Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments
Abstract
1. Introduction
2. Signaling Pathways in MTC
2.1. RET and Its Downstream Signaling Cascades
2.2. MAPK Activation (RAS/RAF/MEK/ERK)
2.3. PI3K/AKT/mTOR Activation
3. RET Mutations in MTC
4. Treatments Available to Current MTC Patients
4.1. Multitarget Kinase Inhibitors (MKIs)
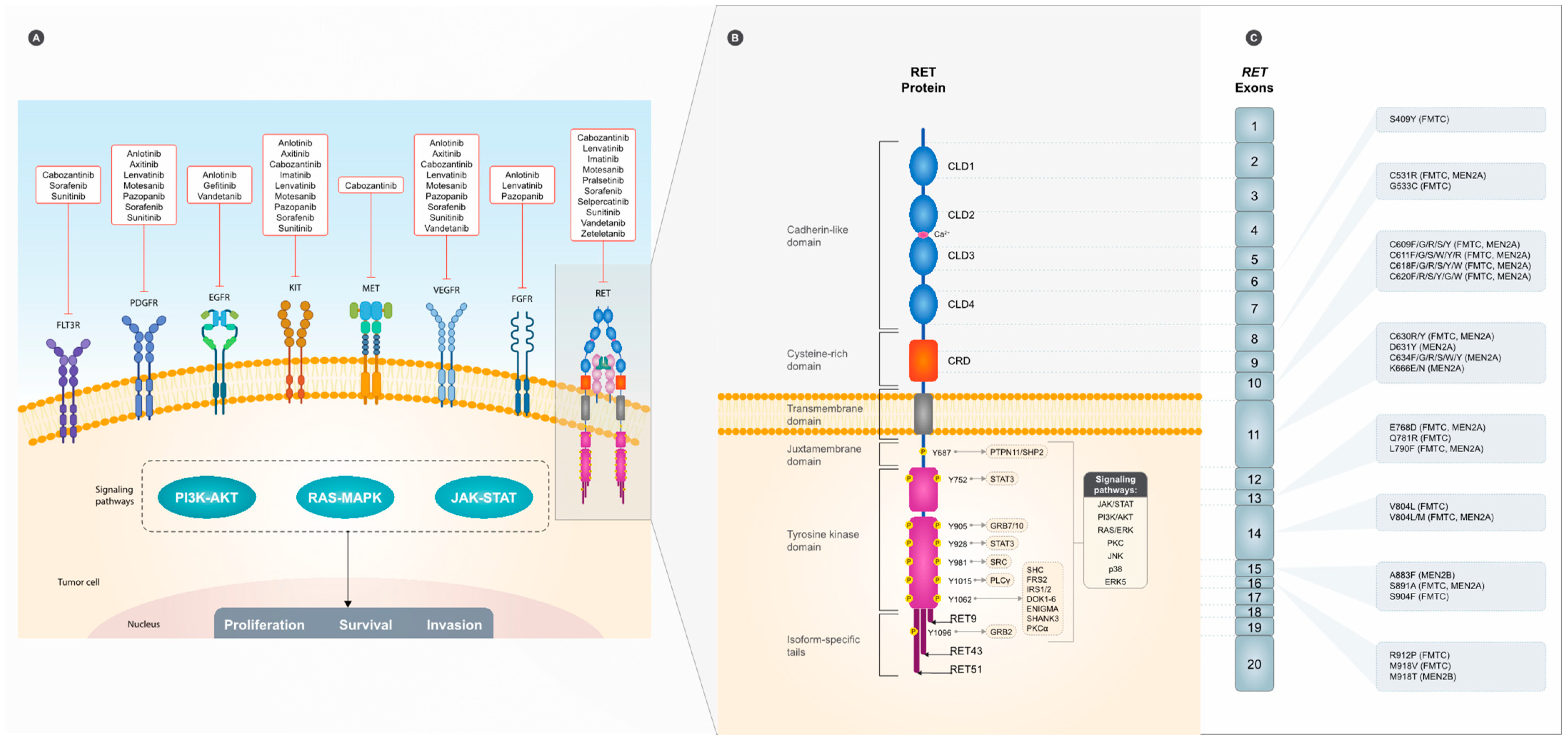
4.2. RET-Specific TKI
4.3. Immunotherapy
4.4. Somatostatin Receptor (SSTR) Inhibitors
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeLellis, R.A.; Al Ghuzlan, G.; Albores Saavedra, J.; Baloch, Z.W.; Basolo, F.; Elisei, R.; Kaserer, K.; LiVolsi, V.; Mati-as-Guiu, X.; Mete, O.; et al. Medullary thyroid carcinoma. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; Lloyd, R.V., Osamura, R.Y., Klöppel, G., Rosai, J., Eds.; IARC: Lyon, France, 2017; Volume 10, pp. 108–113. [Google Scholar]
- Fagin, J.A.; Wells, S.A., Jr. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Ceolin, L.; Duval, M.; Benini, A.F.; Ferreira, C.V.; Maia, A.L. Medullary thyroid carcinoma beyond surgery: Advances, challenges, and perspectives. Endocr. Relat. Cancer 2019, 26, R499–R518. [Google Scholar] [CrossRef]
- Wells, S.A., Jr.; Asa, S.L.; Dralle, H.; Elisei, R.; Evans, D.B.; Gagel, R.F.; Lee, N.; Machens, A.; Moley, J.F.; Pacini, F.; et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015, 25, 567–610. [Google Scholar] [CrossRef]
- Elisei, R.; Tacito, A.; Ramone, T.; Ciampi, R.; Bottici, V.; Cappagli, V.; Viola, D.; Matrone, A.; Lorusso, L.; Valerio, L.; et al. Twenty-Five Years Experience on RET Genetic Screening on Hereditary MTC: An Update on The Prevalence of Germline RET Mutations. Genes 2019, 10, 698. [Google Scholar] [CrossRef]
- Romei, C.; Cosci, B.; Renzini, G.; Bottici, V.; Molinaro, E.; Agate, L.; Passannanti, P.; Viola, D.; Biagini, A.; Basolo, F.; et al. RET genetic screening of sporadic medullary thyroid cancer (MTC) allows the preclinical diagnosis of unsuspected gene carriers and the identification of a relevant percentage of hidden familial MTC (FMTC). Clin. Endocrinol. 2011, 74, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Wiench, M.; Wygoda, Z.; Gubala, E.; Wloch, J.; Lisowska, K.; Krassowski, J.; Scieglinska, D.; Fiszer-Kierzkowska, A.; Lange, D.; Kula, D.; et al. Estimation of risk of inherited medullary thyroid carcinoma in apparent sporadic patients. J. Clin. Oncol. 2001, 19, 1374–1380. [Google Scholar] [CrossRef]
- Niccoli-Sire, P.; Murat, A.; Rohmer, V.; Franc, S.; Chabrier, G.; Baldet, L.; Maes, B.; Savagner, F.; Giraud, S.; Bezieau, S.; et al. Familial medullary thyroid carcinoma with noncysteine ret mutations: Phenotype-genotype relationship in a large series of patients. J. Clin. Endocrinol. Metab. 2001, 86, 3746–3753. [Google Scholar] [CrossRef]
- Toussi, A.; Mans, N.; Welborn, J.; Kiuru, M. Germline mutations predisposing to melanoma. J. Cutan. Pathol. 2020, 47, 606–616. [Google Scholar] [CrossRef]
- Prete, A.; Matrone, A.; Gambale, C.; Bottici, V.; Cappagli, V.; Romei, C.; Torregrossa, L.; Valerio, L.; Minaldi, E.; Campopiano, M.C.; et al. Active Surveillance in RET Gene Carriers Belonging to Families with Multiple Endocrine Neoplasia. Cancers 2021, 13, 5554. [Google Scholar] [CrossRef]
- Kuo, E.J.; Sho, S.; Li, N.; Zanocco, K.A.; Yeh, M.W.; Livhits, M.J. Risk Factors Associated with Reoperation and Disease-Specific Mortality in Patients with Medullary Thyroid Carcinoma. JAMA Surg. 2018, 153, 52–59. [Google Scholar] [CrossRef]
- Girelli, M.E.; Nacamulli, D.; Pelizzo, M.R.; De Vido, D.; Mian, C.; Piccolo, M.; Busnardo, B. Medullary thyroid carcinoma: Clinical features and long-term follow-up of seventy-eight patients treated between 1969 and 1986. Thyroid 1998, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hazard, J.B.; Hawk, W.A.; Crile, G., Jr. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J. Clin. Endocrinol. Metab. 1959, 19, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.C.; Beahrs, O.H.; Sizemore, G.W.; Woolner, L.H. Medullary carcinoma of the thyroid gland. Cancer 1975, 35, 695–704. [Google Scholar] [CrossRef]
- Rossi, R.L.; Cady, B.; Meissner, W.A.; Wool, M.S.; Sedgwick, C.E.; Werber, J. Nonfamilial medullary thyroid carcinoma. Am. J. Surg. 1980, 139, 554–560. [Google Scholar] [CrossRef]
- Koehler, V.F.; Adam, P.; Frank-Raue, K.; Raue, F.; Berg, E.; Hoster, E.; Allelein, S.; Schott, M.; Kroiss, M.; Spitzweg, C. Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid 2021, 31, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin. Cancer Res. 2012, 18, 3722–3730. [Google Scholar] [CrossRef]
- Di Molfetta, S.; Dotto, A.; Fanciulli, G.; Florio, T.; Feola, T.; Colao, A.; Faggiano, A. Immune Checkpoint Inhibitors: New Weapons against Medullary Thyroid Cancer? Front. Endocrinol. 2021, 12, 667784. [Google Scholar] [CrossRef]
