311. [Google Scholar] [CrossRef]
- Barratt, P.A.; Brookes, N.; Newson, A. Conservative treatments for greater trochanteric pain syndrome: A systematic review. Br. J. Sport. Med. 2017, 51, 97–104. [Google Scholar] [CrossRef]

| Group A | Group B | Group C | Group D | Total | p-Value | |
|---|---|---|---|---|---|---|
| Age; average ± SD (range) | 59.1 ± 9.6 (37–69) | 59.5 ± 7.7 (49–73) | 64.0 ± 12.7 (37–74) | 52.1 ± 13.1 (31–74) | 58.9 ± 10.4 (31–74) | 0.196 |
| Female; n (%) | 12 (80.0) | 13 (86.7) | 4 (57.1) | 7 (100.0) | 36 (81.8) | 0.245 |
| BMI; average ± SD (range) | 28.7 ± 6.0 (19.8–39.3) | 27.5 ± 2.4 (24.3–33.0) | 26.2 ± 2.3 (22.0–28.8) | 25.0 ± 2.5 (20.8–28.0) | 27.3 ± 4.1 (19.8–39.3) | 0.218 |
| Diagnoses | Group A (Number of Patients) | Group B (Number of Patients) | Group C (Number of Patients) | Group D (Number of Patients) |
|---|---|---|---|---|
| Gluteus bursitis | 9 | 9 | 2 | 3 |
| Medius and minimus gluteus tendinopathies | 9 | 9 | 3 | 2 |
| Medius and minimus gluteus calcific tendinopathies | 4 | 4 | 2 | 2 |
| Time | Group A (n = 15) | Group B (n = 15) | Group C (n = 7) | Group D (n = 7) | Total (n = 44) | Comparison between Groups | Comparison between Times | Interaction between Groups and Times |
|---|---|---|---|---|---|---|---|---|
| NRS | ||||||||
| T0 | 7.7 ± 1.4 (4–9) | 8.3 ± 0.9 (7–10) | 7.0 ± 1.2 (5–8) | 8.3 ± 1.1 (7–10) | 7.9 ± 1.2 (4–10) | 0.358 | <0.0001 | 0.511 |
| T1 | 5.5 ± 1.6 (1–7) | 5.4 ± 2.0 (1–7) | 5.1 ± 2.0 (1–10) | 6.9 ± 2.9 (1–10) | 5.6 ± 2.0 (1–10) | |||
| T2 | 5.0 ± 2.3 (1–8) | 4.5 ± 2.0 (1–7) | 4.3 ± 1.8 (1–6) | 5.9 ± 2.7 (1–10) | 4.8 ± 2.2 (1–10) | |||
| T3 | 4.3 ± 2.6 (1–9) | 3.9 ± 1.8 (1–6) | 3.7 ± 1.6 (1–6) | 5.3 ± 2.4 (1–9) | 4.2 ± 2.1 (1–9) | |||
| LEFS | ||||||||
| T0 | 48.7 ± 12.3 (24–76) | 32.8 ± 13.8 (17–66) | 40.4 ± 7.8 (32–53) | 36.6 ± 8.9 (24–46) | 40.0 ± 13.3 (17–76) | 0.207 | <0.0001 | 0.393 |
| T1 | 53.8 ± 12.2 (24–76) | 42.9 ± 19.3 (21–80) | 48.7 ± 9.8 (40–66) | 42.4 ± 17.4 (25–76) | 47.5 ± 15.9 (21–80) | |||
| T2 | 55.9 ± 15.5 (22–74) | 48.8 ± 17.7 (21–80) | 53.1 ± 14.4 (30–70) | 48.6 ± 19.6 (28–77) | 51.9 ± 16.5 (21–80) | |||
| T3 | 59.0 ± 15.3 (28–75) | 52.3 ± 15.1 (30–80) | 55.0 ± 14.3 (30–72) | 49.4 ± 19.1 (30–77) | 54.5 ± 15.6 (28–80) | |||
| RMS | ||||||||
| T1 | 2.7 ± 0.6 (2–4) | 2.5 ± 0.8 (1–4) | 2.6 ± 0.5 (2–3) | 2.7 ± 0.8 (1–3) | 2.6 ± 0.7 (1–4) | 0.755 | <0.0001 | 0.964 |
| T2 | 2.3 ± 0.8 (1–4) | 2.1 ± 0.7 (1–3) | 2.1 ± 0.7 (1–4) | 2.4 ± 1.0 (1–3) | 2.2 ± 0.8 (1–4) | |||
| T3 | 2.1 ± 0.9 (1–4) | 2.0 ± 0.8 (1–3) | 2.1 ± 0.7 (1–3) | 2.4 ± 1.0 (1–3) | 2.1 ± 0.8 (1–4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarnicola, A.; Ladisa, I.; Lanzilotta, P.; Bizzoca, D.; Covelli, I.; Bianchi, F.P.; Maccagnano, G.; Farì, G.; Moretti, B. Shock Waves and Therapeutic Exercise in Greater Trochanteric Pain Syndrome: A Prospective Randomized Clinical Trial with Cross-Over. J. Pers. Med. 2023, 13, 976. https://doi.org/10.3390/jpm13060976
Notarnicola A, Ladisa I, Lanzilotta P, Bizzoca D, Covelli I, Bianchi FP, Maccagnano G, Farì G, Moretti B. Shock Waves and Therapeutic Exercise in Greater Trochanteric Pain Syndrome: A Prospective Randomized Clinical Trial with Cross-Over. Journal of Personalized Medicine. 2023; 13(6):976. https://doi.org/10.3390/jpm13060976
Chicago/Turabian StyleNotarnicola, Angela, Ilaria Ladisa, Paola Lanzilotta, Davide Bizzoca, Ilaria Covelli, Francesco Paolo Bianchi, Giuseppe Maccagnano, Giacomo Farì, and Biagio Moretti. 2023. "Shock Waves and Therapeutic Exercise in Greater Trochanteric Pain Syndrome: A Prospective Randomized Clinical Trial with Cross-Over" Journal of Personalized Medicine 13, no. 6: 976. https://doi.org/10.3390/jpm13060976
APA StyleNotarnicola, A., Ladisa, I., Lanzilotta, P., Bizzoca, D., Covelli, I., Bianchi, F. P., Maccagnano, G., Farì, G., & Moretti, B. (2023). Shock Waves and Therapeutic Exercise in Greater Trochanteric Pain Syndrome: A Prospective Randomized Clinical Trial with Cross-Over. Journal of Personalized Medicine, 13(6), 976. https://doi.org/10.3390/jpm13060976










