Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection
Abstract
1. Introduction
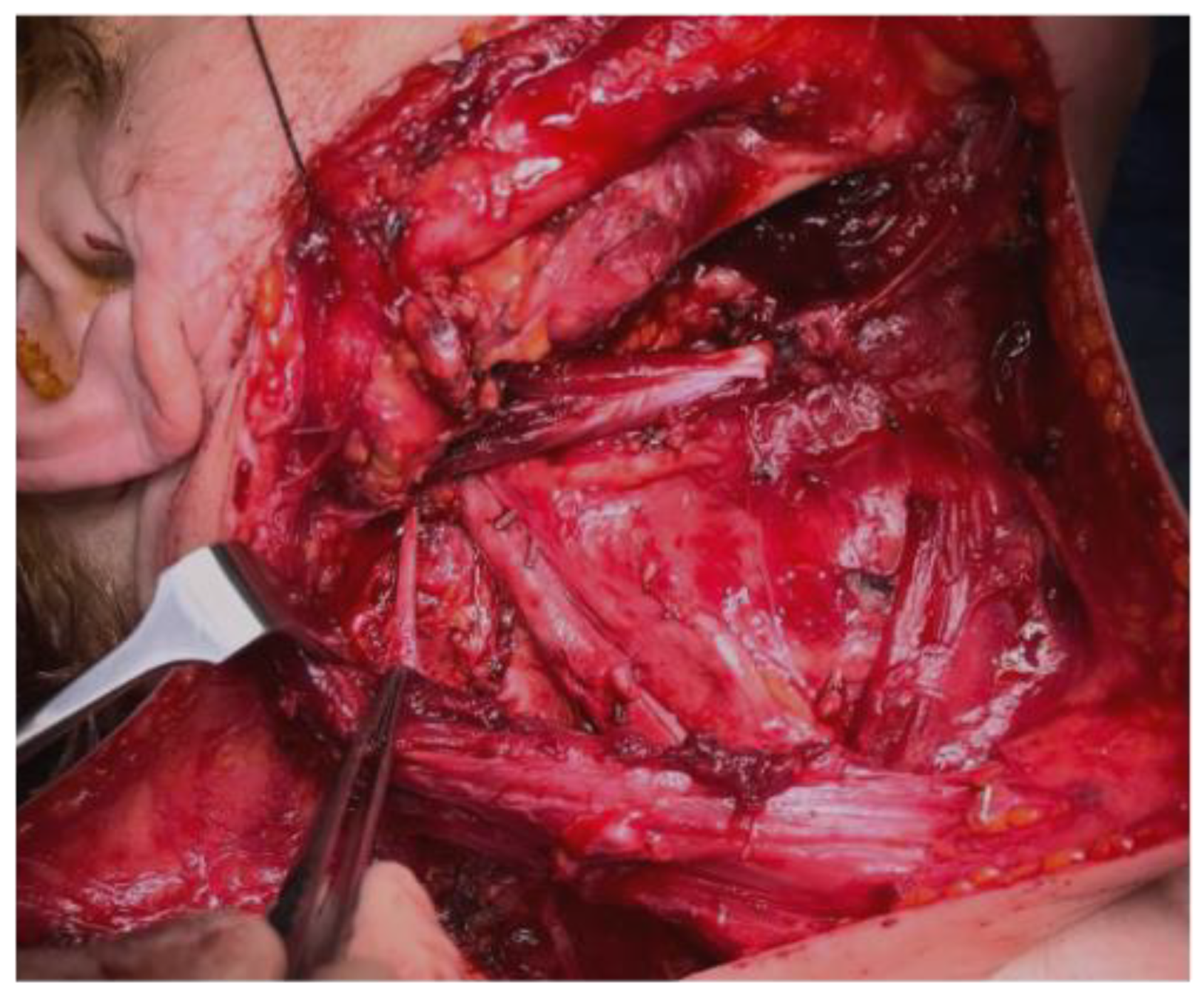
2. Materials and Methods
- The inclusion criteria were as follows:
- ○
- Age > 18 years;
- ○
- BMI < 35;
- ○
- Selective neck dissection of levels I–II (always including IIB) and III;
- ○
- No previous history of neck irradiation or surgery;
- ○
- No history of shoulder fractures or other functional and anatomical limitations;
- ○
- No history of rheumatologic disease affecting joints.