- Pachnis, V.; Mankoo, B.; Costantini, F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 1993, 119, 1005–1017. [Google Scholar] [CrossRef]
- Zordan, P.; Tavella, S.; Brizzolara, A.; Biticchi, R.; Ceccherini, I.; Garofalo, S.; Ravazzolo, R.; Bocciardi, R. The immediate upstream sequence of the mouse Ret gene controls tissue-specific expression in transgenic mice. Int. J. Mol. Med. 2006, 18, 601–608. [Google Scholar] [CrossRef]
- Takahashi, M.; Ritz, J.; Cooper, G.M. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985, 42, 581–588. [Google Scholar] [CrossRef]
- Ishizaka, Y.; Itoh, F.; Tahira, T.; Ikeda, I.; Sugimura, T.; Tucker, J.; Fertitta, A.; Carrano, A.V.; Nagao, M. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 1989, 4, 1519–1521. [Google Scholar]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, C.; Asai, N.; Murakami, H.; Iwashita, T.; Iwata, Y.; Horibe, K.; Klein, R.D.; Rosenthal, A.; Takahashi, M. Calcium-dependent Ret activation by GDNF and neurturin. Oncogene 1998, 16, 293–299. [Google Scholar] [CrossRef]
- Kawamoto, Y.; Takeda, K.; Okuno, Y.; Yamakawa, Y.; Ito, Y.; Taguchi, R.; Kato, M.; Suzuki, H.; Takahashi, M.; Nakashima, I. Identification of RET autophosphorylation sites by mass spectrometry. J. Biol. Chem. 2004, 279, 14213–14224. [Google Scholar] [CrossRef] [PubMed]
- Knowles, P.P.; Murray-Rust, J.; Kjaer, S.; Scott, R.P.; Hanrahan, S.; Santoro, M.; Ibáñez, C.F.; McDonald, N.Q. Structure and chemical inhibition of the RET tyrosine kinase domain. J. Biol. Chem. 2006, 281, 33577–33587. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vega, Q.C.; Decker, R.A.; Pandey, A.; Worby, C.A.; Dixon, J.E. Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J. Biol. Chem. 1996, 271, 5309–5312. [Google Scholar] [CrossRef]
- Arighi, E.; Borrello, M.G.; Sariola, H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor. Rev. 2005, 16, 441–467. [Google Scholar] [CrossRef]
- Prescott, J.D.; Zeiger, M.A. The RET oncogene in papillary thyroid carcinoma. Cancer 2015, 121, 2137–2146. [Google Scholar] [CrossRef]
- Regua, A.T.; Najjar, M.; Lo, H.W. RET signaling pathway and RET inhibitors in human cancer. Front. Oncol. 2022, 12, 932353. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 2013, 5, a009134. [Google Scholar] [CrossRef]
- Takahashi, M. RET receptor signaling: Function in development, metabolic disease, and cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2022, 98, 112–125. [Google Scholar] [CrossRef]
- Iwashita, T.; Asai, N.; Murakami, H.; Matsuyama, M.; Takahashi, M. Identification of tyrosine residues that are essential for transforming activity of the ret proto-oncogene with MEN2A or MEN2B mutation. Oncogene 1996, 12, 481–487. [Google Scholar] [PubMed]
- Encinas, M.; Crowder, R.J.; Milbrandt, J.; Johnson, E.M., Jr. Tyrosine 981, a novel ret autophosphorylation site, binds c-Src to mediate neuronal survival. J. Biol. Chem. 2004, 279, 18262–18269. [Google Scholar] [CrossRef] [PubMed]
- Borrello, M.G.; Alberti, L.; Arighi, E.; Bongarzone, I.; Battistini, C.; Bardelli, A.; Pasini, B.; Piutti, C.; Rizzetti, M.G.; Mondellini, P.; et al. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cgamma. Mol. Cell Biol. 1996, 16, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, G.; Rosário, M.; Grimm, J.; Boeckers, T.M.; Gundelfinger, E.D.; Birchmeier, W. The neuronal scaffold protein Shank3 mediates signaling and biological function of the receptor tyrosine kinase Ret in epithelial cells. J. Cell Biol. 2004, 167, 945–952. [Google Scholar] [CrossRef]
- Asai, N.; Murakami, H.; Iwashita, T.; Takahashi, M. A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with shc adaptor proteins. J. Biol. Chem. 1996, 271, 17644–17649. [Google Scholar] [CrossRef]
- Durick, K.; Wu, R.Y.; Gill, G.N.; Taylor, S.S. Mitogenic signaling by Ret/ptc2 requires association with enigma via a LIM domain. J. Biol. Chem. 1996, 271, 12691–12694. [Google Scholar] [CrossRef]
- Arighi, E.; Alberti, L.; Torriti, F.; Ghizzoni, S.; Rizzetti, M.G.; Pelicci, G.; Pasini, B.; Bongarzone, I.; Piutti, C.; Pierotti, M.A.; et al. Identification of Shc docking site on Ret tyrosine kinase. Oncogene 1997, 14, 773–782. [Google Scholar] [CrossRef]
- Lorenzo, M.J.; Gish, G.D.; Houghton, C.; Stonehouse, T.J.; Pawson, T.; Ponder, B.A.; Smith, D.P. RET alternate splicing influences the interaction of activated RET with the SH2 and PTB domains of Shc, and the SH2 domain of Grb2. Oncogene 1997, 14, 763–771. [Google Scholar] [CrossRef]
- Ohiwa, M.; Murakami, H.; Iwashita, T.; Asai, N.; Iwata, Y.; Imai, T.; Funahashi, H.; Takagi, H.; Takahashi, M. Characterization of Ret-Shc-Grb2 complex induced by GDNF, MEN 2A, and MEN 2B mutations. Biochem. Biophys. Res. Commun. 1997, 237, 747–751. [Google Scholar] [CrossRef]
