Revisited Surgical Anatomy of the Left Colonic Angle for Tailored Carcinologic Colectomy: A Review
Abstract
:1. Introduction
2. Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Reviewing and Extraction
3. Results
3.1. A Bit of Embryology
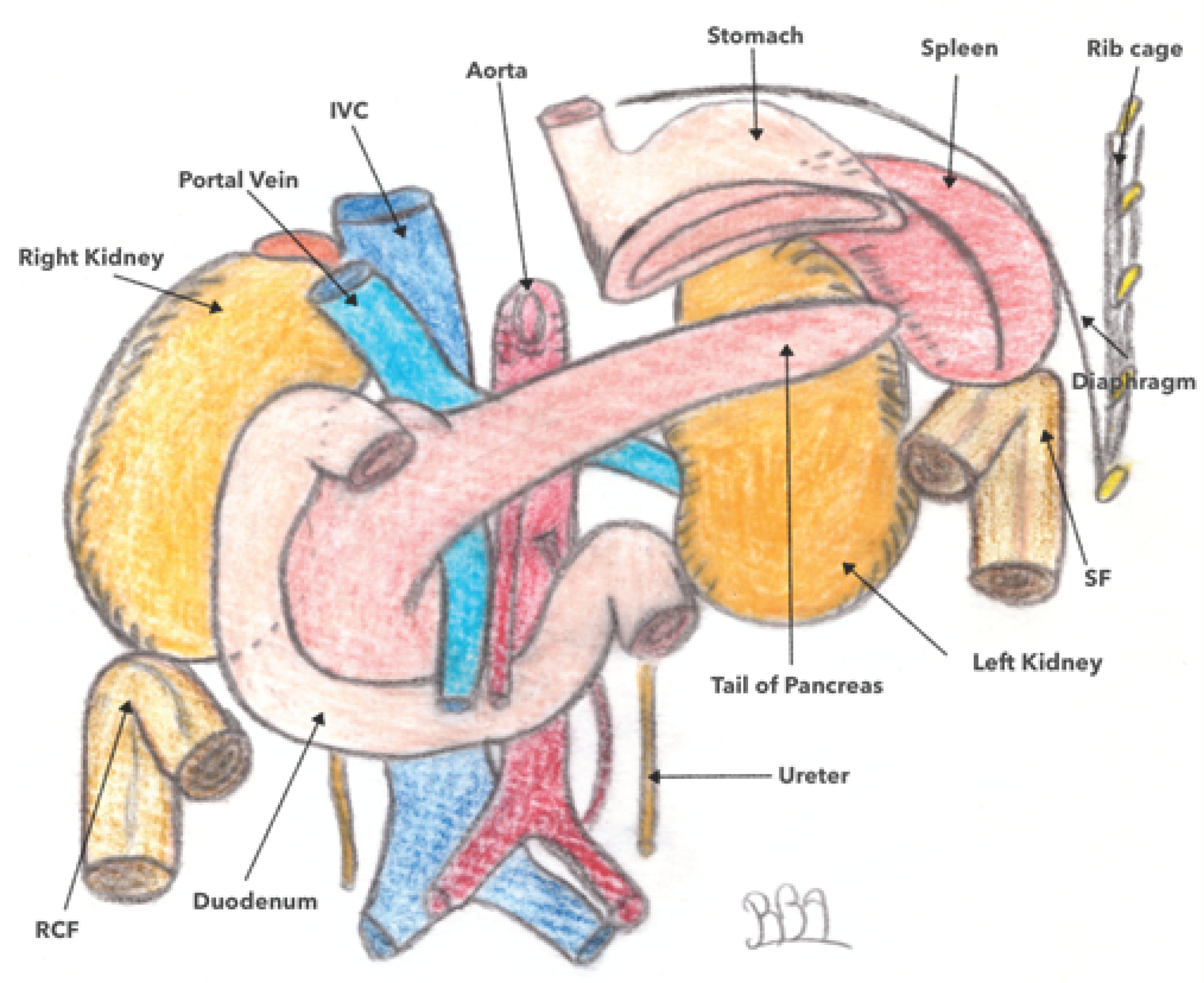
3.2. Location and Anatomical Relationships of the Left Colonic Angle (LCA)
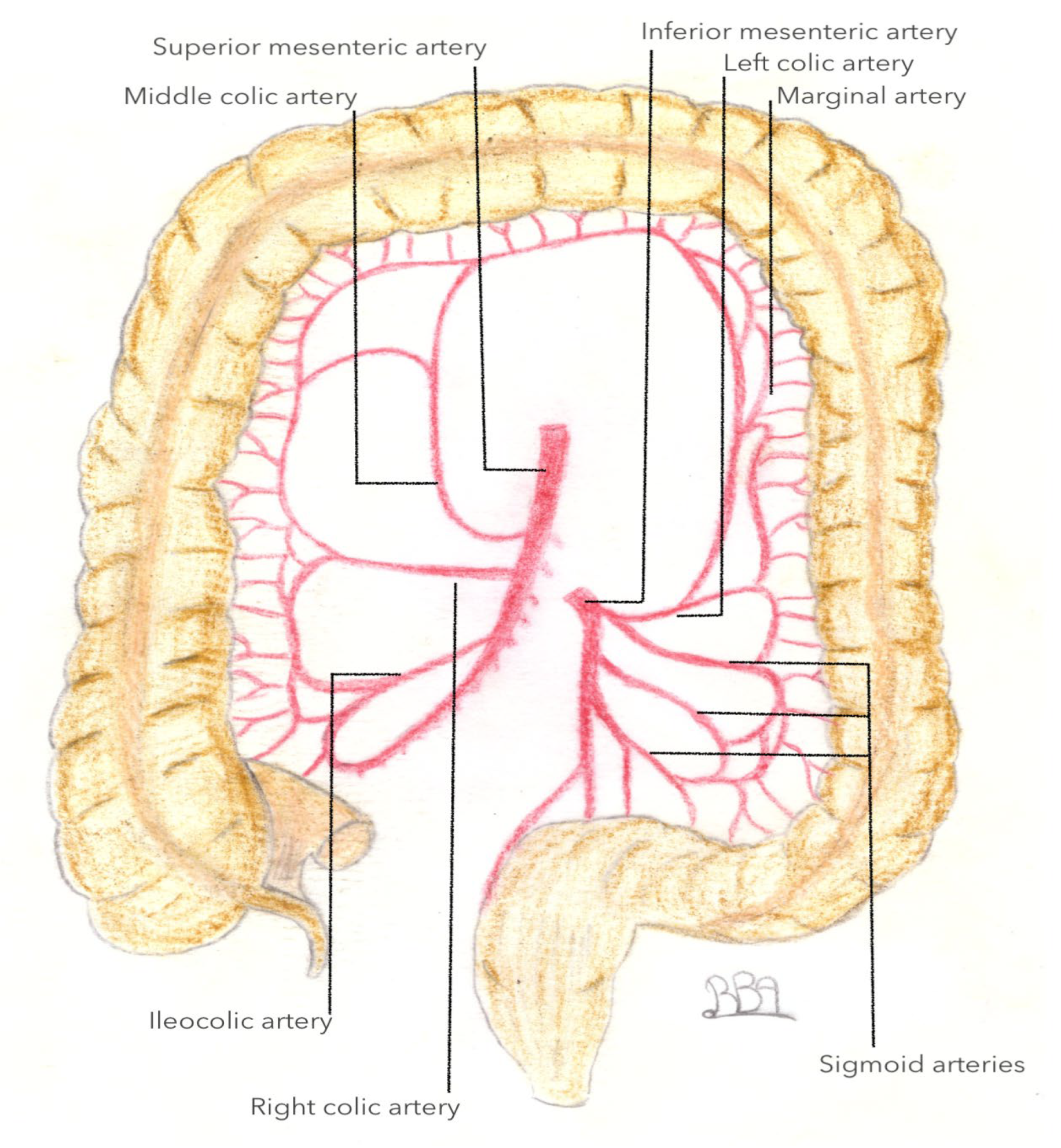
3.3. Arterial Vascularization of the Left Colonic Angle
3.3.1. What We Know
3.3.2. What Is Less Known: A New Artery: The Accessory Middle Colonic Artery (AMCA)
3.4. Venous Vascularization of the Left Colonic Angle
3.5. Lymphatic Drainage of the Left Colonic Angle
4. Perspectives and Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iguchi, K.; Mushiake, H.; Hasegawa, S.; Fukushima, T.; Numata, M.; Tamagawa, H.; Shiozawa, M.; Yukawa, N.; Rino, Y.; Masuda, M. Evaluation of vascular anatomy for colon cancer located in the splenic flexure using the preoperative three-dimensional computed tomography angiography with colonography. Int. J. Colorectal Dis. 2021, 36, 405–411. [Google Scholar] [CrossRef]
- Aldridge, M.C.; Phillips, R.K.; Hittinger, R.; Fry, J.S.; Fielding, L.P. Influence of tumour site on presentation, management and subsequent outcome in large bowel cancer. Br. J. Surg. 1986, 73, 663–670. [Google Scholar] [CrossRef]
- Nakagoe, T.; Sawa, T.; Tsuji, T.; Jibiki, M.; Nanashima, A.; Yamaguchi, H.; Yasutake, T.; Ayabe, H.; Ishikawa, H. Carcinoma of the splenic flexure: Multivariate analysis of predictive factors for clinicopathological characteristics and outcome after surgery. J. Gastroenterol. 2000, 35, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C.; Bokey, E.L.; Chapuis, P.H. Carcinoma of the splenic flexure. Dis. Colon. Rectum. 1987, 30, 872–874. [Google Scholar] [CrossRef]