- The exclusion criteria were as follows:
- ○
- Metastatic and/or Lymph nodal disease (M/N+);
- ○
- Previous history of neck surgery;
- ○
- Previous history of significant shoulder trauma or surgery.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehanna, H.; Paleri, V.; West, C.M.L.; Nutting, C. Head and neck cancer—Part 1: Epidemiology, presentation, and prevention. BMJ 2010, 341, c4684. [Google Scholar] [CrossRef] [PubMed]
- Doll, C.; Mrosk, F.; Wuester, J.; Runge, A.-S.; Neumann, F.; Rubarth, K.; Heiland, M.; Kreutzer, K.; Voss, J.; Raguse, J.-D.; et al. Pattern of cervical lymph node metastases in squamous cell carcinoma of the upper oral cavity—How to manage the neck. Oral Oncol. 2022, 130, 105898. [Google Scholar] [CrossRef] [PubMed]
- Garba, S.M.; Hami, H.; Zaki, H.M.; Soulaymani, A.; Nouhou, H.; Mokhtari, A.; Quyou, A. 283P Descriptive epidemiology of head and neck cancer in Niger: First results from the National Cancer Registry. Ann. Oncol. 2020, 31, S1352. [Google Scholar] [CrossRef]
- Robbins, K.T. Indications for Selective Neck Dissection: When, How, and Why. Oncology 2000, 14, 1455–1464; discussion 1467–1469. [Google Scholar] [PubMed]
- Gane, E.; Michaleff, Z.; Cottrell, M.; McPhail, S.; Hatton, A.; Panizza, B.; O’Leary, S. Prevalence, incidence, and risk factors for shoulder and neck dysfunction after neck dissection: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1199–1218. [Google Scholar] [CrossRef]
- Lubov, J.; Labbé, M.; Sioufi, K.; Morand, G.B.; Hier, M.P.; Khanna, M.; Sultanem, K.; Mlynarek, A.M. Prognostic factors of head and neck cutaneous squamous cell carcinoma: A systematic review. J. Otolaryngol. Head Neck Surg. = J. D’oto-Rhino-Laryngol. Chir. Cervico-Faciale 2021, 50, 54. [Google Scholar] [CrossRef]
- Nason, R.W.; Abdulrauf, B.M.; Stranc, M.F. The anatomy of the accessory nerve and cervical lymph node biopsy. Am. J. Surg. 2000, 180, 241–243. [Google Scholar] [CrossRef]
- Shah, J.P.; Gil, Z. Current concepts in management of oral cancer—Surgery. Oral Oncol. 2009, 45, 394–401. [Google Scholar] [CrossRef]
- Nahum, A.M.; Mullally, W.; Marmor, L. A Syndrome Resulting from Radical Neck Dissection. Arch. Otolaryngol. Head Neck Surg. 1961, 74, 424–428. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Neck and Shoulder Motor Function following Neck Dissection: A Comparison with Healthy Control Subjects. Otolaryngol. Neck Surg. 2019, 160, 1009–1018. [Google Scholar] [CrossRef]
- Stubblefield, M.D. Cancer Rehabilitation. Semin. Oncol. 2011, 38, 386–393. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, G.J.; Leffers, P.; Bouter, L.M. Shoulder disability questionnaire design and responsiveness of a functional status measure. J. Clin. Epidemiol. 2000, 53, 29–38. [Google Scholar] [CrossRef]
- Cho, J.-G.; Lee, N.; Park, M.-W.; Baek, S.-K.; Kwon, S.-Y.; Jung, K.-Y.; Woo, J.-S. Measurement of the trapezius muscle volume: A new assessment strategy of shoulder dysfunction after neck dissection for the treatment of head and neck cancers. Head Neck 2015, 37, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, A.; Naito, K.; Nakamura, S.; Miyahara, K.; Goto, K.; Obata, H.; Nagura, N.; Sugiyama, Y.; Kaneko, K.; Ishijima, M. Influence of aging on the peripheral nerve repair process using an artificial nerve conduit. Exp. Ther. Med. 2020, 21, 168. [Google Scholar] [CrossRef] [PubMed]
- Negredo, P.N.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef]
- Breckenridge, J.D.; McAuley, J.H. Shoulder Pain and Disability Index (SPADI). J. Physiother. 2011, 57, 197. [Google Scholar] [CrossRef]