- Hennige, A.M.; Lammers, R.; Arlt, D.; Höppner, W.; Strack, V.; Niederfellner, G.; Seif, F.J.; Häring, H.U.; Kellerer, M. Ret oncogene signal transduction via a IRS-2/PI 3-kinase/PKB and a SHC/Grb-2 dependent pathway: Possible implication for transforming activity in NIH3T3 cells. Mol. Cell Endocrinol. 2000, 167, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Iwashita, T.; Murakami, H.; Hayashi, H.; Kawai, K.; Takahashi, M. Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 2001, 20, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Melillo, R.M.; Santoro, M.; Ong, S.H.; Billaud, M.; Fusco, A.; Hadari, Y.R.; Schlessinger, J.; Lax, I. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell Biol. 2001, 21, 4177–4187. [Google Scholar] [CrossRef] [PubMed]
- Melillo, R.M.; Carlomagno, F.; De Vita, G.; Formisano, P.; Vecchio, G.; Fusco, A.; Billaud, M.; Santoro, M. The insulin receptor substrate (IRS)-1 recruits phosphatidylinositol 3-kinase to Ret: Evidence for a competition between Shc and IRS-1 for the binding to Ret. Oncogene 2001, 20, 209–218. [Google Scholar] [CrossRef]
- Grimm, J.; Sachs, M.; Britsch, S.; Di Cesare, S.; Schwarz-Romond, T.; Alitalo, K.; Birchmeier, W. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J. Cell Biol. 2001, 154, 345–354. [Google Scholar] [CrossRef]
- Murakami, H.; Yamamura, Y.; Shimono, Y.; Kawai, K.; Kurokawa, K.; Takahashi, M. Role of Dok1 in cell signaling mediated by RET tyrosine kinase. J. Biol. Chem. 2002, 277, 32781–32790. [Google Scholar] [CrossRef]
- Pelicci, G.; Troglio, F.; Bodini, A.; Melillo, R.M.; Pettirossi, V.; Coda, L.; De Giuseppe, A.; Santoro, M.; Pelicci, P.G. The neuron-specific Rai (ShcC) adaptor protein inhibits apoptosis by coupling Ret to the phosphatidylinositol 3-kinase/Akt signaling pathway. Mol. Cell Biol. 2002, 22, 7351–7363. [Google Scholar] [CrossRef]
- Andreozzi, F.; Melillo, R.M.; Carlomagno, F.; Oriente, F.; Miele, C.; Fiory, F.; Santopietro, S.; Castellone, M.D.; Beguinot, F.; Santoro, M.; et al. Protein kinase Calpha activation by RET: Evidence for a negative feedback mechanism controlling RET tyrosine kinase. Oncogene 2003, 22, 2942–2949. [Google Scholar] [CrossRef]
- Crowder, R.J.; Enomoto, H.; Yang, M.; Johnson, E.M., Jr.; Milbrandt, J. Dok-6, a Novel p62 Dok family member, promotes Ret-mediated neurite outgrowth. J. Biol. Chem. 2004, 279, 42072–42081. [Google Scholar] [CrossRef]
- Murakumo, Y.; Jijiwa, M.; Asai, N.; Ichihara, M.; Takahashi, M. RET and neuroendocrine tumors. Pituitary 2006, 9, 179–192. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Husaini, Y.; Sainsbury, A.; Brown, D.A.; Breit, S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018, 28, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Melillo, R.M.; Carlomagno, F.; Vecchio, G.; Fusco, A. Minireview: RET: Normal and abnormal functions. Endocrinology 2004, 145, 5448–5451. [Google Scholar] [CrossRef] [PubMed]
- Mallia, N.; Vassallo, J. Pathogenesis of endocrine thyroid cancer. Malta Med. J. 2015, 27, 28–33. [Google Scholar]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert. Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Halilovic, E.; Solit, D.B. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr. Opin. Pharmacol. 2008, 8, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Gordoa, T.; Díez, J.J.; Durán, M.; Grande, E. Advances in thyroid cancer treatment: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015, 7, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.M.; Cavaco, B.M.; Pinto, A.E.; Leite, V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J. Clin. Endocrinol. Metab. 2011, 96, E863–E868. [Google Scholar] [CrossRef]
- Agrawal, N.; Jiao, Y.; Sausen, M.; Leary, R.; Bettegowda, C.; Roberts, N.J.; Bhan, S.; Ho, A.S.; Khan, Z.; Bishop, J.; et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J. Clin. Endocrinol. Metab. 2013, 98, E364–E369. [Google Scholar] [CrossRef]
- Boichard, A.; Croux, L.; Al Ghuzlan, A.; Broutin, S.; Dupuy, C.; Leboulleux, S.; Schlumberger, M.; Bidart, J.M.; Lacroix, L. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J. Clin. Endocrinol. Metab. 2012, 97, E2031–E2035. [Google Scholar] [CrossRef]
- Lyra, J.; Vinagre, J.; Batista, R.; Pinto, V.; Prazeres, H.; Rodrigues, F.; Eloy, C.; Sobrinho-Simões, M.; Soares, P. mTOR activation in medullary thyroid carcinoma with RAS mutation. Eur. J. Endocrinol. 2014, 171, 633–640. [Google Scholar] [CrossRef]
- Goutas, N.; Vlachodimitropoulos, D.; Bouka, M.; Lazaris, A.C.; Nasioulas, G.; Gazouli, M. BRAF and K-RAS mutation in a Greek papillary and medullary thyroid carcinoma cohort. Anticancer Res. 2008, 28, 305–308. [Google Scholar]
- Arthan, D.; Hong, S.K.; Park, J.I. Leukemia inhibitory factor can mediate Ras/Raf/MEK/ERK-induced growth inhibitory signaling in medullary thyroid cancer cells. Cancer Lett. 2010, 297, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Strock, C.J.; Ball, D.W.; Nelkin, B.D. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol. Cell Biol. 2003, 23, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Kunnimalaiyaan, M.; Vaccaro, A.M.; Ndiaye, M.A.; Chen, H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol. Cancer Ther. 2007, 6, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Yuan, T.L.; Cantley, L.C. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef]
