N-Terminal Pro-B Type Natriuretic Peptide as a Predictive Biomarker of Bronchopulmonary Dysplasia or Death Due to Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
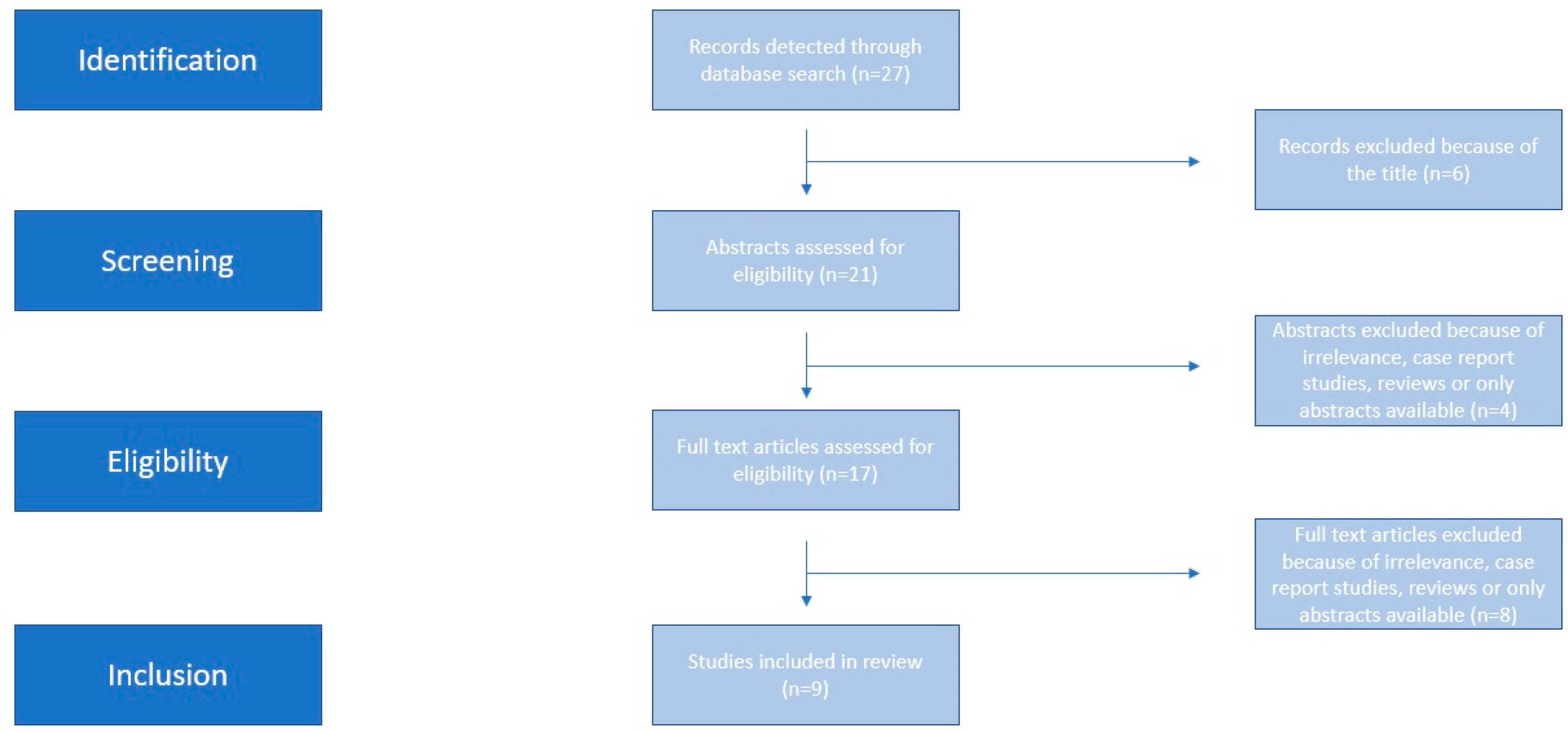
2.4. Study Selection and Data Extraction
2.5. Assessment of Risk of Bias and Quality of Evidence
2.6. Synthesis of Results
2.7. Prediction Intervals
2.8. Meta-Regression Analysis
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| AUC | Area under the curve |
| BPD | Bronchopulmonary dysplasia |
| BNP | B-natriuretic peptide |
| HsPDA | hemodynamically significant patent ductus arteriosus |
| LUS | lung ultrasound score |
| MISC | Multisystemic Inflammatory Systematic disease in children |
| NT-proBNP | N terminal pro B type natriuretic peptide |
| NIH | National Institute of Health |
| PDA | Patent Ductus Arteriosus |
| PPHN | Persistent Pulmonary Hypertension of the Newborn |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SMD | standardized mean difference |
References
- Kalikkot Thekkeveedu, R.; Guaman, M.C.; Shivanna, B. Bronchopulmonary dysplasia: A review of pathogenesis and pathophysiology. Respir. Med. 2017, 132, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Annesi, C.A.; Levin, J.C.; Litt, J.S.; Sheils, C.A.; Hayden, L.P. Long-term respiratory and developmental outcomes in children with bronchopulmonary dysplasia and history of tracheostomy. J. Perinatol. 2021, 41, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shen, Q.; Wang, S.; Dong, T.; Liang, L.; Xu, F.; He, Y.; Li, C.; Luo, F.; Liang, J.; et al. Risk factors that affect the degree of bronchopulmonary dysplasia in very preterm infants: A 5-year retrospective study. BMC Pediatr. 2022, 22, 200. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sallmon, H.; Koestenberger, M.; Avian, A.; Reiterer, F.; Schwaberger, B.; Meinel, K.; Cvirn, G.; Kurath-Koller, S.; Gamillscheg, A.; Hansmann, G. Extremely premature infants born at 23-25 weeks gestation are at substantial risk for pulmonary hypertension. J. Perinatol. 2022, 42, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Gokulakrishnan, G.; Kulkarni, M.; He, S.; Leeflang, M.M.; Cabrera, A.G.; Fernandes, C.J.; Pammi, M. Brain natriuretic peptide and N-terminal brain natriuretic peptide for the diagnosis of haemodynamically significant patent ductus arteriosus in preterm neonates. Cochrane Database Syst. Rev. 2022, 12, CD013129. [Google Scholar]
- Kim, G.; Lee, O.J.; Kang, I.S.; Song, J.; Huh, J. Clinical implications of serial serum N-terminal prohormone brain natriuretic peptide levels in the prediction of outcome in children with dilated cardiomyopathy. Am. J. Cardiol. 2013, 112, 1455–1460. [Google Scholar] [CrossRef]
