Mast Cell Activation Syndrome Update—A Dermatological Perspective
Abstract
:1. Introduction
2. Mast Cell Basic Physiology
3. Mast Cells as Immune Tissue Effectors
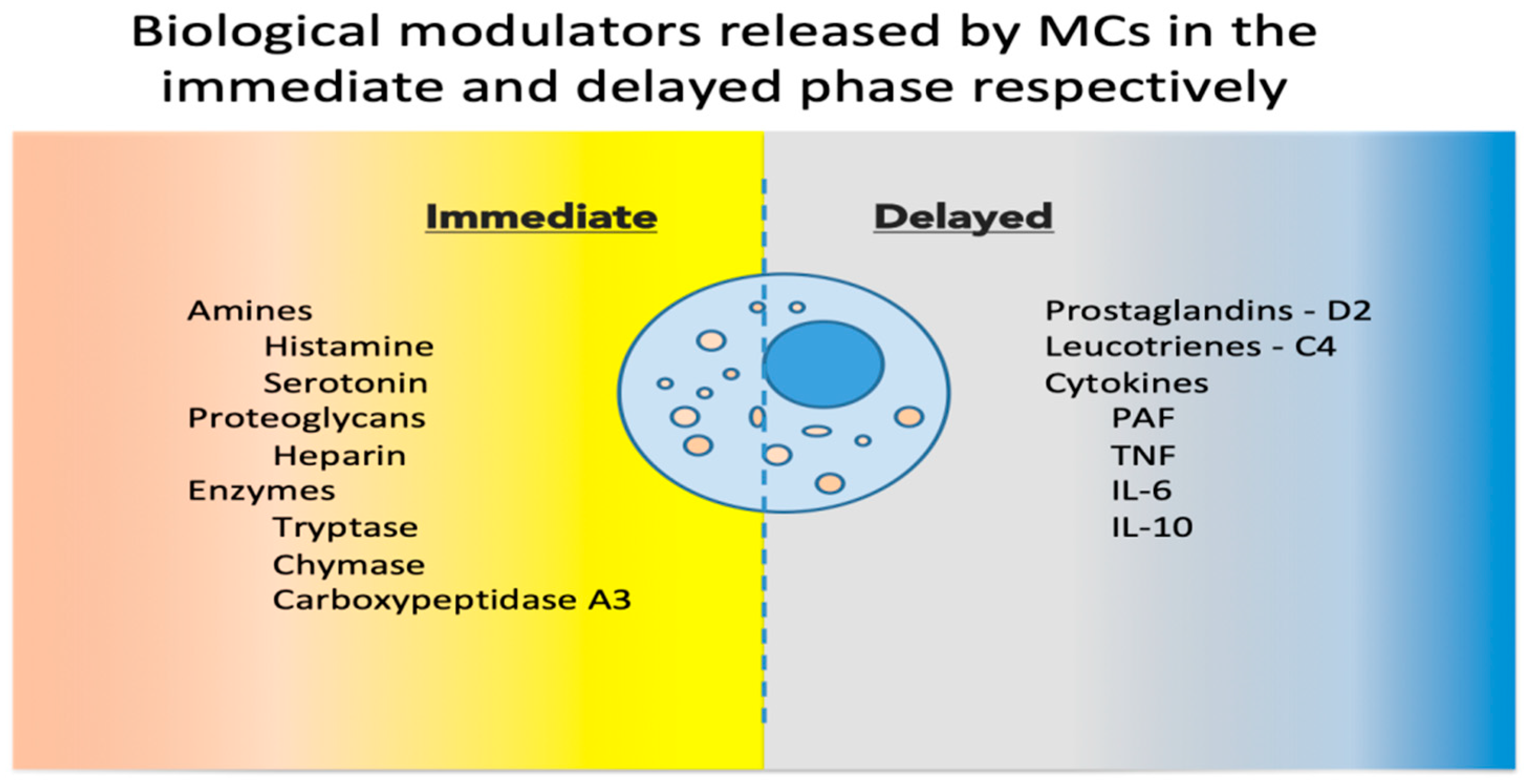
4. Role of Mast Cells in Pathology
5. Mast Cells in the Skin
6. MCAS
6.1. MCAS Clinical Manifestations
6.2. MCAS Diagnostic Criteria
- Clinical: typical MC activation symptoms, which are episodic, recurrent, severe (often taking the form of anaphylaxis) and systemic (involving two organ systems at least);
- Laboratory: markers of MC activation—event-related serum tryptase level elevated above 120% of the individual’s serum baseline + 2 ng/mL;
- Therapeutic: clinical response to drugs that counteract MC mediators or prevent their release.
6.3. MCAS Classification
6.4. Histamine Intolerance
6.5. Hereditary Alpha Tryptasemia (HαT)
6.6. Cutaneous Manifestations
- Maculopapular cutaneous mastocytosis (urticaria pigmentosa)—hyperpigmented macules, papules or nodules usually on the trunk associating Darier’s sign (formation of a wheal in response to stroking/rubbing the skin), although the absence of it does not rule out the diagnosis [163];
- Diffuse cutaneous mastocytosis—a rare variant of cutaneous mastocytosis with onset usually at birth or in early infancy [164], manifesting in children with generalized erythema and thickened skin, variable pigmentation and in some cases with papules and intense dermographism [165]; in adults it presents with extensive bullae, that sometimes can be hemorrhagic, and which progress to erosions, desquamation and finally hyper-pigmentation and also intense dermographism [165]
6.7. Treatment
7. Pharmacological Treatment
- An epinephrine autoinjector should be carried by all patients who had an anaphylaxis episode in their personal history—especially patients with known allergies to insect venoms—or who have a known risk of developing one. Epinephrine should be administered intramuscularly as soon as the first signs are noticed, or after insect bites and stings, in a dose of 0.01 mg/kg to a maximum of 0.5 mg in adults and 0.3 mg in children [167].
- Antihistamines are the first line of treatment for patients with non-life-threatening symptoms. They act by binding to, and blocking histamine receptors in the target tissues, thus reducing the impact of MC degranulation. Decades of clinical practice have established that the use of antihistamines alleviates symptoms such as itching, pain, urticaria, and acid reflux [169]. If symptom control cannot be achieved with the highest recommended doses of H2 antihistamines, a combination of non-sedating H1 and H2 antihistamines can be attempted, especially if the patient associates gastric symptoms [170]. Most patients have benefited from long-term antihistamine use, with good tolerance and minimum side effects [170].
- Leukotriene inhibitors are used in asthma and respiratory symptoms with probable underlying bronchoconstriction with allergic triggers that do not fulfil the criteria for asthma. They are also used in the treatment of allergic rhinitis, psoriasis and atopic dermatitis. Drugs with two mechanisms of action are currently approved: leukotriene receptor blockers (montelukast and zafirlukast) and 5-lipoxygenase inhibitors that block synthesis of leukotrienes from arachidonic acid (zileuton). Leukotriene inhibitors can be prescribed in combination with antihistamines [171,172,173].
- MC stabilizers increase the threshold for MC degranulation signaling. Cromolyn sodium and nedocromil are currently approved. Although very promising on paper, these drugs are not more effective than antihistamines and leukotriene inhibitors, with possibly more significant side effects. Consequently, they are not routinely prescribed. Moreover, ketotifen (a second-generation H1 receptor antagonist with higher brain permeability compared with the newer second-generation antagonists desloratadine and levocetirizine) has MC stabilizer activity. Desloratadine and cetirizine have also been reported as MC stabilizers, although doses significantly higher than the ones currently used for therapy were required [174,175,176,177,178].
- Steroids should only be used short term for acute exacerbations of symptoms not controlled by standard therapy. Potential uses include edema, urticaria, wheezing, diarrhea and severe pain [179].