- Nozhat, Z.; Hedayati, M. PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas. Mol. Diagn. Ther. 2016, 20, 13–26. [Google Scholar] [CrossRef]
- Bjornsti, M.A.; Houghton, P.J. The TOR pathway: A target for cancer therapy. Nat. Rev. Cancer 2004, 4, 335–348. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-κB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Ludwig, L.; Kessler, H.; Wagner, M.; Hoang-Vu, C.; Dralle, H.; Adler, G.; Böhm, B.O.; Schmid, R.M. Nuclear Factor-κB Is Constitutively Active in C-Cell Carcinoma and Required for RET-induced Transformation1. Cancer Res. 2001, 61, 4526–4535. [Google Scholar]
- Tamburrino, A.; Molinolo, A.A.; Salerno, P.; Chernock, R.D.; Raffeld, M.; Xi, L.; Gutkind, J.S.; Moley, J.F.; Wells, S.A., Jr.; Santoro, M. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin. Cancer Res. 2012, 18, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Rapa, I.; Saggiorato, E.; Giachino, D.; Palestini, N.; Orlandi, F.; Papotti, M.; Volante, M. Mammalian target of rapamycin pathway activation is associated to RET mutation status in medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 2011, 96, 2146–2153. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Santoro, M. Targeting the RET pathway in thyroid cancer. Clin. Cancer Res. 2009, 15, 7119–7123. [Google Scholar] [CrossRef]
- Kouvaraki, M.A.; Liakou, C.; Paraschi, A.; Dimas, K.; Patsouris, E.; Tseleni-Balafouta, S.; Rassidakis, G.Z.; Moraitis, D. Activation of mTOR signaling in medullary and aggressive papillary thyroid carcinomas. Surgery 2011, 150, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Moura, M.M.; Cavaco, B.M.; Pinto, A.E.; Domingues, R.; Santos, J.R.; Cid, M.O.; Bugalho, M.J.; Leite, V. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br. J. Cancer 2009, 100, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M.; Marsh, D.J.; Robinson, B.G.; Schuffenecker, I.; Zedenius, J.; Lips, C.J.; Gagel, R.F.; Takai, S.I.; Noll, W.W.; Fink, M.; et al. Genotype-phenotype correlation in multiple endocrine neoplasia type 2: Report of the International RET Mutation Consortium. J. Intern. Med. 1995, 238, 343–346. [Google Scholar] [CrossRef]
- Komminoth, P.; Kunz, E.K.; Matias-Guiu, X.; Hiort, O.; Christiansen, G.; Colomer, A.; Roth, J.; Heitz, P.U. Analysis of RET protooncogene point mutations distinguishes heritable from nonheritable medullary thyroid carcinomas. Cancer 1995, 76, 479–489. [Google Scholar]
- Eng, C.; Clayton, D.; Schuffenecker, I.; Lenoir, G.; Cote, G.; Gagel, R.F.; van Amstel, H.K.; Lips, C.J.; Nishisho, I.; Takai, S.I.; et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996, 276, 1575–1579. [Google Scholar] [CrossRef]
- Mulligan, L.M.; Kwok, J.B.; Healey, C.S.; Elsdon, M.J.; Eng, C.; Gardner, E.; Love, D.R.; Mole, S.E.; Moore, J.K.; Papi, L.; et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993, 363, 458–460. [Google Scholar] [CrossRef]
- Donis-Keller, H.; Dou, S.; Chi, D.; Carlson, K.M.; Toshima, K.; Lairmore, T.C.; Howe, J.R.; Moley, J.F.; Goodfellow, P.; Wells, S.A., Jr. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum. Mol. Genet. 1993, 2, 851–856. [Google Scholar] [CrossRef]
- Mulligan, L.M.; Eng, C.; Healey, C.S.; Clayton, D.; Kwok, J.B.; Gardner, E.; Ponder, M.A.; Frilling, A.; Jackson, C.E.; Lehnert, H.; et al. Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat. Genet. 1994, 6, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Smith, D.P.; Mulligan, L.M.; Healey, C.S.; Zvelebil, M.J.; Stonehouse, T.J.; Ponder, M.A.; Jackson, C.E.; Waterfield, M.D.; Ponder, B.A. A novel point mutation in the tyrosine kinase domain of the RET proto-oncogene in sporadic medullary thyroid carcinoma and in a family with FMTC. Oncogene 1995, 10, 509–513. [Google Scholar] [PubMed]
- Bolino, A.; Schuffenecker, I.; Luo, Y.; Seri, M.; Silengo, M.; Tocco, T.; Chabrier, G.; Houdent, C.; Murat, A.; Schlumberger, M.; et al. RET mutations in exons 13 and 14 of FMTC patients. Oncogene 1995, 10, 2415–2419. [Google Scholar]
- Hofstra, R.M.; Landsvater, R.M.; Ceccherini, I.; Stulp, R.P.; Stelwagen, T.; Luo, Y.; Pasini, B.; Höppener, J.W.; van Amstel, H.K.; Romeo, G.; et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994, 367, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Bürck, J.; Sinn, H.-P.; Clemens, A.; Otto, H.F.; Höppner, W.; Herfarth, C.; Ziegler, R.; Schwab, M.; Raue, F. Prognostic value of codon 918 (ATG→ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. Int. J. Cancer 2001, 95, 62–66. [Google Scholar] [CrossRef]