- Dionne, A.; Dahdah, N. A Decade of NT-proBNP in Acute Kawasaki Disease, from Physiological Response to Clinical Relevance. Children 2018, 5, 141. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, J.; Huang, Y.; Yin, L.; Tang, L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 49, 62–70. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Palas, D.; Ehlinger, V.; Alberge, C.; Truffert, P.; Kayem, G.; Goffinet, F.; Ancel, P.-Y.; Arnaud, C.; Vayssière, C. Efficacy of antenatal corticosteroids in preterm twins: The EPIPAGE-2 cohort study. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef] [PubMed]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Joseph, L.; Nir, A.; Hammerman, C.; Goldberg, S.; Ben Shalom, E.; Picard, E. N-terminal pro-B-type natriuretic peptide as a marker of bronchopulmonary dysplasia in premature infants. Am. J. Perinatol. 2010, 27, 381–386. [Google Scholar] [CrossRef]
- Sellmer, A.; Hjortdal, V.E.; Bjerre, J.V.; Schmidt, M.R.; McNamara, P.J.; Bech, B.H.; Henriksen, T.B. N-Terminal Pro-B Type Natriuretic Peptide as a Marker of Bronchopulmonary Dysplasia or Death in Very Preterm Neonates: A Cohort Study. PLoS ONE. 2015, 10, e0140079. [Google Scholar] [CrossRef]
- Montaner, A.; Pinillos, R.; Galve, Z.; Boix, H.; de la Cuesta, C.R.; Jimenez, L.; Samper, P.; Rite, S. Brain Natriuretic Propeptide as an Early Marker of Bronchopulmonary Dysplasia or Death in the Preterm Newborn. Klin. Padiatr. 2017, 229, 223–228. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, S.; Oulego-Erroz, I.; Alonso-Quintela, P.; Terroba-Seara, S.; Jiménez-González, A.; Palau-Benavides, M. N-terminal-probrain natriuretic peptide as a biomarker of moderate to severe bronchopulmonary dysplasia in preterm infants: A prospective observational study. Pediatr. Pulmonol. 2018, 53, 1073–1081. [Google Scholar] [CrossRef]
- Khan, S.; Concina, V.A.; Schneider, D.; Westgate, P.; Arriagada, S.; Bada, H. Role of NT-proBNP in the prediction of moderate to severe Bronchopulmonary Dysplasia in preterm infants. Pediatr. Pulmonol. 2020, 55, 376–382. [Google Scholar] [CrossRef]
- Méndez-Abad, P.; Zafra-Rodríguez, P.; Lubián-López, S.; Benavente-Fernández, I. NTproBNP is a useful early biomarker of bronchopulmonary dysplasia in very low birth weight infants. Eur. J. Pediatr. 2019, 178, 755–761. [Google Scholar] [CrossRef]
- Zhou, L.; Xiang, X.; Wang, L.; Chen, X.; Zhu, J.; Xia, H. N-Terminal Pro-B-Type Natriuretic Peptide as a Biomarker of Bronchopulmonary Dysplasia or Death in Preterm Infants: A Retrospective Cohort Analysis. Front. Pediatr. 2019, 7, 166. [Google Scholar] [CrossRef]
- Potsiurko, S.; Dobryanskyy, D.; Sekretar, L. Patent ductus arteriosus, systemic NT-proBNP concentrations and development of bronchopulmonary dysplasia in very preterm infants: Retrospective data analysis from a randomized controlled trial. BMC Pediatr. 2021, 21, 286. [Google Scholar] [CrossRef]
