Is There a Role for Intraoperative Neuromonitoring in Intradural Extramedullary Spine Tumors? Results and Indications from an Institutional Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Enrollment and Data Collection
2.2. Intraoperative Monitoring
2.2.1. Motor Evoked Potentials
2.2.2. Somatosensory Evoked Potentials
2.2.3. MEP Amplitude Ratios
2.2.4. Statistical Analysis
3. Results
3.1. Predictors of Preoperative MMS
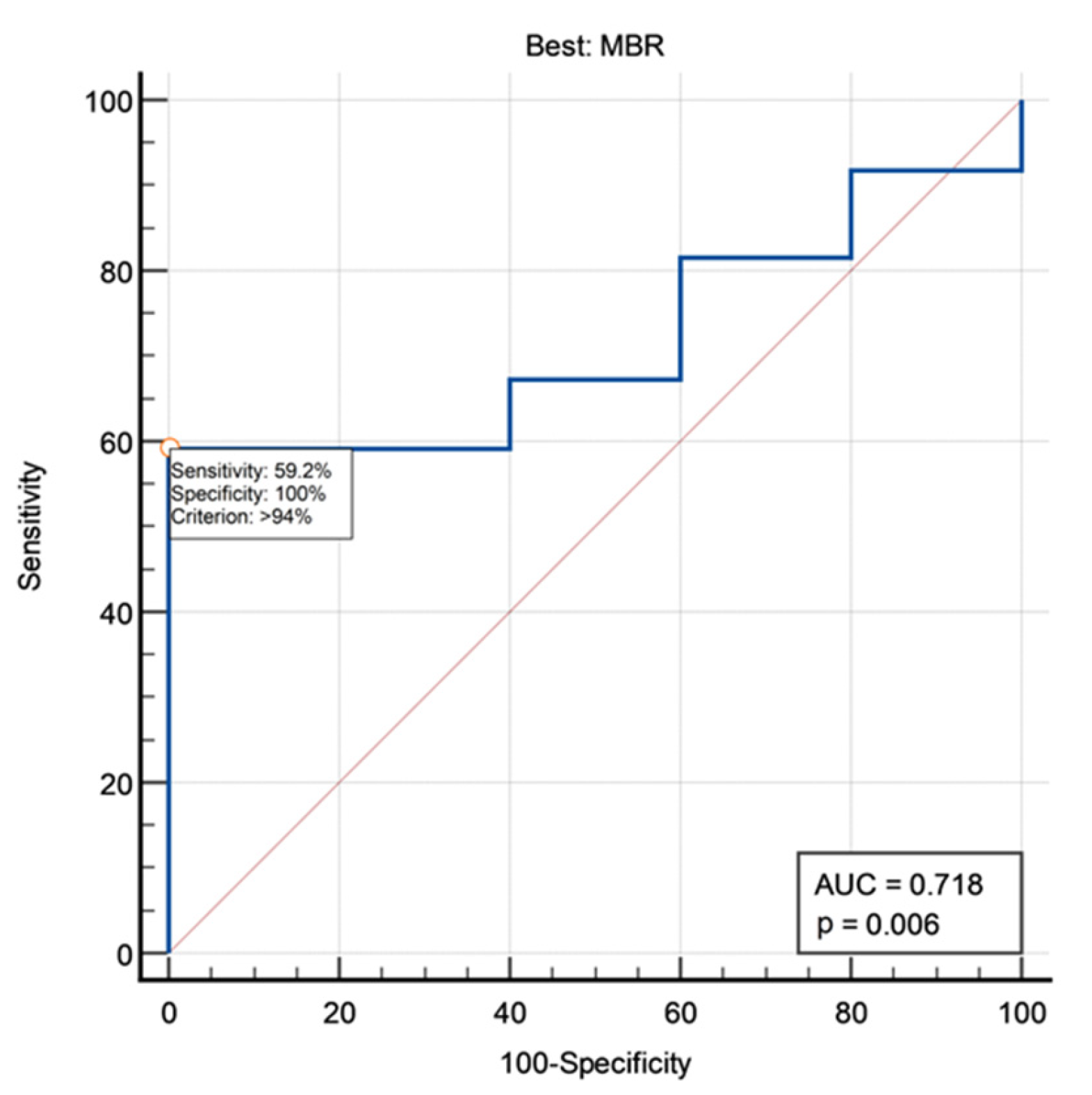
3.2. MEP Amplitude Ratios
3.3. Predictors of MMS at Discharge
3.4. Predictors of MMS at the End of Follow-up
| Intraoperative SEP Variation | Good MMS at Discharge | p | Good MMS at Last Follow-Up | p |
|---|---|---|---|---|
| Stable | 37/49 | 0.0006 | 44/47 | 0.0433 |
| Worsened | 0/6 | 2/4 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jellema, K.; Overbeeke, J.J.; Teepen, H.L.; Visser, L.H. Time to diagnosis of intraspinal tumors. Eur. J. Neurol. 2005, 12, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Montano, N.; Trevisi, G.; Cioni, B.; Lucantoni, C.; Della Pepa, G.M.; Meglio, M.; Papacci, F. The role of laminoplasty in preventing spinal deformity in adult patients submitted to resection of an intradural spinal tumor. Case Ser. Lit. Rev. Clin. Neurol. Neurosurg. 2014, 125, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.T.; Marquardt, G.; Seifert, V.; Szelényi, A. Spinal cord tumor surgery–importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine 2012, 37, E1001–E1008. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Fehlings, M.G. Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg. Focus 2008, 25, E15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korn, A.; Halevi, D.; Lidar, Z.; Biron, T.; Ekstein, P.; Constantini, S. Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: Experience with 100 cases. Acta Neurochir. 2015, 157, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Brodke, D.S.; Norvell, D.C.; Dettori, J.R. The evidence for intraoperative neurophysiological monitoring in spine surgery: Does it make a difference? Spine 2010, 35 (Suppl. S9), S37–S46. [Google Scholar] [CrossRef]
- Deletis, V.; Sala, F. Intraoperative neurophysiological monitoring of the spinal cord during spinal cord and spine surgery: A review focus on the corticospinal tracts. Clin. Neurophysiol. 2008, 119, 248–264. [Google Scholar] [CrossRef]
- MacDonald, D.B.; Dong, C.; Quatrale, R.; Sala, F.; Skinner, S.; Soto, F.; Szelényi, A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin. Neurophysiol. 2019, 130, 161–179. [Google Scholar] [CrossRef]
- D’Alessandris, Q.G.; Menna, G.; Stifano, V.; Della Pepa, G.M.; Burattini, B.; Di Domenico, M.; Izzo, A.; D’Ercole, M.; Lauretti, L.; Montano, N.; et al. A Study on the Role of Intraoperative Corticobulbar Motor Evoked Potentials for Improving Safety of Cerebellopontine Angle Surgery in Elderly Patients. Diagnostics 2023, 13, 710. [Google Scholar] [CrossRef]