- Elisei, R.; Cosci, B.; Romei, C.; Bottici, V.; Renzini, G.; Molinaro, E.; Agate, L.; Vivaldi, A.; Faviana, P.; Basolo, F.; et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 682–687. [Google Scholar] [CrossRef]
- Segouffin-Cariou, C.; Billaud, M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J. Biol. Chem. 2000, 275, 3568–3576. [Google Scholar] [CrossRef]
- Salvatore, D.; Melillo, R.M.; Monaco, C.; Visconti, R.; Fenzi, G.; Vecchio, G.; Fusco, A.; Santoro, M. Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res 2001, 61, 1426–1431. [Google Scholar]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef]
- Sherman, S.I.; Clary, D.O.; Elisei, R.; Schlumberger, M.J.; Cohen, E.E.W.; Schöffski, P.; Wirth, L.J.; Mangeshkar, M.; Aftab, D.T.; Brose, M.S. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 2016, 122, 3856–3864. [Google Scholar] [CrossRef]
- Carlomagno, F.; Guida, T.; Anaganti, S.; Provitera, L.; Kjaer, S.; McDonald, N.Q.; Ryan, A.J.; Santoro, M. Identification of tyrosine 806 as a molecular determinant of RET kinase sensitivity to ZD6474. Endocr.-Relat. Cancer 2009, 16, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Gosnell, J.E.; Gagel, R.F.; Moley, J.; Pfister, D.; Sosa, J.A.; Skinner, M.; Krebs, A.; Vasselli, J.; Schlumberger, M. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J. Clin. Oncol. 2010, 28, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Berse, B.; Brown, L.F.; Van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol. Biol. Cell 1992, 3, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: A critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002, 20, 4368–4380. [Google Scholar] [CrossRef]
- Song, S.; Ewald, A.J.; Stallcup, W.; Werb, Z.; Bergers, G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol. 2005, 7, 870–879. [Google Scholar] [CrossRef]
- Lokker, N.A.; Sullivan, C.M.; Hollenbach, S.J.; Israel, M.A.; Giese, N.A. Platelet-derived growth factor (PDGF) autocrine signaling regulates survival and mitogenic pathways in glioblastoma cells: Evidence that the novel PDGF-C and PDGF-D ligands may play a role in the development of brain tumors. Cancer Res. 2002, 62, 3729–3735. [Google Scholar]
- Perrinjaquet, M.; Vilar, M.; Ibáñez, C.F. Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase. J. Biol. Chem. 2010, 285, 31867–31875. [Google Scholar] [CrossRef]
- Pandey, A.; Liu, X.; Dixon, J.E.; Di Fiore, P.P.; Dixit, V.M. Direct association between the Ret receptor tyrosine kinase and the Src homology 2-containing adapter protein Grb7. J. Biol. Chem. 1996, 271, 10607–10610. [Google Scholar] [CrossRef]
- Pandey, A.; Duan, H.; Di Fiore, P.P.; Dixit, V.M. The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J. Biol. Chem. 1995, 270, 21461–21463. [Google Scholar] [CrossRef]
- Donatello, S.; Fiorino, A.; Degl’Innocenti, D.; Alberti, L.; Miranda, C.; Gorla, L.; Bongarzone, I.; Rizzetti, M.G.; Pierotti, M.A.; Borrello, M.G. SH2B1beta adaptor is a key enhancer of RET tyrosine kinase signaling. Oncogene 2007, 26, 6546–6559. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, T.K.; Nakahata, K.; Fritz, N.; Rebellato, P.; Zhang, S.; Uhlén, P. RET PLCγ phosphotyrosine binding domain regulates Ca2+ signaling and neocortical neuronal migration. PLoS ONE 2012, 7, e31258. [Google Scholar] [CrossRef]
- Elisei, R.; Schlumberger, M.J.; Müller, S.P.; Schöffski, P.; Brose, M.S.; Shah, M.H.; Licitra, L.; Jarzab, B.; Medvedev, V.; Kreissl, M.C.; et al. Cabozantinib in progressive medullary thyroid cancer. J. Clin. Oncol. 2013, 31, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.T.; Ringel, M.D.; Kloos, R.T.; Prior, T.W.; Knopp, M.V.; Liang, J.; Sammet, S.; Hall, N.C.; Wakely, P.E., Jr.; Vasko, V.V.; et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J. Clin. Oncol. 2010, 28, 2323–2330. [Google Scholar] [CrossRef]
- Grasic Kuhar, C.; Lozar, T.; Besic, N.; Music Marolt, M. Outcome of Patients with Locally Advanced Metastatic Medullary Thyroid Cancer and Induction Therapy with Tyrosine Kinase Inhibitors in Slovenia. Adv. Ther. 2021, 38, 5684–5699. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chi, Y.; Hu, C.; Chen, X.; Ge, M.; Zhang, Y.; Guo, Z.; Wang, J.; Chen, J.; Zhang, J.; et al. Anlotinib in patients with medullary thyroid carcinoma with negative prognostic factors: A sub-analysis based on the ALTER01031 study. Front. Oncol. 2022, 12, 852032. [Google Scholar] [CrossRef]
- Capdevila, J.; Trigo, J.M.; Aller, J.; Manzano, J.L.; Adrián, S.G.; Llopis, C.Z.; Reig, Ò.; Bohn, U.; Cajal, T.R.Y.; Duran-Poveda, M.; et al. Axitinib treatment in advanced RAI-resistant differentiated thyroid cancer (DTC) and refractory medullary thyroid cancer (MTC). Eur. J. Endocrinol. 2017, 177, 309–317. [Google Scholar] [CrossRef]
- Bible, K.C.; Suman, V.J.; Molina, J.R.; Smallridge, R.C.; Maples, W.J.; Menefee, M.E.; Rubin, J.; Karlin, N.; Sideras, K.; Morris, J.C., 3rd; et al. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J. Clin. Endocrinol. Metab. 2014, 99, 1687–1693. [Google Scholar] [CrossRef]