- Song, M.; Lei, M.; Luo, C.; Shi, Z.; Cheng, X.; Ding, W.; Cao, W.; Zhang, J.; Ge, J.; Wang, M.; et al. Development of a Nomogram for Moderate-to-Severe Bronchopulmonary Dysplasia or Death: Role of N-Terminal Pro-brain Natriuretic Peptide as a Biomarker. Front. Pediatr. 2021, 9, 727362. [Google Scholar] [CrossRef]
- Cantinotti, M.; Walters, H.L.; Crocetti, M.; Marotta, M.; Murzi, B.; Clerico, A. BNP in children with congenital cardiac disease: Is there now sufficient evidence for its routine use? Cardiol. Young 2015, 25, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, Z.Y.; Luo, C.H.; Wang, L.; Zhang, S.S.; Cheng, X.R.; Zhang, Q.; Xu, Q.Y.; Guo, H.X.; Cheng, X.Y.; et al. Application of NT-proBNP in ventilator weaning for preterm infants with RDS. Pediatr. Pulmonol. 2014, 49, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Vijlbrief, D.C.; Benders, M.J.; Kemperman, H.; van Bel, F.; de Vries, W.B. B-type natriuretic peptide and rebound during treatment for persistent pulmonary hypertension. J. Pediatr. 2012, 160, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.S.; Keller, T.; Kribs, A.; Hünseler, C. Diseases associated with prematurity in correlation with N-terminal pro-brain natriuretic peptide levels during the early postnatal life. Eur. J. Pediatr. 2023, 182, 3075–3082. [Google Scholar] [CrossRef]
- Rite, S.; Martín de Vicente, C.; García-Iñiguez, J.P.; Couce, M.L.; Samper, M.P.; Montaner, A.; Ruiz de la Cuesta, C. The Consensus Definition of Bronchopulmonary Dysplasia Is an Adequate Predictor of Lung Function at Preschool Age. Front. Pediatr. 2022, 10, 830035. [Google Scholar] [CrossRef]
- Rivera, L.; Siddaiah, R.; Oji-Mmuo, C.; Silveyra, G.R.; Silveyra, P. Biomarkers for Bronchopulmonary Dysplasia in the Preterm Infant. Front. Pediatr. 2016, 4, 33. [Google Scholar] [CrossRef]
- Akcan, A.B.; Kardelen, F.; Oygucu, S.E.; Kocabaş, A.; Ozel, D.; Akbaş, H.; Oygür, N. The efficacy of cardiac findings in assessing the outcome in preterms with bronchopulmonary dysplasia. Indian J. Pediatr. 2013, 80, 896–902. [Google Scholar] [CrossRef]
- Harris, S.L.; More, K.; Dixon, B.; Troughton, R.; Pemberton, C.; Horwood, J.; Ellis, N.; Austin, N. Factors affecting N-terminal pro-B-type natriuretic peptide levels in preterm infants and use in determination of haemodynamic significance of patent ductus arteriosus. Eur. J. Pediatr. 2018, 177, 521–532. [Google Scholar] [CrossRef]
- Alonso-Ojembarrena, A.; Méndez-Abad, P.; Alonso-Quintela, P.; Zafra-Rodríguez, P.; Oulego-Erroz, I.; Lubián-López, S.P. Lung ultrasound score has better diagnostic ability than NT-proBNP to predict moderate-severe bronchopulmonary dysplasia. Eur. J. Pediatr. 2022, 181, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.S.; Keller, T.; Kribs, A.; Hünseler, C. Reference values for N-terminal Pro-brain natriuretic peptide in premature infants during their first weeks of life. Eur. J. Pediatr. 2021, 180, 1193–1201. [Google Scholar] [CrossRef] [PubMed]