- de’Angelis, N.; Martínez-Pérez, A.; Winter, D.C.; Landi, F.; Vitali, G.C.; Le Roy, B.; Coccolini, F.; Brunetti, F.; Celentano, V.; Di Saverio, S.; et al. Extended right colectomy, left colectomy, or segmental left colectomy for splenic flexure carcinomas: A European multicenter propensity score matching analysis. Surg. Endosc. 2021, 35, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Manceau, G.; Alves, A.; Meillat, H.; Benhaïm, L.; Ouaïssi, M.; Panis, Y.; Tuech, J.-J.; Dousset, B.; Brigand, C.; Cotte, E.; et al. What is the optimal elective colectomy for splenic flexure cancer: End of the debate? A multicenter study from the GRECCAR group with a propensity score analysis. Dis. Colon. Rectum. 2021. [Google Scholar] [CrossRef] [PubMed]
- Manceau, G.; Benoist, S.; Panis, Y.; Rault, A.; Mathonnet, M.; Goere, D.; Tuech, J.J.; Collet, D.; Penna, C.; Karoui, M. Elective surgery for tumours of the splenic flexure: A French inter-group (AFC, SFCD, FRENCH, GRECCAR) survey. Tech. Coloproctol. 2020, 24, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.D. Surgical anatomy of the blood supply of the distal colon. Ann. R. Coll. Surg. Engl. 1956, 19, 241–256. [Google Scholar] [PubMed]
- Sakorafas, G.H.; Zouros, E.; Peros, G. Applied vascular anatomy of the colon and rectum: Clinical implications for the surgical oncologist. Surg. Oncol. 2006, 15, 243–255. [Google Scholar] [CrossRef]
- Steward, J.A.; Rankin, F.W. Blood supply of the large intestine: Its surgical considerations. Arch. Surg. 1933, 26, 843–891. [Google Scholar] [CrossRef]
- Hirai, K.; Yoshinari, D.; Ogawa, H.; Nakazawa, S.; Takase, Y.; Tanaka, K.; Miyamae, Y.; Takahashi, N.; Tsukagoshi, H.; Toya, H.; et al. Three-dimensional computed tomography for analyzing the vascular anatomy in laparoscopic surgery for right-sided colon cancer. Surg. Laparosc. Endosc. Percutan Tech. 2013, 23, 536–539. [Google Scholar] [CrossRef]
- Lee, S.W.; Shinohara, H.; Matsuki, M.; Okuda, J.; Nomura, E.; Mabuchi, H.; Nishiguchi, K.; Takaori, K.; Narabayashi, I.; Tanigawa, N. Preoperative simulation of vascular anatomy by three-dimensional computed tomography imaging in laparoscopic gastric cancer surgery. J. Am. Coll. Surg. 2003, 197, 927–936. [Google Scholar] [CrossRef]