- Schlumberger, M.J.; Elisei, R.; Bastholt, L.; Wirth, L.J.; Martins, R.G.; Locati, L.D.; Jarzab, B.; Pacini, F.; Daumerie, C.; Droz, J.P.; et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J. Clin. Oncol. 2009, 27, 3794–3801. [Google Scholar] [CrossRef]
- Schlumberger, M.; Elisei, R.; Müller, S.; Schöffski, P.; Brose, M.; Shah, M.; Licitra, L.; Krajewska, J.; Kreissl, M.C.; Niederle, B.; et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma. Ann. Oncol. 2017, 28, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Chuk, M.K.; Widemann, B.C.; Minard, C.G.; Liu, X.; Kim, A.; Bernhardt, M.B.; Kudgus, R.A.; Reid, J.M.; Voss, S.D.; Blaney, S.; et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children’s Oncology Group. Pediatr. Blood Cancer 2018, 65, e27077. [Google Scholar] [CrossRef] [PubMed]
- Kraft, I.L.; Akshintala, S.; Zhu, Y.; Lei, H.; Derse-Anthony, C.; Dombi, E.; Steinberg, S.M.; Lodish, M.; Waguespack, S.G.; Kapustina, O.; et al. Outcomes of Children and Adolescents with Advanced Hereditary Medullary Thyroid Carcinoma Treated with Vandetanib. Clin. Cancer Res. 2018, 24, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Carter, C.; Lynch, M.; Lowinger, T.; Dumas, J.; Smith, R.A.; Schwartz, B.; Simantov, R.; Kelley, S. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat. Rev. Drug Discov. 2006, 5, 835–844. [Google Scholar] [CrossRef]
- Dowell, J.; Minna, J.D.; Kirkpatrick, P. Erlotinib hydrochloride. Nat. Rev. Drug Discov. 2005, 4, 13–14. [Google Scholar] [CrossRef]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef]
- Awada, A.; Hendlisz, A.; Gil, T.; Bartholomeus, S.; Mano, M.; de Valeriola, D.; Strumberg, D.; Brendel, E.; Haase, C.G.; Schwartz, B.; et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br. J. Cancer 2005, 92, 1855–1861. [Google Scholar] [CrossRef]
- Carlomagno, F.; Anaganti, S.; Guida, T.; Salvatore, G.; Troncone, G.; Wilhelm, S.M.; Santoro, M. BAY 43-9006 inhibition of oncogenic RET mutants. J. Natl. Cancer Inst. 2006, 98, 326–334. [Google Scholar] [CrossRef]
- Chow, L.Q.; Eckhardt, S.G. Sunitinib: From rational design to clinical efficacy. J. Clin. Oncol. 2007, 25, 884–896. [Google Scholar] [CrossRef]
- Carr, L.L.; Mankoff, D.A.; Goulart, B.H.; Eaton, K.D.; Capell, P.T.; Kell, E.M.; Bauman, J.E.; Martins, R.G. Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin. Cancer Res. 2010, 16, 5260–5268. [Google Scholar] [CrossRef]
- Masaki, C.; Sugino, K.; Saito, N.; Saito, Y.; Tanaka, T.; Ogimi, Y.; Maeda, T.; Osaku, T.; Akaishi, J.; Hames, K.Y.; et al. Lenvatinib induces early tumor shrinkage in patients with advanced thyroid carcinoma. Endocr. J. 2017, 64, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; Prete, A.; Nervo, A.; Ragni, A.; Agate, L.; Molinaro, E.; Giani, C.; Valerio, L.; Minaldi, E.; Piovesan, A.; et al. Lenvatinib as a salvage therapy for advanced metastatic medullary thyroid cancer. J. Endocrinol. Investig. 2021, 44, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Hui, K.; Hu, C.; Wen, Y.; Yang, S.; Zhu, P.; Wang, L.; Xia, Y.; Qiao, Y.; Sun, W.; et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2019, 38, 71. [Google Scholar] [CrossRef]
- Taurin, S.; Yang, C.H.; Reyes, M.; Cho, S.; Coombs, D.M.; Jarboe, E.A.; Werner, T.L.; Peterson, C.M.; Janát-Amsbury, M.M. Endometrial Cancers Harboring Mutated Fibroblast Growth Factor Receptor 2 Protein Are Successfully Treated with a New Small Tyrosine Kinase Inhibitor in an Orthotopic Mouse Model. Int. J. Gynecol. Cancer 2018, 28, 152–160. [Google Scholar] [CrossRef]
- Sun, D.; Guo, J.; Liang, W.; Chen, Y.; Chen, X.; Wang, L. Anlotinib Alleviates Renal Fibrosis via Inhibition of the ERK and AKT Signaling Pathways. Oxid. Med. Cell Longev. 2023, 2023, 1686804. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Rosen, L.S.; Vokes, E.E.; Kies, M.S.; Forastiere, A.A.; Worden, F.P.; Kane, M.A.; Sherman, E.; Kim, S.; Bycott, P.; et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: Results from a phase II study. J. Clin. Oncol. 2008, 26, 4708–4713. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Knick, V.B.; Rudolph, S.K.; Johnson, J.H.; Crosby, R.M.; Crouthamel, M.C.; Hopper, T.M.; Miller, C.G.; Harrington, L.E.; Onori, J.A.; et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol. Cancer Ther. 2007, 6, 2012–2021. [Google Scholar] [CrossRef]
- Coxon, A.; Bready, J.; Hughes, P.; Estrada, J.; Wang, L.; DeMelfi, T.; Doerr, N.; Kaufman, S.; Radinsky, R.; Kendall, R. Motesanib diphosphate (AMG 706) inhibits the growth of medullary thyroid carcinoma in a nude mouse model. Cancer Res. 2007, 67, LB-283. [Google Scholar]
- U.S. Food & Drug Administration. FDA Approves Selpercatinib for Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-lung-and-thyroid-cancers-ret-gene-mutations-or-fusions (accessed on 14 April 2023).