| Year, Author | Country | Type of Study | Exclusion Criteria | Type of Sample | Timing of Collection |
|---|---|---|---|---|---|
| 2009, Joseph [14] | Israel | Prospective cohort | Major anomalies Congenital heart defects Current sepsis Current PDA Lack of informed consent of parents/guardians | Serum | 28 days of life |
| 2015, Sellmer [15] | Denmark | Prospective cohort | Chromosomal abnormalities Congenital heart defects (except for ASD) Lack of informed consent of parents/guardians | Serum | 3 days of life |
| 2017, Montaner [16] | Spain | Retrospective cohort | Chromosomal disorders and other malformations, Cardiac disease causing circulatory overload other than PDA, Incomplete echocardiographic and biochemical evaluation | Serum | 2 to 3 days of life |
| 2018, Blanco [17] | Spain | Prospective cohort | Major congenital defects, death during first 48 h, incomplete data, Lack of informed consent of parents/guardians | Serum | 5–10 days of life |
| 2019, Khan [18] | USA | Prospective | Congenital anomalies, sepsis, perinatal asphyxia, complex congenital heart disease and PDA | Serum | 28 days of life |
| 2019, Abad [19] | Spain | Prospective cohort | Congenital heart defects (except for PFO, PDA, ASD, VSD < 2 mm) Genetic syndrome Major anomalies Death in 1st week of life Lack of informed consent of parents/guardians | Serum | 1, 3, 7, 14, 28, 35, 42, and 49 days after birth |
| 2019, Zhou Lin [20] | China | Retrospective cohort | Genetic disorders, congenital anomalies including complex congenital heart disease, death on the first day of life | Serum | 1st day of life |
| 2021, Potchiurko [21] | Ukraine | Retrospective cohort | Congenital heart defects (except for PDA) Haemorrhaging syndrome Necrotizing enterocolitis Lung hypoplasia Platelets < 50,000/mm3 Oliguria < 1 mL/kg/h Lack of informed consent of parents/guardians | Serum | 2, 3, 8, 9 days of life |
| 2021, Song [22] | China | Retrospective cohort | Congenital metabolic defects Congenital heart defects(except for PFO and PDA) Severe renal insufficiency Death in 1st week of life Incomplete data Lack of informed consent of parents/guardians | Serum | 1, 3, 7, 14, 21, 28 days of life |
| Year, Author | Patients No (BPD. vs. Control) | Birth Weight(gr) (BPD. vs. Control) | Nature of Birth (BPD. vs. Control) | Gestational Age at Delivery (BPD. vs. Control) | Apgar Score (Median) (BPD. vs. Control) | Antenatal Steroids (n) (BPD. vs. Control) | Surfactant Replacement Therapy(n) (BPD vs. Control) | Presence of Patent Ductus Arteriosus (PDA) (n) (BPD vs. Control) |
|---|---|---|---|---|---|---|---|---|
| 2009, Joseph [14] | 11 vs. 23 | 944 ± 337 vs. 1512 ± 314 | N/A | 27.4 ± 2.9 vs. 31.1 ± 1.4 | A1:6.5 vs. 8 A5: 7 vs. 8 | N/A | N/A | 0 |
| 2015, Sellmer [15] | 48 vs. 86 | 835 vs. 1170 | CS: 31 vs. 56 | 26 vs. 29 | A1: 7 vs. 8 A5: 10 vs. 10 | 33 vs. 82 | 30 vs. 31 | 31 vs. 29 |