- Penfield, W.; Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 1937, 60, 389–443. [Google Scholar] [CrossRef]
- Nash, C.L.; Brodkey, J.S.; Croft, T.J. A model for electrical monitoring of spinal cord function in scoliosis patients undergoing correctio. J. Bone Jt. Surg. 1972, 54, 197–198. [Google Scholar]
- Lesser, R.P.; Raudzens, P.; Lüders, H.; Nuwer, M.R.; Goldie, W.D.; Morris, H.H., III; Dinner, D.S.; Klem, G.; Hahn, J.F.; Shetter, A.G.; et al. Postoperative neurological deficits may occur despite unchanged intraoperative somatosensory evoked potentials. Ann. Neurol. 1986, 19, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Zornow, M.H.; Grafe, M.R.; Tybor, C.; Swenson, M.R. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 137–139. [Google Scholar] [CrossRef]
- Pelosi, L.; Jardine, A.; Webb, J.K. Neurological complications of anterior spinal surgery for kyphosis with normal somatosensory evoked potentials (SEPs). J. Neurol. Neurosurg. Psychiatry 1999, 66, 662–664. [Google Scholar] [CrossRef] [Green Version]
- Kothbauer, K.F.; Deletis, V.; Epstein, F.J. Motor-evoked potential monitoring for intramedullary spinal cord tumor surgery: Correlation of clinical and neurophysiological data in a series of 100 consecutive procedures. Neurosurg. Focus 1998, 4, E3. [Google Scholar] [CrossRef] [PubMed]
- Quiñones-Hinojosa, A.; Lyon, R.; Zada, G.; Lamborn, K.R.; Gupta, N.; Parsa, A.T.; McDermott, M.W.; Weinstein, P.R. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery 2005, 56, 982–993. [Google Scholar]
- Sala, F.; Palandri, G.; Basso, E.; Lanteri, P.; Deletis, V.; Faccioli, F.; Bricolo, A. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: A historical control study. Neurosurgery 2006, 58, 1129–1143. [Google Scholar] [CrossRef]
- Tamaki, T.; Yamane, T. Proceedings: Clinical utilization of the evoked spinal cord action potential in spine and spinal cord surgery. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 539. [Google Scholar]
- Szelenyi, A.; Heukamp, C.; Seifert, V.; Marquardt, G. S100B, intraoperative neuromonitoring findings and their relation to clin-ical outcome in surgically treated intradural spinal lesions. Acta Neurochir. 2014, 156, 733–739. [Google Scholar] [CrossRef]
- Lakomkin, N.; Mistry, A.M.; Zuckerman, S.L.; Ladner, T.; Kothari, P.; Lee, N.J.; Stannard, B.; Vasquez, R.A.; Cheng, J.S. Utility of intraoperative monitoring in the resection of spinal cord tumors: An analysis by tumor location and anatomical region. Spine 2018, 43, 287–294. [Google Scholar] [CrossRef]