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Subbiah, V.; Velcheti, V.; Tuch, B.B.; Ebata, K.; Busaidy, N.L.; Cabanillas, M.E.; Wirth, L.J.; Stock, S.; Smith, S.; Lauriault, V.; et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann. Oncol. 2018, 29, 1869–1876. [Google Scholar] [CrossRef]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.S.; Ha, J.; Ha, S.; Bae, J.S.; Jung, C.K.; Lim, D.J. Initial Experiences of Selective RET Inhibitor Selpercatinib in Adults with Metastatic Differentiated Thyroid Carcinoma and Medullary Thyroid Carcinoma: Real-World Case Series in Korea. Curr. Oncol. 2023, 30, 3020–3031. [Google Scholar] [CrossRef] [PubMed]
- Schoffski, P.; Cho, B.C.; Italiano, A.; Loong, H.H.F.; Massard, C.; Rodriguez, L.M.; Shih, J.-Y.; Subbiah, V.; Verlingue, L.; Andreas, K.; et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J. Clin. Oncol. 2021, 39, 3008. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Rahal, R.; Brubaker, J.D.; Kim, J.L.; Maynard, M.; Hu, W.; Cao, Q.; Sheets, M.P.; Wilson, D.; et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov. 2018, 8, 836–849. [Google Scholar] [CrossRef]
- Hu, M.; Subbiah, V.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Brose, M.S.; Curigliano, G.; Leboulleux, S.; Zhu, V.W.; Keam, B.; et al. 1913O Results from the registrational phase I/II ARROW trial of pralsetinib (BLU-667) in patients (pts) with advanced RET mutation-positive medullary thyroid cancer (RET+ MTC). Ann Oncol 2020, 31, S1084. [Google Scholar] [CrossRef]
- Fonseca, L.; Freitas, C.; Caramelo, A.; Eloy, C. Expression of PD-L1 in medullary thyroid carcinoma-a new therapeutic target? Endocrinol. Diabetes Metab. 2021, 4, e00241. [Google Scholar] [CrossRef]
- Shi, X.; Yu, P.C.; Lei, B.W.; Li, C.W.; Zhang, Y.; Tan, L.C.; Shi, R.L.; Wang, J.; Ma, B.; Xu, W.B.; et al. Association between Programmed Death-Ligand 1 Expression and Clinicopathological Characteristics, Structural Recurrence, and Biochemical Recurrence/Persistent Disease in Medullary Thyroid Carcinoma. Thyroid 2019, 29, 1269–1278. [Google Scholar] [CrossRef]
- Naoum, G.E.; Morkos, M.; Kim, B.; Arafat, W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol. Cancer 2018, 17, 51. [Google Scholar] [CrossRef]
- Del Rivero, J.; Donahue, R.N.; Marté, J.L.; Gramza, A.W.; Bilusic, M.; Rauckhorst, M.; Cordes, L.; Merino, M.J.; Dahut, W.L.; Schlom, J.; et al. A Case Report of Sequential Use of a Yeast-CEA Therapeutic Cancer Vaccine and Anti-PD-L1 Inhibitor in Metastatic Medullary Thyroid Cancer. Front. Endocrinol. 2020, 11, 490. [Google Scholar] [CrossRef]
- Yamamoto, N.; Nokihara, H.; Yamada, Y.; Shibata, T.; Tamura, Y.; Seki, Y.; Honda, K.; Tanabe, Y.; Wakui, H.; Tamura, T. Phase I study of Nivolumab, an anti-PD-1 antibody, in patients with malignant solid tumors. Investig. New Drugs 2017, 35, 207–216. [Google Scholar] [CrossRef]
- Schott, M.; Seissler, J.; Lettmann, M.; Fouxon, V.; Scherbaum, W.A.; Feldkamp, J. Immunotherapy for medullary thyroid carcinoma by dendritic cell vaccination. J. Clin. Endocrinol. Metab. 2001, 86, 4965–4969. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, C.W.; Tan, L.C.; Wen, S.S.; Liao, T.; Zhang, Y.; Chen, T.Z.; Ma, B.; Yu, P.C.; Lu, Z.W.; et al. Immune Co-inhibitory Receptors PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT in Medullary Thyroid Cancers: A Large Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, 120–132. [Google Scholar] [CrossRef]
- Lorch, J.H.; Barletta, J.A.; Nehs, M.; Uppaluri, R.; Alexander, E.K.; Haddad, R.I.; Hanna, G.J.; Margalit, D.N.; Tishler, R.B.; Schoenfeld, J.D.; et al. A phase II study of nivolumab (N) plus ipilimumab (I) in radioidine refractory differentiated thyroid cancer (RAIR DTC) with exploratory cohorts in anaplastic (ATC) and medullary thyroid cancer (MTC). J. Clin. Oncol. 2020, 38, 6513. [Google Scholar] [CrossRef]
- Chatal, J.F.; Campion, L.; Kraeber-Bodéré, F.; Bardet, S.; Vuillez, J.P.; Charbonnel, B.; Rohmer, V.; Chang, C.H.; Sharkey, R.M.; Goldenberg, D.M.; et al. Survival improvement in patients with medullary thyroid carcinoma who undergo pretargeted anti-carcinoembryonic-antigen radioimmunotherapy: A collaborative study with the French Endocrine Tumor Group. J. Clin. Oncol. 2006, 24, 1705–1711. [Google Scholar] [CrossRef]
- Eroglu, Z.; Kim, D.W.; Wang, X.; Camacho, L.H.; Chmielowski, B.; Seja, E.; Villanueva, A.; Ruchalski, K.; Glaspy, J.A.; Kim, K.B.; et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur. J. Cancer 2015, 51, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.C.D.; Hofland, L.J.; Lamberts, S.W.J. Chapter 11—Somatostatin and Somatostatin Receptors: From Basic Concepts to Clinical Applications. Prog. Brain Res. 2010, 182, 255–280. [Google Scholar] [CrossRef]
- Herac, M.; Niederle, B.; Raderer, M.; Krebs, M.; Kaserer, K.; Koperek, O. Expression of somatostatin receptor 2A in medullary thyroid carcinoma is associated with lymph node metastasis. APMIS 2016, 124, 839–845. [Google Scholar] [CrossRef]
- Cakir, M.; Dworakowska, D.; Grossman, A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: Part 1—Molecular pathways. J. Cell Mol. Med. 2010, 14, 2570–2584. [Google Scholar] [CrossRef]
- Giardino, E.; Catalano, R.; Mangili, F.; Barbieri, A.M.; Treppiedi, D.; Elli, F.M.; Dolci, A.; Contarino, A.; Spada, A.; Arosio, M.; et al. Octreotide and pasireotide effects on medullary thyroid carcinoma (MTC) cells growth, migration and invasion. Mol. Cell Endocrinol. 2021, 520, 111092. [Google Scholar] [CrossRef]
- Vitale, G.; Lupoli, G.; Guarrasi, R.; Colao, A.; Dicitore, A.; Gaudenzi, G.; Misso, G.; Castellano, M.; Addeo, R.; Facchini, G.; et al. Interleukin-2 and lanreotide in the treatment of medullary thyroid cancer: In vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2013, 98, E1567–E1574. [Google Scholar] [CrossRef]