| 2017, Montaner [16] | 40 (BPD or death) vs. 77 (no BPD or death) | 755 vs. 1040 | N/A | 26.6 vs. 28.1 | N/A | 33 vs. 64 | 39 vs. 53 | 39 vs. 38 |
| 2018, Blanco [17] | 28 (BPD and/or death) vs. 82 (no BPD) | 840 (690–1050) vs. 1280 | N/A | 26.7 vs. 30.4 | A1: N/A A5: 7.5 vs. 9 | 21 vs. 57 | 22 vs. 41 | 27 vs. 32 |
| 2019, Khan [18] | 44 (moderate to severe BPD) vs. 24 (no to mild BPD) | 955 ± 249 vs. 1158 ± 230 | CS: 36 vs. 15 Vaginal: 8 vs. 8 | 26 ± 2 vs. 28 ±1 | A1: 3.5 vs. 5 A5: 6 vs. 7 | 27 vs. 15 | 1st dose: 44 vs. 20 2nd dose: 18 vs. 1 | N/A |
| 2019, Abad [19] | 15 vs. 86 | 850 vs. 1200 | CS: 12 vs. 72 | 27.27 ± 1.3 vs. 29.13 ± 1.79 | A5: 7 vs. 8 | 14 vs. 72 | N/A | 7 vs. 14 |
| 2019, Zhou Lin [20] | 48 (moderate to severe BPD) vs. 99 (no to mild BPD) | 1110 ± 273 vs. 1398 ± 304 | CS: 33 vs. 61 | 29+1 vs. 30 | A1: 7 vs. 9 A5: 9 Vs. 10 | N/A | 42 vs. 86 | 34 vs. 34 |
| 2021, Potchiurko [21] | 25 vs. 27 | 820 vs. 1200 | CS: 5 vs. 17 | 27 vs. 30 | A1: 4 vs. 6 A5: 5 vs. 6 | 15 vs. 21 | 24 vs. 19 | 17 vs. 5 |
| 2021, Song [22] | 138 (moderate to severe BPD) vs. 418 (no to mild BPD) | 1100 vs. 1250 | CS: 100 vs. 331 | 29.1 vs. 30 | A1: 7 vs. 8 A5: 8 vs. 9 | 133 vs. 170 | 324 vs. 452 | 65 vs. 112 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodolaki, K.; Pergialiotis, V.; Sapantzoglou, I.; Theodora, M.; Antsaklis, P.; Pappa, K.; Daskalakis, G.; Papapanagiotou, A. N-Terminal Pro-B Type Natriuretic Peptide as a Predictive Biomarker of Bronchopulmonary Dysplasia or Death Due to Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 1287. https://doi.org/10.3390/jpm13091287
Rodolaki K, Pergialiotis V, Sapantzoglou I, Theodora M, Antsaklis P, Pappa K, Daskalakis G, Papapanagiotou A. N-Terminal Pro-B Type Natriuretic Peptide as a Predictive Biomarker of Bronchopulmonary Dysplasia or Death Due to Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine. 2023; 13(9):1287. https://doi.org/10.3390/jpm13091287
Chicago/Turabian StyleRodolaki, Kalliopi, Vasilios Pergialiotis, Ioakeim Sapantzoglou, Marianna Theodora, Panagiotis Antsaklis, Kalliopi Pappa, Georgios Daskalakis, and Aggeliki Papapanagiotou. 2023. "N-Terminal Pro-B Type Natriuretic Peptide as a Predictive Biomarker of Bronchopulmonary Dysplasia or Death Due to Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Meta-Analysis" Journal of Personalized Medicine 13, no. 9: 1287. https://doi.org/10.3390/jpm13091287
APA StyleRodolaki, K., Pergialiotis, V., Sapantzoglou, I., Theodora, M., Antsaklis, P., Pappa, K., Daskalakis, G., & Papapanagiotou, A. (2023). N-Terminal Pro-B Type Natriuretic Peptide as a Predictive Biomarker of Bronchopulmonary Dysplasia or Death Due to Bronchopulmonary Dysplasia in Preterm Neonates: A Systematic Review and Meta-Analysis. Journal of Personalized Medicine, 13(9), 1287. https://doi.org/10.3390/jpm13091287