- Ishida, W.; Casaos, J.; Chandra, A.; D’Sa, A.; Ramhmdani, S.; Perdomo-Pantoja, A.; Theodore, N.; Jallo, G.; Gokaslan, Z.L.; Wolinsky, J.P.; et al. Diagnostic and therapeutic values of intraoperative electrophysiological neuromoni-toring during resection of intradural extramedullary spinal tumors: A single center retrospective cohort and meta-analysis. J. Neurosurg. Spine 2019, 30, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.A.; Jeyanandarajan, D.; Hansen, C.; Zada, G.; Hsieh, P.C. Intraoperative neurophysiological monitoring during spine surgery: A review. Neurosurg. Focus 2009, 27, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, M.J.; Wolf, S.; Schul, D.B.; Gerstner, W.; Lumenta, C.B. Significance of intraoperative motor function monitoring using transcranial electrical motor evoked potentials (MEP) in patients with spinal and cranial lesions near the motor pathways. Br. J. Neurosurg. 2009, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kothbauer, K.F.; Deletis, V. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv. Syst. 2010, 26, 247–253. [Google Scholar] [CrossRef]
- Chiappa, K.; Hill, R. Short Latency Somatosensory Evoked Potentials: Methodology. In Evoked Potentials in Clinical Medicine; Chiappa, K., Ed.; Lippincott-Raven: Philadelphia, PA, USA, 1997. [Google Scholar]
- Hyun, S.J.; Rhim, S.C. Combined motor and somatosensory evoked potential monitoring for intramedullary spinal cord tu-mor surgery: Correlation of clinical and neurophysiological data in 17 consecutive procedures. Br. J. Neurosurg. 2009, 23, 393–400. [Google Scholar] [CrossRef]
- Sandalcioglu, I.E.; Hunold, A.; Muller, O.; Bassiouni, H.; Stolke, D.; Asgari, S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur. Spine J. 2008, 17, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Safaee, M.M.; Lyon, R.; Barbaro, N.M.; Chou, D.; Mummaneni, P.V.; Weinstein, P.R.; Chin, C.T.; Tihan, T.; Ames, C.P. Neurological outcomes and surgical complications in 221 spinal nerve sheath tu-mors. J. Neurosurg. Spine 2017, 26, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Peretta, P.; Faccani, G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur. Spine J. 2013, 22, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Scibilia, A.; Terranova, C.; Rizzo, V.; Raffa, G.; Morelli, A.; Esposito, F.; Mallamace, R.; Buda, G.; Conti, A.; Quartarone, A.; et al. Intraoperative neurophysiological mapping and monitoring in spinal tumor surgery: Sirens or indispensable tools? Neurosurg. Focus 2016, 41, E18. [Google Scholar] [CrossRef] [Green Version]
- Ghadirpour, R.; Nasi, D.; Iaccarino, C.; Romano, A.; Motti, L.; Sabadini, R.; Valzania, F.; Servadei, F. Intraoperative neurophysiolog-ical monitoring for intradural extramedullary spinal tumors: Predictive value and relevance of D-wave amplitude on sur-gical outcome during a 10-year experience. J. Neurosurg. Spine 2018, 30, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Ghadirpour, R.; Nasi, D.; Iaccarino, C.; Giraldi, D.; Sabadini, R.; Motti, L.; Sala, F.; Servadei, F. Intraoperative neurophysiological monitoring for intradural extramedullary tumors: Why not? Clin. Neurol. Neurosurg. 2015, 130, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Slin’ko, E.I.; Al-Qashqish, I.I. Intradural ventral and ventrolateral tumors of the spinal cord: Surgical treatment and results. Neurosurg. Focus 2004, 17, ECP2. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Feature | Value |