- Frank-Raue, K.; Ziegler, R.; Raue, F. The use of octreotide in the treatment of medullary thyroid carcinoma. Horm. Metab. Res. Suppl. 1993, 27, 44–47. [Google Scholar] [PubMed]
- Resteghini, C.; Cavalieri, S.; Galbiati, D.; Granata, R.; Alfieri, S.; Bergamini, C.; Bossi, P.; Licitra, L.; Locati, L.D. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best. Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.Y.; Won, H.H.; Zheng, Y.; Cocco, E.; Selcuklu, D.; Gong, Y.; Friedman, N.D.; de Bruijn, I.; Sumer, O.; Bielski, C.M.; et al. The evolution of RET inhibitor resistance in RET-driven lung and thyroid cancers. Nat. Commun. 2022, 13, 1450. [Google Scholar] [CrossRef]
| Tyrosine Kinase Inhibitor | Target of Inhibition | PFS | ORR | Observations | Most Common Adverse Events | Ref. |
|---|---|---|---|---|---|---|
| Vandetanib * | VEGFR2-3 EGFR EGFR2 RET MMPs | 30.5 mo. | 46.4% (hereditary) 51.8% (sporadic) | 37% PD and 15% had died. OS was immature at data cutoff. | Diarrhea, rash, nausea, hypertension and headache | [90] |
| Cabozantinib *& | MET VEGFR2 RET KIT FLT-3 | 11.2 mo. | 28% | 100% PR in cabozantinib group | Diarrhea, palmar–plantar erythrodysesthesia (hand–foot syndrome), fatigue and weight loss | [104] |
| Sorafenib & | VEGFR2 PDGFR RAF1 KIT FLT-3 RET BRAF | 17.9 mo. | NR | 6.3% PR; 87.5% SD regarding cabozantinib group | Diarrhea, hand–foot–skin reaction, rash and hypertension | [105] |
| Sunitinib | VEGFR1-3 PDGFR KIT FLT-3 CSF-1 RET | 10.6 mo. | 50% | 25% SD; 25% PD. Chemotherapy patients only presented with 3.5 mo. PFS. | Fatigue, hypertension, dysgeusia and cutaneous papules | [106] |
| Lenvatinib & | VEGFR1-3 PDGFRα FGFR1-4 KIT RET | 18.3 mo. | 64.8% | 4 patients CR; 165 patients PR | Hypertension, diarrhea, fatigue or asthenia, decreased appetite, weight loss and nausea | [107] |
| Anlotinib | VEGFR1-3 EGFR FGFR1-4 PDGFR KIT | 17.5 mo. & 20.7 mo. | 48.4% | 88,7% DCR; 17.5 mo. PFS for patients older than 50 y/o; 20.7 mo. PFS for bone metastases | Hypertension, palmar- plantar erythrodysesthesia syndrome, proteinuria, hypertriglyceridemia, QTc prolongation, diarrhea and fatigue | [108] |
| Axitinib | VEGFR PDGFR KIT | 9.4 mo. | 23.1% | 0% CR; 23.07% PR; 38.46% SD; 30.77% PD; 1 patient NR. Best results achieved as first-line treatment. | Diarrhea, hypertension, mucositis and weight loss | [109] |
| Pazopanib | VEGFR PDGFR KIT FGFR | 9.4 mo. | 15%; 20%; and 10% | 19.9 mo. OS; ORR: 15% without prior treatment; 20% in TKI naïve and 10% in prior TKI treatment patients; 1 death after withdrawal, potentially treatment related | Hypertension, fatigue, diarrhea and abnormal liver tests | [110] |
| Motesanib | VEGFR PDGFR KIT RET WT | 48 weeks | 2% | 81% SD | Diarrhea, fatigue, hypothyroidism, hypertension and anorexia | [111] |
| Tyrosine Kinase Inhibitor | Biochemical Target | IC50 (nmol/L) |
|---|---|---|
| Pralsetinib | WT RET | 0.4 |
| RET M918T | 0.4 | |
| VEGFR2 | 35 | |
| Cabozantinib | WT RET | 11 |
| RET M918T | 8 | |
| VEGFR2 | 2 | |
| Vandetanib | WT RET | 4 |
| RET M918T | 7 | |
| VEGFR2 | 4 |
| Tyrosine Kinase Inhibitor | PFS | ORR | Observations | Most Common Adverse Events | Ref. |
|---|---|---|---|---|---|
| Selpercatinib | 92% | 73% | PFS was attained in percentage; 91% had calcitonin decrease and 66% had CEA reduction; at 1 year, 64% remained progression-free. | Hypertension, increased aspartate and alanine aminotransferase, hyponatremia and diarrhea | [133] |
| Zeteletinib | NR | 44% | ORR was higher in MTC vs. NSCLC patients (44% vs. 30%). | Creatinine phosphokinase increase, dyspnea, aspartate aminotransferase increase, diarrhea, anemia and fatigue | [135] |
| Pralsetinib | NR | 65% | 97% DCR; 99% had tumor size reduction; 75% remained on treatment. | Aspartate and alanine aminotransferase increased, anemia, hypertension and constipation | [137] |
| Immune Checkpoints | Immunotherapy Drugs | Observations | Most Common Adverse Events | Ref. |
|---|---|---|---|---|
| PD-1/PD-L1 | Nivolumab | PR for >12 months | Ventricular extrasystoles, constipation, diarrhea and fatigue. | [142] |
| CTLA-4 | Nivolumab + Ipilumab | Considerable activity in ATC, but no response was observable in MTC. | NR | [147] |
| SSTR Inhibitors | Observations | Most Common Adverse Events | Ref. |
|---|---|---|---|
| Octreotide | Octreotide does not improve the natural course of advanced stages of medullary thyroid carcinoma. | Diarrhea and weight loss. | [153] |
| Lanreotide | After 6 months of therapy, partial response and stable disease have been recorded in 2 and 3 patients, respectively, with significant decreases in calcitonin levels in 3 patients. | Diarrhea, weight loss and fatigue. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.S.; Jesus, T.T.; Cardoso, L.; Soares, P.; Vinagre, J. Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments. J. Pers. Med. 2023, 13, 1132. https://doi.org/10.3390/jpm13071132
Martins RS, Jesus TT, Cardoso L, Soares P, Vinagre J. Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments. Journal of Personalized Medicine. 2023; 13(7):1132. https://doi.org/10.3390/jpm13071132
Chicago/Turabian StyleMartins, Rui Sousa, Tito Teles Jesus, Luís Cardoso, Paula Soares, and João Vinagre. 2023. "Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments" Journal of Personalized Medicine 13, no. 7: 1132. https://doi.org/10.3390/jpm13071132
APA StyleMartins, R. S., Jesus, T. T., Cardoso, L., Soares, P., & Vinagre, J. (2023). Personalized Medicine in Medullary Thyroid Carcinoma: A Broad Review of Emerging Treatments. Journal of Personalized Medicine, 13(7), 1132. https://doi.org/10.3390/jpm13071132