- Stelzner, S.; Hohenberger, W.; Weber, K.; West, N.P.; Witzigmann, H.; Wedel, T. Anatomy of the transverse colon revisited with respect to complete mesocolic excision and possible pathways of aberrant lymphatic tumor spread. Int. J. Colorectal Dis. 2016, 31, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, T.; Sumi, Y.; Yamashita, K.; Hasegawa, H.; Yamamoto, M.; Matsuda, Y.; Kanaji, S.; Oshikiri, T.; Nakamura, T.; Suzuki, S.; et al. Anatomical and embryological perspectives in laparoscopic complete mesocoloic excision of splenic flexure cancers. Surg. Endosc. 2018, 32, 1202–1208. [Google Scholar] [CrossRef]
- Masoomi, H.; Carmichael, J.C.; Mills, S.; Ketana, N.; Dolich, M.O.; Stamos, M.J. Predictive factors of splenic injury in colorectal surgery: Data from the nationwide inpatient sample, 2006–2008. Arch. Surg. 2012, 147, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Matsuda, T.; Hasegawa, H.; Yamashita, K.; Nakamura, T.; Suzuki, S.; Kakeji, Y. Arterial anatomy of the splenic flexure using preoperative three-dimensional computed tomography. Int. J. Colorectal Dis. 2019, 34, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Latarjet, A. Traite D’anatomie Humaine; G Doin & Cie: Paris, France, 1949. [Google Scholar]
- Miyake, H.; Murono, K.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Otani, K.; Sasaki, K.; Kaneko, M.; Emoto, S.; et al. Evaluation of the vascular anatomy of the left-sided colon focused on the accessory middle colic artery: A single-centre study of 734 patients. Color. Dis. 2018, 20, 1041–1046. [Google Scholar] [CrossRef]
- Patroni, A.; Bonnet, S.; Bourillon, C.; Bruzzi, M.; Zinzindohoué, F.; Chevallier, J.M.; Douard, R.; Berger, A. Technical difficulties of left colic artery preservation during left colectomy for colon cancer. Surg. Radiol. Anat. 2016, 38, 477–484. [Google Scholar] [CrossRef]
- Murono, K.; Miyake, H.; Hojo, D.; Nozawa, H.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Shuno, Y.; Sasaki, K.; et al. Vascular anatomy of the splenic flexure, focusing on the accessory middle colic artery and vein. Color. Dis. 2020, 22, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.C.; Vlad, M.; Voinea, L.M.; Curcă, G.C.; Sişu, A.M. Detailed anatomy of a left accessory aberrant colic artery. Surg. Radiol. Anat. 2008, 30, 595–599. [Google Scholar] [CrossRef]