|---|---|
| Age (mean ± SD) | 56.3 ± 15.9 |
| Male:Female (%) | 21:39 (35:65%) |
| Tumor location | |
| Cervical | 12 (20%) |
| Upper thoracic | 14 (23.3%) |
| Lower thoracic | 14 (23.3%) |
| Lumbosacral | 20 (33.3%) |
| Number of involved levels | |
| 1 | 16 (26.7%) |
| 2 | 36 (60%) |
| 3 | 6 (10%) |
| 4 | 1 (1.7%) |
| 5 | 1 (1.7%) |
| Pathology | |
| Meningioma | 19 (31.7%) |
| Schwannoma | 28 (46.7%) |
| Paraganglioma | 2 (3.3%) |
| Hemangiopericytoma | 2 (3.3%) |
| Neurofibroma | 1 (1.7%) |
| Other | 8 (13.3%) |
| Modified McCormick Scale | Preoperative | Postoperative | At Follow-Up |
|---|---|---|---|
| Good | 47 (78.3%) | 41 (68.3%) | 50 (90.9%) |
| Poor | 13 (21.7%) | 19 (31.7%) | 5 (9.1%) |
| Improved * | NA | 8 (13.3%) | 17 (30.9%) |
| Stable * | NA | 43 (71.7%) | 36 (65.5%) |
| Worsened * | NA | 9 (15%) | 2 (3.6%) |
| Amplitude Ratio | Mean | Median | SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Mean FBR | 234.8% | 123.0% | 371.8% | 27.2% | 2619.3% |
| Median FBR | 113.3% | 95.8% | 64.8% | 17.2% | 476.5% |
| Best FBR | 961.0% | 197.9% | 2225.9% | 65.0% | 14,674.2% |
| Worst FBR | 49.6% | 48.5% | 33.2% | 1.7% | 131.6% |
| Mean MBR | 58.1% | 58.7% | 21.0% | 14.0% | 96.6% |
| Median MBR | 58.3% | 62.6% | 26.5% | 5.1% | 100.0% |
| Best MBR | 88.6% | 96.6% | 15.6% | 36.3% | 100.0% |
| Worst MBR | 25.7% | 17.2% | 22.2% | 0.6% | 76.0% |
| Mean RV | 176.8% | 55.9% | 370.0% | 2.6% | 2541.8% |
| Median RV | 52.8% | 31.7% | 65.7% | 0.0% | 465.2% |
| Best RV | 890.4% | 145.1% | 2219.2% | 11.7% | 14,576.6% |
| Worst RV | 9.5% | 2.1% | 19.2% | 0.0% | 120.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ercole, M.; D’Alessandris, Q.G.; Di Domenico, M.; Burattini, B.; Menna, G.; Izzo, A.; Polli, F.M.; Della Pepa, G.M.; Olivi, A.; Montano, N. Is There a Role for Intraoperative Neuromonitoring in Intradural Extramedullary Spine Tumors? Results and Indications from an Institutional Series. J. Pers. Med. 2023, 13, 1103. https://doi.org/10.3390/jpm13071103
D’Ercole M, D’Alessandris QG, Di Domenico M, Burattini B, Menna G, Izzo A, Polli FM, Della Pepa GM, Olivi A, Montano N. Is There a Role for Intraoperative Neuromonitoring in Intradural Extramedullary Spine Tumors? Results and Indications from an Institutional Series. Journal of Personalized Medicine. 2023; 13(7):1103. https://doi.org/10.3390/jpm13071103
Chicago/Turabian StyleD’Ercole, Manuela, Quintino Giorgio D’Alessandris, Michele Di Domenico, Benedetta Burattini, Grazia Menna, Alessandro Izzo, Filippo Maria Polli, Giuseppe Maria Della Pepa, Alessandro Olivi, and Nicola Montano. 2023. "Is There a Role for Intraoperative Neuromonitoring in Intradural Extramedullary Spine Tumors? Results and Indications from an Institutional Series" Journal of Personalized Medicine 13, no. 7: 1103. https://doi.org/10.3390/jpm13071103