- Biologics: omalizumab, an IgE receptor blocker, effectively controls MC-mediated symptoms, improves the patients’ quality of life, and should be recommended as low-dose long-term therapy to persons at risk of anaphylaxis [180,181]. Emerging kinase inhibitors (imatinib, mitostaurine, avapritinib) inhibit MC proliferation and IgE-dependent MC activation. These drugs may represent future effective therapies for patients with advanced systemic mastocytosis [168].
- DAO oral supplements should be considered in the case of HIT [182].
8. Discussion
9. Conclusions
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Stassen, M.; Hultner, L.; Schmitt, E. Classical and Alternative Pathways of Mast Cell Activation. Crit. Rev. Immunol. 2002, 22, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. The Mast Cell-IgE Paradox from Homeostasis to Anaphylaxis. Am. J. Pathol. 2016, 186, 212–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, A.; Köberle, M.; Goldmann, O.; Meyer, N.; Dudeck, J.; Lemmens, S.; Rohde, M.; Roldán, N.G.; Dietze-Schwonberg, K.; Orinska, Z.; et al. Mast Cells as Protectors of Health. J. Allergy Clin. Immunol. 2019, 144, S4–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurish, M.F.; Austen, K.F. Developmental Origin and Functional Specialization of Mast Cell Subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Friend, D.S.; Gurish, M.F.; Austen, K.F.; Hunt, J.; Stevens, R.L. Senescent Jejunal Mast Cells and Eosinophils in the Mouse Preferentially Translocate to the Spleen and Draining Lymph Node, Respectively, during the Recovery Phase of Helminth Infection. J. Immunol. 2000, 165, 344–352. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory Mast Cells: Negative, as Well as Positive, Regulators of Immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Gould, H.J.; Sutton, B.J. IgE in Allergy and Asthma Today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef]
- Abraham, S.N.; St. John, A.L. Mast Cell-Orchestrated Immunity to Pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Wernersson, S.; Pejler, G. Mast Cell Secretory Granules: Armed for Battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Irani, A.A.; Schechter, N.M.; Craig, S.S.; DeBlois, G.; Schwartz, L.B. Two Types of Human Mast Cells That Have Distinct Neutral Protease Compositions. Proc. Natl. Acad. Sci. USA 1986, 83, 4464–4468. [Google Scholar] [CrossRef]
- Douaiher, J.; Succar, J.; Lancerotto, L.; Gurish, M.F.; Orgill, D.P.; Hamilton, M.J.; Krilis, S.A.; Stevens, R.L. Development of Mast Cells and Importance of Their Tryptase and Chymase Serine Proteases in Inflammation and Wound Healing. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 122, pp. 211–252. [Google Scholar]
- Pejler, G.; Rönnberg, E.; Waern, I.; Wernersson, S. Mast Cell Proteases: Multifaceted Regulators of Inflammatory Disease. Blood 2010, 115, 4981–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast Cells in the Development of Adaptive Immune Responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Lyons, J.J. Hereditary Alpha-Tryptasemia: A Commonly Inherited Modifier of Anaphylaxis. Curr. Allergy Asthma Rep. 2021, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Nedoszytko, B.; Bonadonna, P.; Hartmann, K.; Niedoszytko, M.; Brockow, K.; Siebenhaar, F.; Triggiani, M.; Arock, M.; et al. Diagnosis, Classification and Management of Mast Cell Activation Syndromes (Mcas) in the Era of Personalized Medicine. Int. J. Mol. Sci. 2020, 21, 9030. [Google Scholar] [CrossRef]
- Grujic, M.; Paivandy, A.; Gustafson, A.M.; Thomsen, A.R.; Öhrvik, H.; Pejler, G. The Combined Action of Mast Cell Chymase, Tryptase and Carboxypeptidase A3 Protects against Melanoma Colonization of the Lung. Oncotarget 2017, 8, 25066–25079. [Google Scholar] [CrossRef] [Green Version]
- Caughey, G.H. Mast Cell Tryptases and Chymases in Inflammation and Host Defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated Basal Serum Tryptase Identifies a Multisystem Disorder Associated with Increased TPSAB1 Copy Number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, N.N.; Tamraz, B.; Chu, C.; Kwok, P.Y.; Caughey, G.H. Human Subjects Are Protected from Mast Cell Tryptase Deficiency despite Frequent Inheritance of Loss-of-Function Mutations. J. Allergy Clin. Immunol. 2009, 124, 1099–1105.e4. [Google Scholar] [CrossRef] [Green Version]
- Sabato, V.; Van De Vijver, E.; Hagendorens, M.; Vrelust, I.; Reyniers, E.; Fransen, E.; Bridts, C.; De Clerck, L.; Mortier, G.; Valent, P.; et al. Familial Hypertryptasemia with Associated Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. 2014, 134, 1448–1450.e3. [Google Scholar] [CrossRef]
- Lyons, J.J.; Stotz, S.C.; Chovanec, J.; Liu, Y.; Lewis, K.L.; Nelson, C.; DiMaggio, T.; Jones, N.; Stone, K.D.; Sung, H.; et al. A Common Haplotype Containing Functional CACNA1H Variants Is Frequently Coinherited with Increased TPSAB1 Copy Number. Genet. Med. 2018, 20, 503–512. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Romani, L. The Contribution of Mast Cells to Bacterial and Fungal Infection Immunity. Immunol. Rev. 2018, 282, 188–197. [Google Scholar] [CrossRef]
- Malaviya, R.; Ideda, R.; Ross, E.; Abraham, S.N. Mast Cell Modulation of Neutrophil Influx and Bacterial Clearance at Sites of Infection through TNF-Alpha. Pneumologie 1997, 51, 869. [Google Scholar]
- Biedermann, T.; Kneilling, M.; Mailhammer, R.; Maier, K.; Sander, C.A.; Kollias, G.; Kunkel, S.L.; Hültner, L.; Röcken, M. Mast Cells Control Neutrophil Recruitment during T Cell-Mediated Delayed-Type Hypersensitivity Reactions through Tumor Necrosis Factor and Macrophage Inflammatory Protein 2. J. Exp. Med. 2000, 192, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlos, D.; Frantz, F.G.; Souza-Júnior, D.A.; Jamur, M.C.; Oliver, C.; Ramos, S.G.; Quesniaux, V.F.; Ryffel, B.; Silva, C.L.; Bozza, M.T.; et al. TLR2-Dependent Mast Cell Activation Contributes to the Control of Mycobacterium Tuberculosis Infection. Microbes Infect. 2009, 11, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Shegarfi, H.; Sydnes, K.; Løvik, M.; Inngjerdingen, M.; Rolstad, B.; Naper, C. The Role of Natural Killer Cells in Resistance to the Intracellular Bacterium Listeria Monocytogenes in Rats. Scand. J. Immunol. 2009, 70, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast Cell: An Emerging Partner in Immune Interaction. Front. Immunol. 2012, 3, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, R.E.; Olsen, J.S.; McKinstry, A.; Villalta, S.A.; Wolters, P.J. Mast Cell IL-6 Improves Survival from Klebsiella Pneumonia and Sepsis by Enhancing Neutrophil Killing. J. Immunol. 2008, 181, 5598–5605. [Google Scholar] [CrossRef] [Green Version]