- Fiorillo, L. Fi-Index: A New Method to Evaluate Authors Hirsch-Index Reliability. Publ. Res. Q. 2022, 38, 465–474. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cicciù, M. The Use of Fi-Index Tool to Assess Per-manuscript Self-citations. Publ. Res. Q. 2022, 38, 684–692. [Google Scholar] [CrossRef]
- Eickmeyer, S.M.; Mpt, C.K.W.; Myers, K.B.; Lindstrom, D.R.; Layde, P.; Campbell, B.H.; Bsn, R.K.B.M.; Layde, M.P.; Campbell, F.B.H. Quality of Life, Shoulder Range of Motion, and Spinal Accessory Nerve Status in 5-Year Survivors of Head and Neck Cancer. PMR J. Inj. Funct. Rehabil. 2014, 6, 1073–1080. [Google Scholar] [CrossRef]
- Celik, B.; Coskun, H.; Kumas, F.F.; Irdesel, J.; Zarifoglu, M.; Erisen, L.; Onart, S. Accessory nerve function after level 2b-preserving selective neck dissection. Head Neck 2009, 31, 1496–1501. [Google Scholar] [CrossRef]
- Thawley, S.E.; Panje, W.R.; Batsakis, J.G. Comprehensive Management of Head and Neck Tumors. II; W.B. Saunders Company: Philadelphia, PA, USA, 1999; pp. 1147–1172. [Google Scholar]
- Cummings, C.W.; Fredrickson, J.M. Otolaryngology–Head and neck surgery. In Year Book. II, 2nd ed.; St. Louis-Baltimore-Boston; Mosby: Maryland Heights, MO, USA, 1993; pp. 1043–1078. [Google Scholar]
- Boström, D.; Dahlin, L.B. Iatrogenic injury to the accessory nerve. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2007, 41, 82–87. [Google Scholar] [CrossRef]
- Skinner, S.A. Neurophysiologic Monitoring of the Spinal Accessory Nerve, Hypoglossal Nerve, and the Spinomedullary Region. J. Clin. Neurophysiol. 2011, 28, 587–598. [Google Scholar] [CrossRef]
- Patten, C.; Hillel, A.D. The 11th nerve syndrome. Accessory nerve palsy or adhesive capsulitis? Arch. Otolaryngol. Head Neck Surg. 1993, 119, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Camp, S.J.; Birch, R. Injuries to the spinal accessory nerve: A lesson to surgeons. J. Bone Jt. Surg. Br. 2011, 93, 62–67. [Google Scholar] [CrossRef]
- Vastamaki, M.; Solonen, K.A. Accessory nerve injury. Acta Orthop. Scand. 1984, 55, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.A.; Ruchelsman, D.E.; Schwarzkopf, R. Iatrogenic spinal accessory nerve injury in children. J. Pediatr. Surg. 2008, 43, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Dailiana, Z.H.; Mehdian, H.; Gilbert, A. Surgical anatomy of spinal accessory nerve: Is trapezius functional deficit inevitable after division of the nerve? J. Hand Surg. Br. 2001, 26, 137–141. [Google Scholar] [CrossRef]
- Soo, K.C.; Guiloff, R.J.; Oh, A.; Della Rovere, G.Q.; Westbury, G. Innervation of the trapezius muscle: A study in patients undergoing neck dissections. Head Neck 1990, 12, 488–495. [Google Scholar] [CrossRef]
- Kelley, M.J.; Kane, T.E.; Leggin, B.G. Spinal Accessory Nerve Palsy: Associated Signs and Symptoms. J. Orthop. Sport. Phys. Ther. 2008, 38, 78–86. [Google Scholar] [CrossRef]
- Teboul, F.; Bizot, P.; Kakkar, R.; Sedel, L. Surgical management of trapezius palsy. J. Bone Jt. Surg. Am. 2004, 86, 1884–1890. [Google Scholar] [CrossRef]
- Wiater, J.M.; Bigliani, L.U. Spinal accessory nerve injury. Clin. Orthop. Relat. Res. 1999, 368, 5–16. [Google Scholar] [CrossRef]
- Chandawarkar, R.Y.; Cervino, L.A.; Pennington, G.A. Management of Iatrogenic Injury to the Spinal Accessory Nerve. Plast. Reconstr. Surg. 2003, 111, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Akgun, K.; Aktas, I.; Uluc, K. Conservative Treatment for Late-Diagnosed Spinal Accessory Nerve Injury. Am. J. Phys. Med. Rehabil. 2008, 87, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Osgaard, O.; Eskesen, V.; Rosenørn, J. Microsurgical repair of iatrogenic accessory nerve lesions in the posterior triangle of the neck. Acta Chir. Scand. 1987, 153, 171–173. [Google Scholar]