- Cheruiyot, I.; Cirocchi, R.; Munguti, J.; Davies, R.J.; Randolph, J.; Ndung’u, B.; Henry, B.M. Surgical anatomy of the accessory middle colic artery: A meta-analysis with implications for splenic flexure cancer surgery. Colorectal Dis. 2021, 23, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, A.; Sasaki, T.; Tsukikawa, S.; Miyajima, N.; Ostubo, T. Evaluating distribution of the left branch of the middle colic artery and the left colic artery by CT angiography and colonography to classify blood supply to the splenic flexure. Asian J. Endosc. Surg. 2017, 10, 148–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, Y.; Ehara, K.; Yamada, T.; Matsuzawa, N.; Arai, S.; Ban, D.; Kudo, A.; Tanabe, M.; Kawashima, Y.; Sakamoto, H. Three-dimensional computed tomography analysis of the vascular anatomy of the splenic hilum for gastric cancer surgery. Surg. Today 2018, 48, 841–847. [Google Scholar] [CrossRef]
- Amonoo-Kuofi, H.S.; el-Badawi, M.G.; el-Naggar, M.E. Anomalous origins of colic arteries. Clin. Anat. 1995, 8, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Horiguchi, M. Accessory arteries supplying the human transverse colon. Acta Anat. 1990, 137, 246–251. [Google Scholar] [CrossRef]
- Vandamme, J.P.; Bonte, J.; van der Schueren, G. Re-evaluation of the colic irrigation from the inferior mesenteric artery. Acta Anat. 1982, 112, 18–30. [Google Scholar] [CrossRef]
- Arimoto, A.; Matsuda, T.; Hasegawa, H.; Yamashita, K.; Nakamura, T.; Sumi, Y.; Suzuki, S.; Kakeji, Y. Evaluation of the venous drainage pattern of the splenic flexure by preoperative three-dimensional computed tomography. Asian J. Endosc. Surg. 2019, 12, 412–416. [Google Scholar] [CrossRef]
- Watanabe, J.; Ota, M.; Suwa, Y.; Ishibe, A.; Masui, H.; Nagahori, K. Evaluation of lymph flow patterns in splenic flexural colon cancers using laparoscopic real-time indocyanine green fluorescence imaging. Int. J. Colorectal Dis. 2017, 32, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Hauschild, A.; Lindenblatt, N.; Pentheroudakis, G.; Keilholz, U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, 126–132. [Google Scholar] [CrossRef]