- Doener, F.; Michel, A.; Reuter, S.; Friedrich, P.; Böhm, L.; Relle, M.; Codarri, L.; Tenzer, S.; Klein, M.; Bopp, T.; et al. Mast Cell-Derived Mediators Promote Murine Neutrophil Effector Functions. Int. Immunol. 2013, 25, 553–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudeck, A.; Dudeck, J.; Scholten, J.; Petzold, A.; Surianarayanan, S.; Köhler, A.; Peschke, K.; Vöhringer, D.; Waskow, C.; Krieg, T.; et al. Mast Cells Are Key Promoters of Contact Allergy That Mediate the Adjuvant Effects of Haptens. Immunity 2011, 34, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Dudeck, J.; Medyukhina, A.; Fröbel, J.; Svensson, C.M.; Kotrba, J.; Gerlach, M.; Gradtke, A.C.; Schröder, B.; Speier, S.; Figge, M.T.; et al. Mast Cells Acquire MHC II from Dendritic Cells during Skin Inflammation. J. Exp. Med. 2017, 214, 3791–3811. [Google Scholar] [CrossRef]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of Mast Cell Secretory Granules on Skin Inflammation Boosts Dendritic Cell Migration and Priming Efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864.e4. [Google Scholar] [CrossRef] [PubMed]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast Cells and Dendritic Cells Form Synapses That Facilitate Antigen Transfer for T Cell Activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC Class II Expression by Mast Cells Supports Effector and Regulatory T Cell Activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudenzio, N.; Espagnolle, N.; Mars, L.T.; Liblau, R.; Valitutti, S.; Espinosa, E. Cell-Cell Cooperation at the T Helper Cell/Mast Cell Immunological Synapse. Blood 2009, 114, 4979–4988. [Google Scholar] [CrossRef] [Green Version]
- Jutel, M.; Watanabe, T.; Klunker, S.; Akdis, M.; Thomet, O.A.R.; Malolepszy, J.; Zak-Nejmark, T.; Koga, R.; Kobayashi, T.; Blaser, K.; et al. Histamine Regulates T-Cell and Antibody Responses by Differential Expression of H1 and H2 Receptors. Nature 2001, 413, 420–425. [Google Scholar] [CrossRef]
- Skokos, D.; Le Panse, S.; Villa, I.; Rousselle, J.-C.; Peronet, R.; David, B.; Namane, A.; Mécheri, S. Mast Cell-Dependent B and T Lymphocyte Activation Is Mediated by the Secretion of Immunologically Active Exosomes. J. Immunol. 2001, 166, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Leveson-Gower, D.B.; Sega, E.I.; Kalesnikoff, J.; Florek, M.; Pan, Y.; Pierini, A.; Galli, S.J.; Negrin, R.S. Mast Cells Suppress Murine GVHD in a Mechanism Independent of CD4+CD25+ Regulatory T Cells. Blood 2013, 122, 3659–3665. [Google Scholar] [CrossRef] [Green Version]
- Marshall, J.S. Mast-Cell Responses to Pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef]
- Migalovich-Sheikhet, H.; Friedman, S.; Mankuta, D.; Levi-Schaffer, F. Novel Identified Receptors on Mast Cells. Front. Immunol. 2012, 3, 238. [Google Scholar] [CrossRef] [Green Version]
- Abel, J.; Goldmann, O.; Ziegler, C.; Höltje, C.; Smeltzer, M.S.; Cheung, A.L.; Bruhn, D.; Rohde, M.; Medina, E. Staphylococcus Aureus Evades the Extracellular Antimicrobial Activity of Mast Cells by Promoting Its Own Uptake. J. Innate Immun. 2011, 3, 495–507. [Google Scholar] [CrossRef]
- Hepworth, M.R.; Maurer, M.; Hartmann, S. Regulation of Type 2 Immunity to Helminths by Mast Cells. Gut Microbes 2012, 3, 476–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, M.; Cato, A.C.B.; Ainooson, G.K.; Freichel, M.; Tsvilovskyy, V.; Jessberger, R.; Riedlinger, E.; Sommerhoff, C.P.; Bischoff, S.C. Regulation of the Pleiotropic Effects of Tissue-Resident Mast Cells. J. Allergy Clin. Immunol. 2019, 144, S31–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaviya, R.; Ross, E.A.; MacGregor, J.I.; Ikeda, T.; Little, J.R.; Jakschik, B.A.; Abraham, S.N. Mast Cell Phagocytosis of FimH-Expressing Enterobacteria. J. Immunol. 1994, 152, 1907–1914. [Google Scholar] [CrossRef]
- Von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-Independent Antimicrobial Activity of Mast Cells by Means of Extracellular Trap Formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, A.; Yamasaki, K.; Dorschner, R.A.; Lai, Y.; Gallo, R.L. Mast Cell Cathelicidin Antimicrobial Peptide Prevents Invasive Group A Streptococcus Infection of the Skin. J. Immunol. 2008, 180, 7565–7573. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.L.; Wang, Z.; Igawa, S.; Choi, J.E.; Werbel, T.; Di Nardo, A. Lipocalin 2: A New Antimicrobial in Mast Cells. Int. J. Mol. Sci. 2019, 20, 2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, M.; Piliponsky, A.M.; Chan, C.C.; Lammel, V.; Åbrink, M.; Pejler, G.; Tsai, M.; Galli, S.J. Mast Cells Can Enhance Resistance to Snake and Honeybee Venoms. Science 2006, 313, 526–530. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.A.; Schlenner, S.M.; Feyerabend, T.B.; Wunderlin, M.; Rodewald, H.R. Molecular Mechanism of Mast Cell-Mediated Innate Defense against Endothelin and Snake Venom Sarafotoxin. J. Exp. Med. 2007, 204, 2629–2639. [Google Scholar] [CrossRef] [Green Version]
- Akahoshi, M.; Song, C.H.; Piliponsky, A.M.; Metz, M.; Guzzetta, A.; Åbrink, M.; Schlenner, S.M.; Feyerabend, T.B.; Rodewald, H.R.; Pejler, G.; et al. Mast Cell Chymase Reduces the Toxicity of Gila Monster Venom, Scorpion Venom, and Vasoactive Intestinal Polypeptide in Mice. J. Clin. Investig. 2011, 121, 4180–4191. [Google Scholar] [CrossRef] [Green Version]
- Marichal, T.; Starkl, P.; Reber, L.L.; Kalesnikoff, J.; Oettgen, H.C.; Tsai, M.; Metz, M.; Galli, S.J. A Beneficial Role for Immunoglobulin E in Host Defense against Honeybee Venom. Immunity 2013, 39, 963–975. [Google Scholar] [CrossRef] [Green Version]
- Starkl, P.; Marichal, T.; Gaudenzio, N.; Reber, L.L.; Sibilano, R.; Tsai, M.; Galli, S.J. IgE Antibodies, FcεRIα, and IgE-Mediated Local Anaphylaxis Can Limit Snake Venom Toxicity. J. Allergy Clin. Immunol. 2016, 137, 246–257.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbi, S.; Eksteen, E.C.; Oberholzer, H.M.; Taute, H.; Bester, M.J. Premature Collagen Fibril Formation, Fibroblast-Mast Cell Interactions and Mast Cell-Mediated Phagocytosis of Collagen in Keloids. Ultrastruct. Pathol. 2015, 39, 95–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradding, P.; Pejler, G. The Controversial Role of Mast Cells in Fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdsworth, S.R.; Summers, S.A. Role of Mast Cells in Progressive Renal Diseases. J. Am. Soc. Nephrol. 2008, 19, 2254–2261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, P.Y.; O’Sullivan, K.M.; Ooi, J.D.; Alikhan, M.A.; Odobasic, D.; Summers, S.A.; Kitching, A.R.; Holdsworth, S.R. Mast Cell Stabilization Ameliorates Autoimmune Anti-Myeloperoxidase Glomerulonephritis. J. Am. Soc. Nephrol. 2016, 27, 1321–1333. [Google Scholar] [CrossRef]
- van der Elst, G.; Varol, H.; Hermans, M.; Baan, C.C.; Duong-van Huyen, J.P.; Hesselink, D.A.; Kramann, R.; Rabant, M.; Reinders, M.E.J.; von der Thüsen, J.H.; et al. The Mast Cell: A Janus in Kidney Transplants. Front. Immunol. 2023, 14, 1122409. [Google Scholar] [CrossRef]