- Bazner, U.M.; Braun, V.; Richter, H.P.; Antoniadis, G. Management iatrogener N.-accessorius-Läsionen. Nervenarzt 2005, 76, 462–466. [Google Scholar] [CrossRef]
- Harpf, C.; Rhomberg, M.; Rumer, A.; Rainer, C.; Hussl, H. Iatrogene Läsion des Nervus accessorius bei der cervicalen Lymphknotenbiopsie. Chirurg 1999, 70, 690–693. [Google Scholar] [CrossRef]
- Norden, A. Peripheral injuries to the spinal accessory nerve. Acta Chir. Scand. 1946, 94, 515–532. [Google Scholar]
- Okajima, S.; Tamai, K.; Fujiwara, H.; Kobashi, H.; Hirata, M.; Kubo, T. Surgical treatment for spinal accessory nerve injury. Microsurgery 2006, 26, 273–277. [Google Scholar] [CrossRef]
- Ducic, I.; Maloney, C.T.; Dellon, A.L. Reconstruction of the Spinal Accessory Nerve with Autograft or Neurotube? Two Case Reports. J. Reconstr. Microsurg. 2005, 21, 29–33. [Google Scholar] [CrossRef]
- Nakamichi, K.-I.; Tachibana, S. Iatrogenic Injury of the Spinal Accessory Nerve. Results of Repair. J. Bone Jt. Surg. 1998, 80, 1616–1621. [Google Scholar] [CrossRef]
- Dziegielewski, P.T.; McNeely, M.; Ashworth, N.; O’connell, D.A.; Barber, B.; Courneya, K.S.; Debenham, B.J.; Seikaly, H. 2b or not 2b? Shoulder function after level 2b neck dissection: A double-blind randomized controlled clinical trial. Cancer 2020, 126, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Paleri, V.; Subramaniam, S.K.; Oozeer, N.; Rees, G.; Krishnan, S. Dissection of the submuscular recess (sublevel IIb) in squamous cell cancer of the upper aerodigestive tract: Prospective study and systematic review of the literature. Head Neck 2008, 30, 194–200. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, A.C.; Hoffman, G.R.; Osmotherly, P.G.; Chiarelli, P.E. Maximizing shoulder function after accessory nerve injury and neck dissection surgery: A multicenter randomized controlled trial: Physical Therapy for Accessory Nerve Injury after Neck Dissection. Head Neck 2015, 37, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Tedman, B.; Scott, B.; Lowe, D.; Rogers, S. A double blind randomised trial of IIb or not IIb neck dissections on electromyography, clinical examination, and questionnaire-based outcomes: A feasibility study. Br. J. Oral Maxillofac. Surg. 2012, 50, 394–403. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crimi, S.; Battaglia, S.; Maugeri, C.; Mirabella, S.; Fiorillo, L.; Cervino, G.; Bianchi, A. Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection. J. Pers. Med. 2023, 13, 1082. https://doi.org/10.3390/jpm13071082
Crimi S, Battaglia S, Maugeri C, Mirabella S, Fiorillo L, Cervino G, Bianchi A. Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection. Journal of Personalized Medicine. 2023; 13(7):1082. https://doi.org/10.3390/jpm13071082
Chicago/Turabian StyleCrimi, Salvatore, Salvatore Battaglia, Claudia Maugeri, Sergio Mirabella, Luca Fiorillo, Gabriele Cervino, and Alberto Bianchi. 2023. "Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection" Journal of Personalized Medicine 13, no. 7: 1082. https://doi.org/10.3390/jpm13071082
APA StyleCrimi, S., Battaglia, S., Maugeri, C., Mirabella, S., Fiorillo, L., Cervino, G., & Bianchi, A. (2023). Does Age Affect the Rate of Spinal Nerve Injury after Selective Neck Dissection? Age as a Prognostic Factor of Spinal Nerve Injury after Selective Neck Dissection. Journal of Personalized Medicine, 13(7), 1082. https://doi.org/10.3390/jpm13071082