- Cahill, R.A.; Leroy, J.; Marescaux, J. Could lymphatic mapping and sentinel node biopsy provide oncological providence for local resectional techniques for colon cancer? A review of the literature. BMC Surg. 2008, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Orsini, F.; Guidoccio, F.; Vidal-Sicart, S.; Valdés Olmos, R.A.; Mariani, G. General concepts on radioguided sentinel lymph node biopsy: Preoperative imaging, intraoperative gamma-probe guidance, intraoperative imaging, and multimodality imaging. In Atlas of Lymphoscintigraphy and Sentinel Node Mapping: A Pictorial Case-Based Approach; Mariani, G., Manca, G., Orsini, F., Vidal-Sicart, S., Valdés Olmos, R.A., Eds.; Springer: Milan, Italy, 2013; pp. 95–110. [Google Scholar]
- West, N.P.; Hohenberger, W.; Weber, K.; Perrakis, A.; Finan, P.J.; Quirke, P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010, 28, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Inomata, M.; Katayama, H.; Mizusawa, J.; Etoh, T.; Konishi, F.; Sugihara, K.; Watanabe, M.; Moriya, Y.; Kitano, S. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan clinical oncology group study JCOG 0404. Ann. Surg. 2014, 260, 23–30. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belhadjamor, R.; Manceau, G.; Menahem, B.; Sabbagh, C.; Alves, A. Revisited Surgical Anatomy of the Left Colonic Angle for Tailored Carcinologic Colectomy: A Review. J. Pers. Med. 2023, 13, 1198. https://doi.org/10.3390/jpm13081198
Belhadjamor R, Manceau G, Menahem B, Sabbagh C, Alves A. Revisited Surgical Anatomy of the Left Colonic Angle for Tailored Carcinologic Colectomy: A Review. Journal of Personalized Medicine. 2023; 13(8):1198. https://doi.org/10.3390/jpm13081198
Chicago/Turabian StyleBelhadjamor, Roukaya, Gilles Manceau, Benjamin Menahem, Charles Sabbagh, and Arnaud Alves. 2023. "Revisited Surgical Anatomy of the Left Colonic Angle for Tailored Carcinologic Colectomy: A Review" Journal of Personalized Medicine 13, no. 8: 1198. https://doi.org/10.3390/jpm13081198