- Khazaie, K.; Blatner, N.R.; Khan, M.W.; Gounari, F.; Gounaris, E.; Dennis, K.; Bonertz, A.; Tsai, F.N.; Strouch, M.J.; Cheon, E.; et al. The Significant Role of Mast Cells in Cancer. Cancer Metastasis Rev. 2011, 30, 45–60. [Google Scholar] [CrossRef]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.S.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.M.; Grady, E.F.; Bunnett, N.W.; et al. Activated Mast Cells in Proximity to Colonic Nerves Correlate with Abdominal Pain in Irritable Bowel Syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [CrossRef]
- Wezel, A.; Lagraauw, H.M.; van der Velden, D.; de Jager, S.C.A.; Quax, P.H.A.; Kuiper, J.; Bot, I. Mast Cells Mediate Neutrophil Recruitment during Atherosclerotic Plaque Progression. Atherosclerosis 2015, 241, 289–296. [Google Scholar] [CrossRef]
- Woolley, D.E.; Tetlow, L.C. Mast Cell Activation and Its Relation to Proinflammatory Cytokine Production in the Rheumatoid Lesion. Arthritis Res. 2000, 2, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, V.; Haffner-Luntzer, M. Interaction between Bone and Immune Cells: Implications for Postmenopausal Osteoporosis. Semin. Cell Dev. Biol. 2022, 123, 14–21. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Zhang, B.; Kempuraj, D.; Tagen, M.; Vasiadi, M.; Angelidou, A.; Alysandratos, K.D.; Kalogeromitros, D.; Asadi, S.; Stavrianeas, N.; et al. IL-33 Augments Substance P-Induced VEGF Secretion from Human Mast Cells and Is Increased in Psoriatic Skin. Proc. Natl. Acad. Sci. USA 2010, 107, 4448–4453. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, S.; Bouguermouh, S.; Rubio, M.; Baba, N.; Bissonnette, R.; Sarfati, M. Human Mast Cells Are Major IL-22 Producers in Patients with Psoriasis and Atopic Dermatitis. J. Allergy Clin. Immunol. 2015, 136, 351–359.e1. [Google Scholar] [CrossRef]
- Lin, A.M.; Rubin, C.J.; Khandpur, R.; Wang, J.Y.; Riblett, M.; Yalavarthi, S.; Villanueva, E.C.; Shah, P.; Kaplan, M.J.; Bruce, A.T. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J. Immunol. 2011, 187, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Cheung, K.L.; Jarrett, R.; Subramaniam, S.; Salimi, M.; Gutowska-Owsiak, D.; Chen, Y.L.; Hardman, C.; Xue, L.; Cerundolo, V.; Ogg, G. Psoriatic T Cells Recognize Neolipid Antigens Generated by Mast Cell Phospholipase Delivered by Exosomes and Presented by CD1a. J. Exp. Med. 2016, 213, 2399–2412. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early Immune Events in the Induction of Allergic Contact Dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of Immune Events in the Murine Contact Hypersensitivity Model: Toward the Understanding of Allergic Contact Dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Horsmanhetmo, L.; Harvima, I.T.; Järvikallio, A.; Harvima, R.J.; Naukkarinen, A.; HORSMANHEIMO, M. Mast Cells Are One Major Source of Interleukin-4 in Atopic Dermatitis. Br. J. Dermatol. 1994, 131, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Barata, L.T.; Ying, S.; Meng, Q.; Barkans, J.; Rajakulasingam, K.; Durham, S.R.; Kay, A.B. IL-4- and IL-5-Positive T Lymphocytes, Eosinophils, and Mast Cells in Allergen-Induced Late-Phase Cutaneous Reactions in Atopic Subjects. J. Allergy Clin. Immunol. 1998, 101, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Obara, W.; Kawa, Y.; Ra, C.; Nishioka, K.; Soma, Y.; Mizoguchi, M. T Cells and Mast Cells as a Major Source of Interleukin-13 in Atopic Dermatitis. Dermatology 2002, 205, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.A.; Fu, W.; Schonefeldt, S.; Feyerabend, T.B.; Ortiz-Lopez, A.; Lampi, Y.; Liston, A.; Mathis, D.; Rodewald, H.R. Type 1 Diabetes in NOD Mice Unaffected by Mast Cell Deficiency. Diabetes 2014, 63, 3827–3834. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Maitland, A.; Afrin, L. Mast Cell Disorders in Ehlers–Danlos Syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 226–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, A.; Chang, C. The Relationship Between Hypermobile Ehlers-Danlos Syndrome (HEDS), Postural Orthostatic Tachycardia Syndrome (POTS), and Mast Cell Activation Syndrome (MCAS). Clin. Rev. Allergy Immunol. 2020, 58, 273–297. [Google Scholar] [CrossRef]
- Fedorowski, A. Postural Orthostatic Tachycardia Syndrome: Clinical Presentation, Aetiology and Management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Rodgers, K.R.; Gui, J.; Dinulos, M.B.P.; Chou, R.C. Ehlers-Danlos Syndrome Hypermobility Type Is Associated with Rheumatic Diseases. Sci. Rep. 2017, 7, 39636. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Johnston, S.; Chacko, A.; Gibson, D.; Cepon, J.; Smith, P.; Staines, D.; Marshall-Gradisnik, S. Novel Characterisation of Mast Cell Phenotypes from Peripheral Blood Mononuclear Cells in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis Patients. Asian Pacific J. Allergy Immunol. 2017, 35, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast Cell Activation Symptoms Are Prevalent in Long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef]
- Murdaca, G.; Di Gioacchino, M.; Greco, M.; Borro, M.; Paladin, F.; Petrarca, C.; Gangemi, S. Basophils and Mast Cells in COVID-19 Pathogenesis. Cells 2021, 10, 2754. [Google Scholar] [CrossRef]
- Liu, S.; Suzuki, Y.; Takemasa, E.; Watanabe, R.; Mogi, M. Mast Cells Promote Viral Entry of SARS-CoV-2 via Formation of Chymase/Spike Protein Complex. Eur. J. Pharmacol. 2022, 930, 175169. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. COVID-19 and Multisystem Inflammatory Syndrome, or Is It Mast Cell Activation Syndrome? J. Biol. Regul. Homeost. Agents 2020, 34, 1633–1636. [Google Scholar]
- Yong, S.J. Long-Haul COVID-19: Putative Pathophysiology, Risk Factors, and Treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Salmon, J.K.; Armstrong, C.A.; Ansel, J.C. The Skin as an Immune Organ. West. J. Med. 1994, 160, 146–152. [Google Scholar]
- Galli, S.J.; Starkl, P.; Marichal, T.; Tsai, M. Mast Cells and IgE Can Enhance Survival during Innate and Acquired Host Responses to Venoms. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 193–221. [Google Scholar]
- Weller, C.L.; Collington, S.J.; Williams, T.; Lamb, J.R. Mast Cells in Health and Disease. Clin. Sci. 2011, 120, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Gao, W.; Li, H.; Tang, J. The Role of the Mast Cell in Skin Aging. J. Dermatol. Res. Ther. 2016, 2, 35. [Google Scholar] [CrossRef]
- Janssens, A.S.; Heide, R.; Den Hollander, J.C.; Mulder, P.G.M.; Tank, B.; Oranje, A.P. Mast Cell Distribution in Normal Adult Skin. J. Clin. Pathol. 2005, 58, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; De Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef]
- Cho, K.A.; Kim, H.J.; Kim, Y.H.; Park, M.; Woo, S.Y. Dexamethasone Promotes Keratinocyte Proliferation by Triggering Keratinocyte Growth Factor in Mast Cells. Int. Arch. Allergy Immunol. 2019, 179, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Feuerherm, A.J.; Jørgensen, K.M.; Sommerfelt, R.M.; Eidem, L.E.; Lægreid, A.; Johansen, B. Platelet-Activating Factor Induces Proliferation in Differentiated Keratinocytes. Mol. Cell. Biochem. 2013, 384, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, M.; Hyttinen, M.; Nilsson, G.; Butterfield, J.H.; Horsmanheimo, M.; Harvima, I.T. Inhibition of Keratinocyte Growth in Cell Culture and Whole Skin Culture by Mast Cell Mediators. Exp. Dermatol. 2001, 10, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Mildner, M.; Mlitz, V.; Gruber, F.; Eckhart, L.; Werfel, T.; Gutzmer, R.; Elias, P.M.; Tschachler, E. Histamine Suppresses Epidermal Keratinocyte Differentiation and Impairs Skin Barrier Function in a Human Skin Model. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 37–47. [Google Scholar] [CrossRef]
- Kneilling, M.; Mailhammer, R.; Hültner, L.; Schönberger, T.; Fuchs, K.; Schaller, M.; Bukala, D.; Massberg, S.; Sander, C.A.; Braumüller, H.; et al. Direct Crosstalk between Mast Cell-TNF and TNFR1-Expressing Endothelia Mediates Local Tissue Inflammation. Blood 2009, 114, 1696–1706. [Google Scholar] [CrossRef]
- Detoraki, A.; Staiano, R.I.; Granata, F.; Giannattasio, G.; Prevete, N.; de Paulis, A.; Ribatti, D.; Genovese, A.; Triggiani, M.; Marone, G. Vascular Endothelial Growth Factors Synthesized by Human Lung Mast Cells Exert Angiogenic Effects. J. Allergy Clin. Immunol. 2009, 123, 1142–1149.e5. [Google Scholar] [CrossRef]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar]
- Hiromatsu, Y.; Toda, S. Mast Cells and Angiogenesis. Microsc. Res. Tech. 2003, 60, 64–69. [Google Scholar] [CrossRef]
- Koh, M.; Noguchi, S.; Araki, M.; Otsuka, H.; Yokosuka, M.; Soeta, S. Expressions of Vascular Endothelial Growth Factor Receptors, Flk1 and Flt1, in Rat Skin Mast Cells during Development. J. Vet. Med. Sci. 2020, 82, 745–753. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ud-Din, S.; Bayat, A. A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. Int. J. Mol. Sci. 2020, 21, 9673. [Google Scholar] [CrossRef]
- Lateef, Z.; Stuart, G.; Jones, N.; Mercer, A.; Fleming, S.; Wise, L. The Cutaneous Inflammatory Response to Thermal Burn Injury in a Murine Model. Int. J. Mol. Sci. 2019, 20, 538. [Google Scholar] [CrossRef] [Green Version]
- Trautmann, A.; Toksoy, A.; Engelhardt, E.; Bröcker, E.B.; Gillitzer, R. Mast Cell Involvement in Normal Human Skin Wound Healing: Expression of Monocyte Chemoattractant Protein-I Is Correlated with Recruitment of Mast Cells Which Synthesize Interleukin-4 in vivo. J. Pathol. 2000, 190, 100–106. [Google Scholar] [CrossRef]
- Zimmermann, C.; Troeltzsch, D.; Giménez-Rivera, V.A.; Galli, S.J.; Metz, M.; Maurer, M.; Siebenhaar, F. Mast Cells Are Critical for Controlling the Bacterial Burden and the Healing of Infected Wounds. Proc. Natl. Acad. Sci. USA 2019, 116, 20500–20504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagabir, R.; Byers, R.J.; Chaudhry, I.H.; Müller, W.; Paus, R.; Bayat, A. Site-Specific Immunophenotyping of Keloid Disease Demonstrates Immune Upregulation and the Presence of Lymphoid Aggregates. Br. J. Dermatol. 2012, 167, 1053–1066. [Google Scholar] [CrossRef]
- Hermes, B.; Feldmann-Böddeker, I.; Welker, P.; Algermissen, B.; Steckelings, M.U.; Grabbe, J.; Henz, B.M. Altered Expression of Mast Cell Chymase and Tryptase and of C-Kit in Human Cutaneous Scar Tissue. J. Investig. Dermatol. 2000, 114, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermes, B.; Welker, P.; Feldmann-Böddeker, I.; Krüger-Krasagakis, S.; Hartmann, K.; Zuberbier, T.; Henz, B.M. Expression of Mast Cell Growth Modulating and Chemotactic Factors and Their Receptors in Human Cutaneous Scars. J. Investig. Dermatol. 2001, 116, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ud-Din, S.; Foden, P.; Mazhari, M.; Al-Habba, S.; Baguneid, M.; Bulfone-Paus, S.; McGeorge, D.; Bayat, A. A Double-Blind, Randomized Trial Shows the Role of Zonal Priming and Direct Topical Application of Epigallocatechin-3-Gallate in the Modulation of Cutaneous Scarring in Human Skin. J. Investig. Dermatol. 2019, 139, 1680–1690.e16. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Schrementi, M.E.; Ranzer, M.J.; Wilgus, T.A.; DiPietro, L.A. Blockade of Mast Cell Activation Reduces Cutaneous Scar Formation. PLoS ONE 2014, 9, e85226. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Hildebrand, K.A.; Hart, D.A. The Mast Cell Stabilizer Ketotifen Prevents Development of Excessive Skin Wound Contraction and Fibrosis in Red Duroc Pigs. Wound Repair Regen. 2008, 16, 226–233. [Google Scholar] [CrossRef]
- Cohen, I.K.; Beaven, M.A.; Horakova, Z.; Keiser, H.R. Histamine and Collagen Synthesis in Keloid and Hypertrophic Scar. Surg. Forum 1972, 23, 509–510. [Google Scholar] [CrossRef]
- Smith, C.J.; Smith, J.C.; Finn, M.C. The Possible Role of Mast Cells (Allergy) in the Production of Keloid and Hypertrophie Scarring. J. Burn Care Rehabil. 1987, 8, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, D.S.; Eaglstein, W.H.; Falanga, V. Exogenous Electric Current Can Reduce the Formation of Hypertrophic Scars. J. Dermatol. Surg. Oncol. 1989, 15, 1272–1276. [Google Scholar] [CrossRef]
- Hellström, M.; Hellström, S.; Engström-Laurent, A.; Bertheim, U. The Structure of the Basement Membrane Zone Differs between Keloids, Hypertrophic Scars and Normal Skin: A Possible Background to an Impaired Function. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1564–1572. [Google Scholar] [CrossRef]
- Theoret, C.L.; Olutoye, O.O.; Parnell, L.K.S.; Hicks, J. Equine Exuberant Granulation Tissue and Human Keloids: A Comparative Histopathologic Study. Vet. Surg. 2013, 42, 783–789. [Google Scholar] [CrossRef]
- Craig, S.S.; DeBlois, G.; Schwartz, L.B. Mast Cells in Human Keloid, Small Intestine, and Lung by an Immunoperoxidase Technique Using a Murine Monoclonal Antibody against Tryptase. Am. J. Pathol. 1986, 124, 427–435. [Google Scholar] [PubMed]
- Kemal Ozbilgin, M.; Inan, S. The Roles of Transforming Growth Factor Type Β3(TGF-Β3) and Mast Cells in the Pathogenesis of Scleroderma. Clin. Rheumatol. 2003, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Hatamochi, A.; Ueki, H. Increased Number of Mast Cells Accompany Enhanced Collagen Synthesis in Linear Localized Scleroderma. Arch. Dermatol. Res. 1989, 281, 288–290. [Google Scholar] [CrossRef]
- Strattan, E.; Palaniyandi, S.; Kumari, R.; Du, J.; Hakim, N.; Huang, T.; Kesler, M.V.; Jennings, C.D.; Sturgill, J.L.; Hildebrandt, G.C. Mast Cells Are Mediators of Fibrosis and Effector Cell Recruitment in Dermal Chronic Graft-vs.-Host Disease. Front. Immunol. 2019, 10, 2470. [Google Scholar] [CrossRef]
- Akin, C.; Valent, P.; Metcalfe, D.D. Mast Cell Activation Syndrome: Proposed Diagnostic Criteria. J. Allergy Clin. Immunol. 2010, 126, 1099–1104. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Tsilioni, I.; Ren, H. Recent Advances in Our Understanding of Mast Cell Activation–or Should It Be Mast Cell Mediator Disorders? Expert Rev. Clin. Immunol. 2019, 15, 639–656. [Google Scholar]
- Molderings, G.J.; Haenisch, B.; Bogdanow, M.; Fimmers, R.; Nöthen, M.M. Familial Occurrence of Systemic Mast Cell Activation Disease. PLoS ONE 2013, 8, e76241. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, L.B.; Pace, L.A.; Rezaie, A.; Afrin, L.B.; Molderings, G.J. Mast Cell Activation Syndrome: A Primer for the Gastroenterologist. Dig. Dis. Sci. 2021, 66, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.W.; Pratt, C.M.; Rupprecht, C.P.; Pattanaik, D.; Krishnaswamy, G. Mastocytosis and Mast Cell Activation Disorders: Clearing the Air. Int. J. Mol. Sci. 2021, 22, 11270. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Akin, C.; Bonadonna, P.; Siebenhaar, F.; Broesby-Olsen, S.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, H.N.G.; Butterfield, J.H.; et al. Selecting the Right Criteria and Proper Classification to Diagnose Mast Cell Activation Syndromes: A Critical Review. J. Allergy Clin. Immunol. Pract. 2021, 9, 3918–3928. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Self, S.; Menk, J.; Lazarchick, J. Characterization of Mast Cell Activation Syndrome. Am. J. Med. Sci. 2017, 353, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, Criteria and Global Classification of Mast Cell Disorders with Special Reference to Mast Cell Activation Syndromes: A Consensus Proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Gülen, T.; Brockow, K.; Alvarez-Twose, I.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM–Adjusted Proposal of the ECNM-AIM Consortium. J. Allergy Clin. Immunol. Pract. 2022, 10, 1941–1950. [Google Scholar] [CrossRef]
- Valent, P. Mast Cell Activation Syndromes: Definition and Classification. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 417–424. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133.e1. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Hatziagelaki, E. Brain “Fog,” Inflammation a Nd Obesity: Key Aspects of Neuropsychiatric Disorders Improved by Luteolin. Front. Neurosci. 2015, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Jennings, S.; Russell, N.; Jennings, B.; Slee, V.; Sterling, L.; Castells, M.; Valent, P.; Akin, C. The Mastocytosis Society Survey on Mast Cell Disorders: Patient Experiences and Perceptions. J. Allergy Clin. Immunol. Pract. 2014, 2, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, L.B.; Pöhlau, D.; Raithel, M.; Haenisch, B.; Dumoulin, F.L.; Homann, J.; Mauer, U.M.; Harzer, S.; Molderings, G.J. Mast Cell Activation Disease: An Underappreciated Cause of Neurologic and Psychiatric Symptoms and Diseases. Brain. Behav. Immun. 2015, 50, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.R.; Austen, K.F.; Akin, C.; Barkoff, M.S.; Bernstein, J.A.; Bonadonna, P.; Butterfield, J.H.; Carter, M.; Fox, C.C.; Maitland, A.; et al. AAAAI Mast Cell Disorders Committee Work Group Report: Mast Cell Activation Syndrome (MCAS) Diagnosis and Management. J. Allergy Clin. Immunol. 2019, 144, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Molderings, G.J.; Brettner, S.; Homann, J.; Afrin, L.B. Mast Cell Activation Disease: A Concise Practical Guide for Diagnostic Workup and Therapeutic Options. J. Hematol. Oncol. 2011, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a Mast-Cell-Specific Receptor Crucial for Pseudo-Allergic Drug Reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Weiler, C.R. Mast Cell Activation Syndrome: Tools for Diagnosis and Differential Diagnosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Colombo, F.M.; Cattaneo, P.; Confalonieri, E.; Bernardi, C. Histamine Food Poisonings: A Systematic Review and Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 1131–1151. [Google Scholar] [CrossRef] [Green Version]
- Reese, I.; Ballmer-Weber, B.; Beyer, K.; Fuchs, T.; Kleine-Tebbe, J.; Klimek, L.; Lepp, U.; Niggemann, B.; Saloga, J.; Schäfer, C.; et al. Leitlinie Zum Vorgehen Bei Verdacht Auf Unverträglichkeit Gegenüber Oral Aufgenommenem Histamin: Leitlinie Der Deutschen Gesellschaft Für Allergologie Und Klinische Immunologie (DGAKI), Der Gesellschaft Für Pädiatrische Allergologie Und Umweltmedizin (GPA), Des Ärzteverbandes Deutscher Allergologen (AeDA) Und Der Schweizerischen Gesellschaft Für Allergologie Und Immunologie (SGAI). Allergo J. 2017, 26, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance—The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef]
- Komericki, P.; Klein, G.; Reider, N.; Hawranek, T.; Strimitzer, T.; Lang, R.; Kranzelbinder, B.; Aberer, W. Histamine Intolerance: Lack of Reproducibility of Single Symptoms by Oral Provocation with Histamine: A Randomised, Double-Blind, Placebo-Controlled Cross-over Study. Wien. Klin. Wochenschr. 2011, 123, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Manzotti, G.; Breda, D.; Di Gioacchino, M.; Burastero, S.E. Serum Diamine Oxidase Activity in Patients with Histamine Intolerance. Int. J. Immunopathol. Pharmacol. 2016, 29, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, M.J.; Scarlata, K. Mast Cell Activation Syndrome–What It Is and Isn’t. Pract. Gastroenterol. 2020, 44, 26–32. [Google Scholar]
- Luskin, K.T.; White, A.A.; Lyons, J.J. The Genetic Basis and Clinical Impact of Hereditary Alpha-Tryptasemia. J. Allergy Clin. Immunol. Pract. 2021, 9, 2235–2242. [Google Scholar] [CrossRef] [PubMed]
- Vadas, P.; Perelman, B.; Liss, G. Platelet-Activating Factor, Histamine, and Tryptase Levels in Human Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 144–149. [Google Scholar] [CrossRef]
- Laroche, D.; Gomis, P.; Gallimidi, E.; Malinovsky, J.M.; Mertes, P.M. Diagnostic Value of Histamine and Tryptase Concentrations in Severe Anaphylaxis with Shock or Cardiac Arrest during Anesthesia. Anesthesiology 2014, 121, 272–279. [Google Scholar] [CrossRef]
- Dua, S.; Dowey, J.; Foley, L.; Islam, S.; King, Y.; Ewan, P.; Clark, A.T. Diagnostic Value of Tryptase in Food Allergic Reactions: A Prospective Study of 160 Adult Peanut Challenges. J. Allergy Clin. Immunol. Pract. 2018, 6, 1692–1698.e1. [Google Scholar] [CrossRef]
- Francis, A.; Fatovich, D.M.; Arendts, G.; Macdonald, S.P.J.; Bosio, E.; Nagree, Y.; Mitenko, H.M.A.; Brown, S.G.A. Serum Mast Cell Tryptase Measurements: Sensitivity and Specificity for a Diagnosis of Anaphylaxis in Emergency Department Patients with Shock or Hypoxaemia. EMA-Emerg. Med. Australas. 2018, 30, 366–374. [Google Scholar] [CrossRef]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable Risk for Severe Anaphylaxis Associated with Increased α-Tryptase–Encoding Germline Copy Number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef]
- Molderings, G.J.; Kolck, U.W.; Scheurlen, C.; Brüss, M.; Homann, J.; Von Kügelgen, I. Multiple Novel Alterations in Kit Tyrosine Kinase in Patients with Gastrointestinally Pronounced Systemic Mast Cell Activation Disorder. Scand. J. Gastroenterol. 2007, 42, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.H.; Weiler, C.R. Prevention of Mast Cell Activation Disorder-Associated Clinical Sequelae of Excessive Prostaglandin D2 Production. Int. Arch. Allergy Immunol. 2008, 147, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B. Sclerosing Mediastinitis and Mast Cell Activation Syndrome. Pathol. Res. Pract. 2012, 208, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B. Burning Mouth Syndrome and Mast Cell Activation Disorder. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 111, 465–472. [Google Scholar] [CrossRef]
- Mönkemüller, K.; Kassalik, M.; Baraksei, D.; Fry, L.C.; Moege, J. Mast Cell Activation Syndrome (MCAS) Diagnosed Using Double-Balloon Enteroscopy. Endoscopy 2012, 44, E72–E73. [Google Scholar] [CrossRef]
- Afrin, L.B.; Cichocki, F.M.; Patel, K.; Molderings, G.J. Successful Treatment of Mast Cell Activation Syndrome with Sunitinib. Eur. J. Haematol. 2015, 95, 595–597. [Google Scholar] [CrossRef]
- Afrin, L.B. Mast Cell Activation Syndrome Masquerading as Agranulocytosis. Mil. Med. 2012, 177, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, D.; Dreßen, P.; Brettner, S.; Rath, N.F.; Molderings, G.J.; Jensen, K.; Ziemann, C. Prostaglandin D2-Supplemented “Functional Eicosanoid Testing and Typing” Assay with Peripheral Blood Leukocytes as a New Tool in the Diagnosis of Systemic Mast Cell Activation Disease: An Explorative Diagnostic Study. J. Transl. Med. 2014, 12, 213. [Google Scholar] [CrossRef]
- Afrin, L.B.; Fox, R.W.; Zito, S.L.; Choe, L.; Glover, S.C. Successful Targeted Treatment of Mast Cell Activation Syndrome with Tofacitinib. Eur. J. Haematol. 2017, 99, 190–193. [Google Scholar] [CrossRef]
- Moon, T.C.; Dean Befus, A.; Kulka, M. Mast Cell Mediators: Their Differential Release and the Secretory Pathways Involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef] [Green Version]
- Pardanani, A. Systemic Mastocytosis in Adults: 2021 Update on Diagnosis, Risk Stratification and Management. Am. J. Hematol. 2021, 96, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Kiszewski, A.E.; Durán-McKinster, C.; Orozco-Covarrubias, L.; Gutiérrez-Castrellón, P.; Ruiz-Maldonado, R. Cutaneous Mastocytosis in Children: A Children Analysis of 71 Cases. J. Eur. Acad. Dermatology Venereol. 2004, 18, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Hannaford, R.; Rogers, M. Presentation of Cutaneous Mastocytosis in 173 Children. Australas. J. Dermatol. 2001, 42, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.M.; Makdisi, J.; Ortenzio, F.; de Feraudy, S.; Smith, J.; Linden, K. Diffuse Cutaneous Mastocytosis: Case Report and Literature Review. Pediatr. Dermatol. 2018, 35, e348–e352. [Google Scholar] [CrossRef]
- Di Raimondo, C.; Del Duca, E.; Silvaggio, D.; Di Prete, M.; Lombardo, P.; Mazzeo, M.; Spallone, G.; Campione, E.; Botti, E.; Bianchi, L. Cutaneous Mastocytosis: A Dermatological Perspective. Australas. J. Dermatol. 2021, 62, e1–e7. [Google Scholar] [CrossRef]
- Shaker, M.S.; Wallace, D.V.; Golden, D.B.K.; Oppenheimer, J.; Bernstein, J.A.; Campbell, R.L.; Dinakar, C.; Ellis, A.; Greenhawt, M.; Khan, D.A.; et al. Anaphylaxis—A 2020 Practice Parameter Update, Systematic Review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Analysis. J. Allergy Clin. Immunol. 2020, 145, 1082–1123. [Google Scholar] [CrossRef] [Green Version]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Niedoszytko, M.; Triggiani, M.; Arock, M.; Brockow, K. Mast Cell Activation Syndromes: Collegium Internationale Allergologicum Update 2022. Int. Arch. Allergy Immunol. 2022, 183, 693–705. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, M.J. Nonclonal Mast Cell Activation Syndrome: A Growing Body of Evidence. Immunol. Allergy Clin. N. Am. 2018, 38, 469–481. [Google Scholar] [CrossRef]
- Virchow, J.C.; Bachert, C. Efficacy and Safety of Montelukast in Adults with Asthma and Allergic Rhinitis. Respir. Med. 2006, 100, 1952–1959. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Schrag, M.L.; Slaughter, D.E.; Raab, C.E.; Shou, M.; Rodrigues, A.D. Mechanism-Based Inhibition of Human Liver Microsomal Cytochrome P450 1A2 by Zileuton, A 5-Lipoxygenase Inhibitor. Drug Metab. Dispos. 2003, 31, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardet, J.C.; Akin, C.; Lee, M.J. Mastocytosis: Update on Pharmacotherapy and Future Directions. Expert. Opin. Pharmacother. 2013, 14, 2033–2045. [Google Scholar]
- Weller, K.; Maurer, M. Desloratadine Inhibits Human Skin Mast Cell Activation and Histamine Release. J. Investig. Dermatol. 2009, 129, 2723–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujimura, R.; Asada, A.; Aizawa, M.; Kazama, I. Cetirizine More Potently Exerts Mast Cell-Stabilizing Property than Diphenhydramine. Drug Discov. Ther. 2022, 16, 245–250. [Google Scholar] [CrossRef]
- Sokol, K.C.; Amar, N.K.; Starkey, J.; Grant, J.A. Ketotifen in the Management of Chronic Urticaria: Resurrection of an Old Drug. Ann. Allergy Asthma Immunol. 2013, 111, 433–436. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Ozaki, T.; Mohammad, S.; Tsuno, S.; Ikeda-Sagara, M.; Honda, K.; Ikeda, M. First and Second Generation H1 Histamine Receptor Antagonists Produce Different Sleep-Inducing Profiles in Rats. Eur. J. Pharmacol. 2012, 683, 179–185. [Google Scholar] [CrossRef]
- Okayama, Y.; Benyon, R.C.; Rees, P.H.; Lowman, M.A.; Hillier, K.; Church, M.K. Inhibition Profiles of Sodium Cromoglycate and Nedocromil Sodium on Mediator Release from Mast Cells of Human Skin, Lung, Tonsil, Adenoid and Intestine. Clin. Exp. Allergy 1992, 22, 401–409. [Google Scholar] [CrossRef]
- Castells, M.; Butterfield, J. Mast Cell Activation Syndrome and Mastocytosis: Initial Treatment Options and Long-Term Management. J. Allergy Clin. Immunol. Pract. 2019, 7, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Hollingsworth, P.; Lucas, M. Successful Treatment of Idiopathic Mast Cell Activation Syndrome with Low-Dose Omalizumab. Clin. Transl. Immunol. 2019, 8, e01075. [Google Scholar] [CrossRef] [Green Version]
- Lemal, R.; Fouquet, G.; Terriou, L.; Vaes, M.; Livideanu, C.B.; Frenzel, L.; Barete, S.; Canioni, D.; Lhermitte, L.; Rossignol, J.; et al. Omalizumab Therapy for Mast Cell-Mediator Symptoms in Patients with ISM, CM, MMAS, and MCAS. J. Allergy Clin. Immunol. Pract. 2019, 7, 2387–2395.e3. [Google Scholar] [CrossRef] [PubMed]
- Kettner, L.; Seitl, I.; Fischer, L. Toward Oral Supplementation of Diamine Oxidase for the Treatment of Histamine Intolerance. Nutrients 2022, 14, 2621. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Che, D.; Yu, Y.; Liu, L.; Mi, S.; Zhang, Y.; Hao, J.; Li, W.; Ji, M.; Geng, S.; et al. Luteolin Inhibits FcεRΙ- and MRGPRX2-Mediated Mast Cell Activation by Regulating Calcium Signaling Pathways. Phyther. Res. 2022, 36, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Shirley, D.; McHale, C.; Gomez, G. Resveratrol Preferentially Inhibits IgE-Dependent PGD2 Biosynthesis but Enhances TNF Production from Human Skin Mast Cells. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda-Yamamoto, M. Human Clinical Studies of Tea Polyphenols in Allergy or Life Style-Related Diseases. Curr. Pharm. Des. 2013, 19, 6148–6155. [Google Scholar] [CrossRef]
- Profet, M. The Function of Allergy: Immunological Defense against Toxins. Q. Rev. Biol. 1991, 66, 23–62. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Starkl, P.; Marichal, T.; Galli, S.J. IgE and Mast Cells in Host Defense against Parasites and Venoms. Semin. Immunopathol. 2016, 38, 581–603. [Google Scholar] [CrossRef] [Green Version]
- Buttgereit, T.; Gu, S.; Carneiro-Leão, L.; Gutsche, A.; Maurer, M.; Siebenhaar, F. Idiopathic mast cell activation syndrome is more often suspected than diagnosed-A prospective real-life study. Allergy 2022, 77, 2794–2802. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 Hyperinflammation and Post-COVID-19 Illness May Be Rooted in Mast Cell Activation Syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Wechsler, J.B.; Butuci, M.; Wong, A.; Kamboj, A.P.; Youngblood, B.A. Mast Cell Activation Is Associated with Post-Acute COVID-19 Syndrome. Allergy 2022, 77, 1288. [Google Scholar] [CrossRef]

| Organ/System | Diagnostic Symptoms | Non-Diagnostic Symptoms |
|---|---|---|
| Cutaneous | Flushing, pruritus, hives, angioedema | Dermographism, urticaria, rashes, edema/migratory edema, alopecia, poor healing, onychodystrophy |
| Respiratory | Shortness of breath, laryngeal edema, wheezing, hypoxia, nasal congestion, sneezing | Dyspnea, asthma, cough |
| Gastrointestinal | Vomiting, abdominal cramps, diarrhea | Constipation, alternating diarrhea/constipation, gastroesophageal reflux, dyspepsia, dysphagia, heartburn, nausea, elevated transaminases, hepatomegaly, multiple chemical/food sensitivity |
| Cardiovascular | Hypotension, syncope, collapse, incontinence | Chest pain, palpitations/dysrhythmias, systolic hypertension (mild/moderate), tachycardia |
| General | Fatigue, weight loss or gain, obesity, fever, chills, sweats, frequent or odd infections, heat and/or cold intolerance | |
| Allergologic | Multiple/odd drug reactions | |
| Neuro-psychiatric | Headaches, migraines, cognitive disfunction/brain fog, insomnia, anxiety, depression, dysautonomia symptoms (POTS), tremor, paresthesia | |
| Musculoskeletal | Myalgia, arthralgia, joint hypermobility, fibromyalgia-type pain | |
| Urogenital | Dysuria, frequency, urgency, prostatitis, polycystic ovarian syndrome, endometriosis | |
| Ophthalmologic | Eye irritation, conjunctivitis, visual anomalies | |
| Ears, nose, throat | Tinnitus, hearing loss, rhinitis, sinusitis, sore throat, oral irritation/sores | |
| Hematological | Easy bleeding/bruising | |
| Lymphatic system | Adenopathy/adenitis | |
| Stomatological | Dental deterioration |
| Flushing | fever, emotion, exercise, temperature changes, foods (spicy, fish—scombroid poisoning) or beverages (alcohol ingestion), medications (e.g., calcium channel blockers, nicotinic acid, disulfiram combined with alcohol) rosacea, climacteric flushing, carcinoid syndrome, pheochromocytoma, mastocytosis, anaphylaxis, thyroid—medullary carcinoma, VIPoma, renal cell carcinoma, neuro-psychiatric (anxiety disorders, Parkinson’s, multiple sclerosis, migraine, trigeminal nerve damage, Frey syndrome, Horner syndrome, Streeten syndrome, autonomic epilepsy, orthostatic hypotension), sarcoid, dumping syndrome, arsenic intoxication, basophilic granulocytic leukemia, malignant histiocytoma, idiopathic flushing |
| Pruritus | xerosis, scabies, noncutaneous causes (e.g., cholestatic/non-cholestatic hepatobiliary diseases, hyperthyroidism, uremic pruritus, myeloproliferative disorders such as Hodgkin/non-Hodgkin lymphoma, lymphocytic leukemia, polycythemia vera ± aquagenic pruritus, essential thrombocytosis, diabetes), psychogenic (depression, anxiety, obsessive-compulsive disorder, somatic symptom disorders, psychosis, substance use), subclinical mastocytosis, drug induced—either directly or via cholestasis (e.g., morphine-based analgesics, angiotensin-converting enzyme inhibitors, selective serotonin re-uptake inhibitors, nonsteroidal anti-inflammatory drugs can induce chronic pruritus without associated cutaneous lesions); for localized pruritus: spinal nerve compressions, notalgia paresthetica, small fiber neuropathy, parasitoses (e.g., enterobiasis) |
| Urticarial lesions | acute/chronic urticaria, urticarial vasculitis, urticarial dermatitis, urticaria multiforme, drug and viral exanthems, autoinflammatory syndromes (cryopyrin-associated periodic syndromes, Schnitzler syndrome, adult-onset Still disease, Gleich syndrome, neutrophilic urticarial dermatosis), hypereosinophilic syndromes, autoimmune progesterone dermatitis, polymorphic light eruption |
| Angioedema | bradykinin-mediated angioedema, hereditary angioedema, granulomatous cheilitis at an initial stage, eosinophilic cellulitis (Wells syndrome), allergic contact dermatitis (e.g., to hair dye), photoallergy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihele, D.M.; Nistor, P.A.; Bruma, G.; Mitran, C.I.; Mitran, M.I.; Condrat, C.E.; Tovaru, M.; Tampa, M.; Georgescu, S.R. Mast Cell Activation Syndrome Update—A Dermatological Perspective. J. Pers. Med. 2023, 13, 1116. https://doi.org/10.3390/jpm13071116
Mihele DM, Nistor PA, Bruma G, Mitran CI, Mitran MI, Condrat CE, Tovaru M, Tampa M, Georgescu SR. Mast Cell Activation Syndrome Update—A Dermatological Perspective. Journal of Personalized Medicine. 2023; 13(7):1116. https://doi.org/10.3390/jpm13071116
Chicago/Turabian StyleMihele, Dana Mihaela, Paul Andrei Nistor, Gabriela Bruma, Cristina Iulia Mitran, Madalina Irina Mitran, Carmen Elena Condrat, Mihaela Tovaru, Mircea Tampa, and Simona Roxana Georgescu. 2023. "Mast Cell Activation Syndrome Update—A Dermatological Perspective" Journal of Personalized Medicine 13, no. 7: 1116. https://doi.org/10.3390/jpm13071116
APA StyleMihele, D. M., Nistor, P. A., Bruma, G., Mitran, C. I., Mitran, M. I., Condrat, C. E., Tovaru, M., Tampa, M., & Georgescu, S. R. (2023). Mast Cell Activation Syndrome Update—A Dermatological Perspective. Journal of Personalized Medicine, 13(7), 1116. https://doi.org/10.3390/jpm13071116








