Robot-Assisted versus Laparoscopic Gastrointestinal Surgery: A Systematic Review and Metanalysis of Intra- and Post-Operative Complications
Abstract
1. Introduction
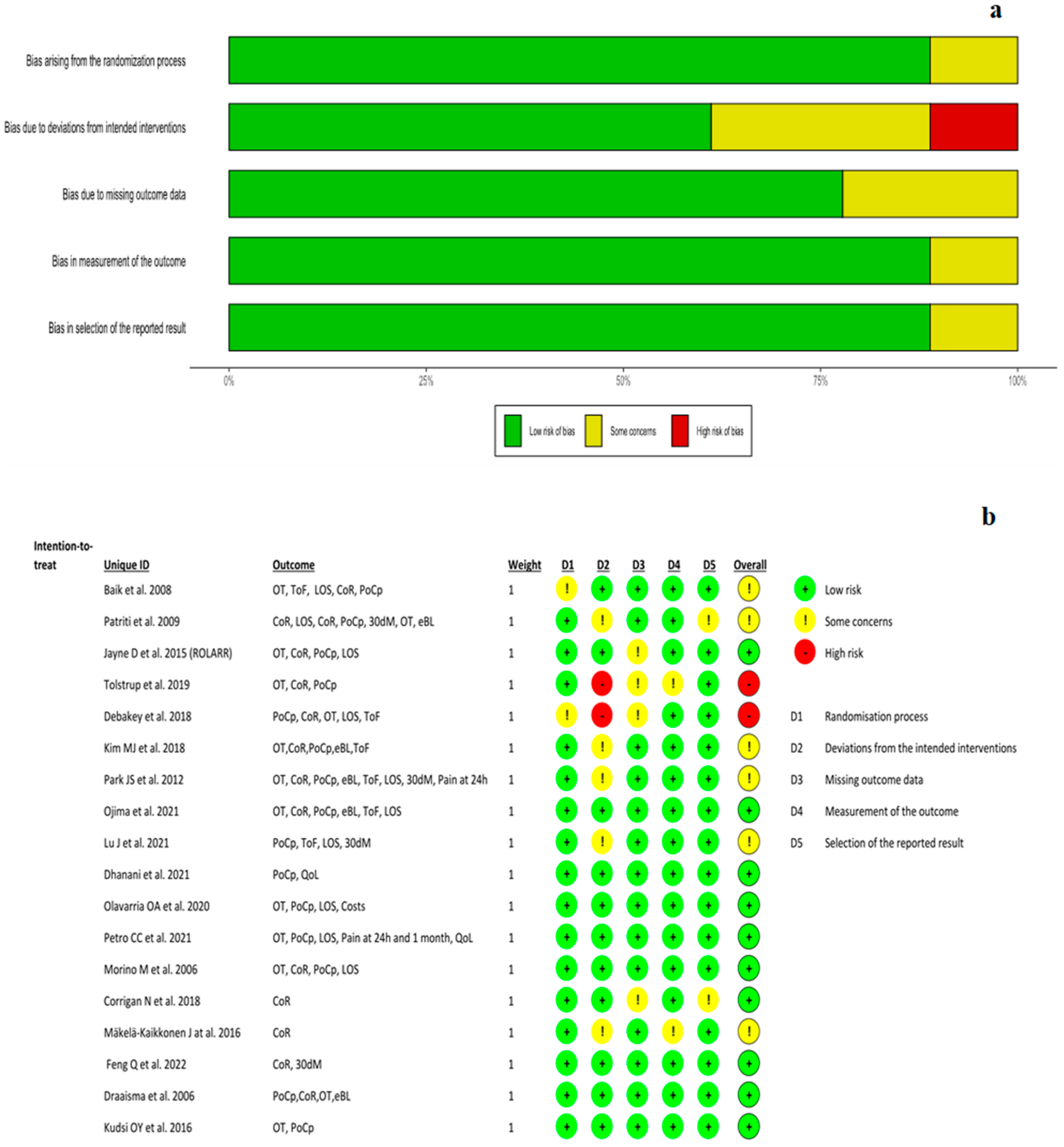
2. Materials and Methods
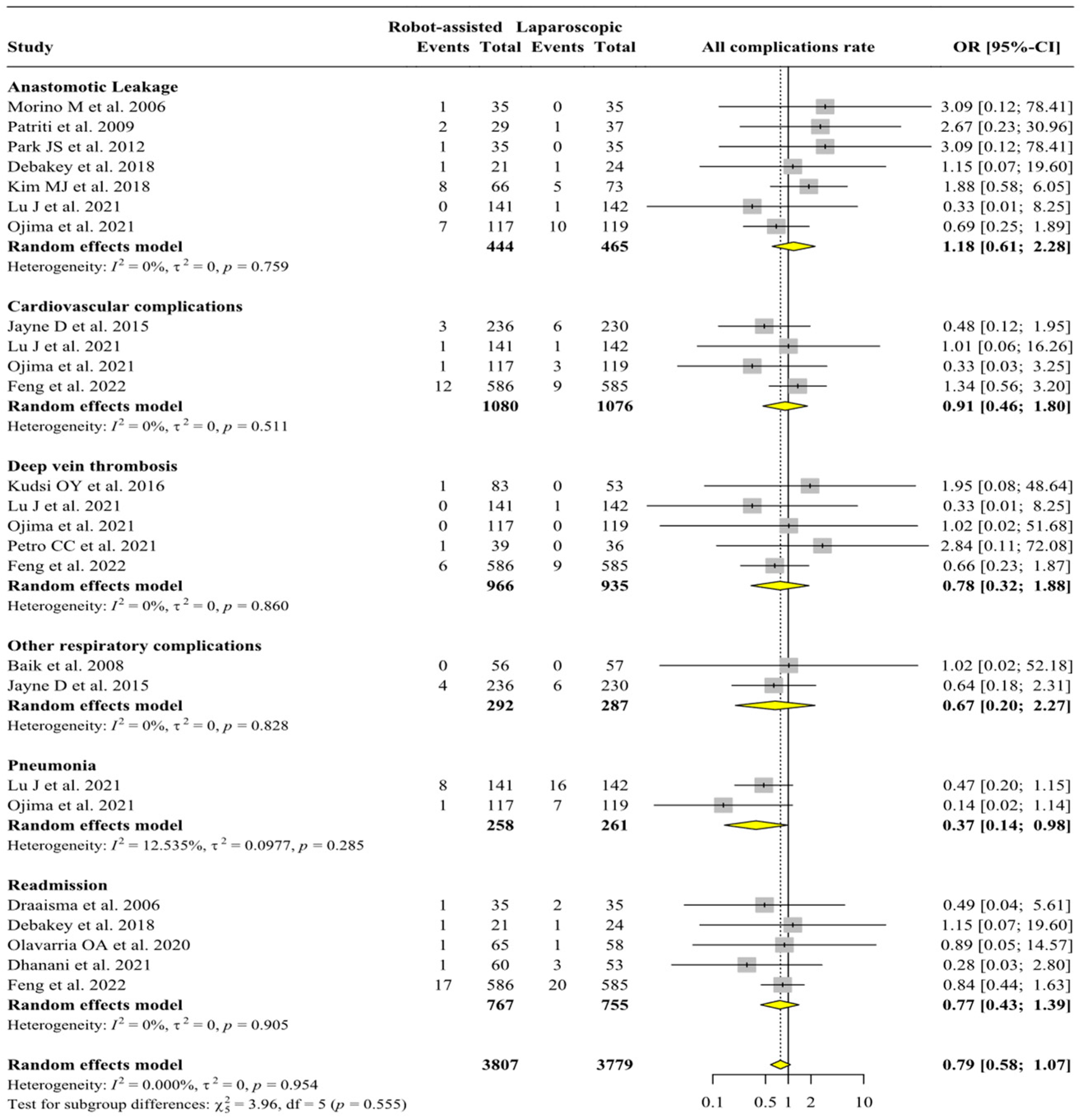
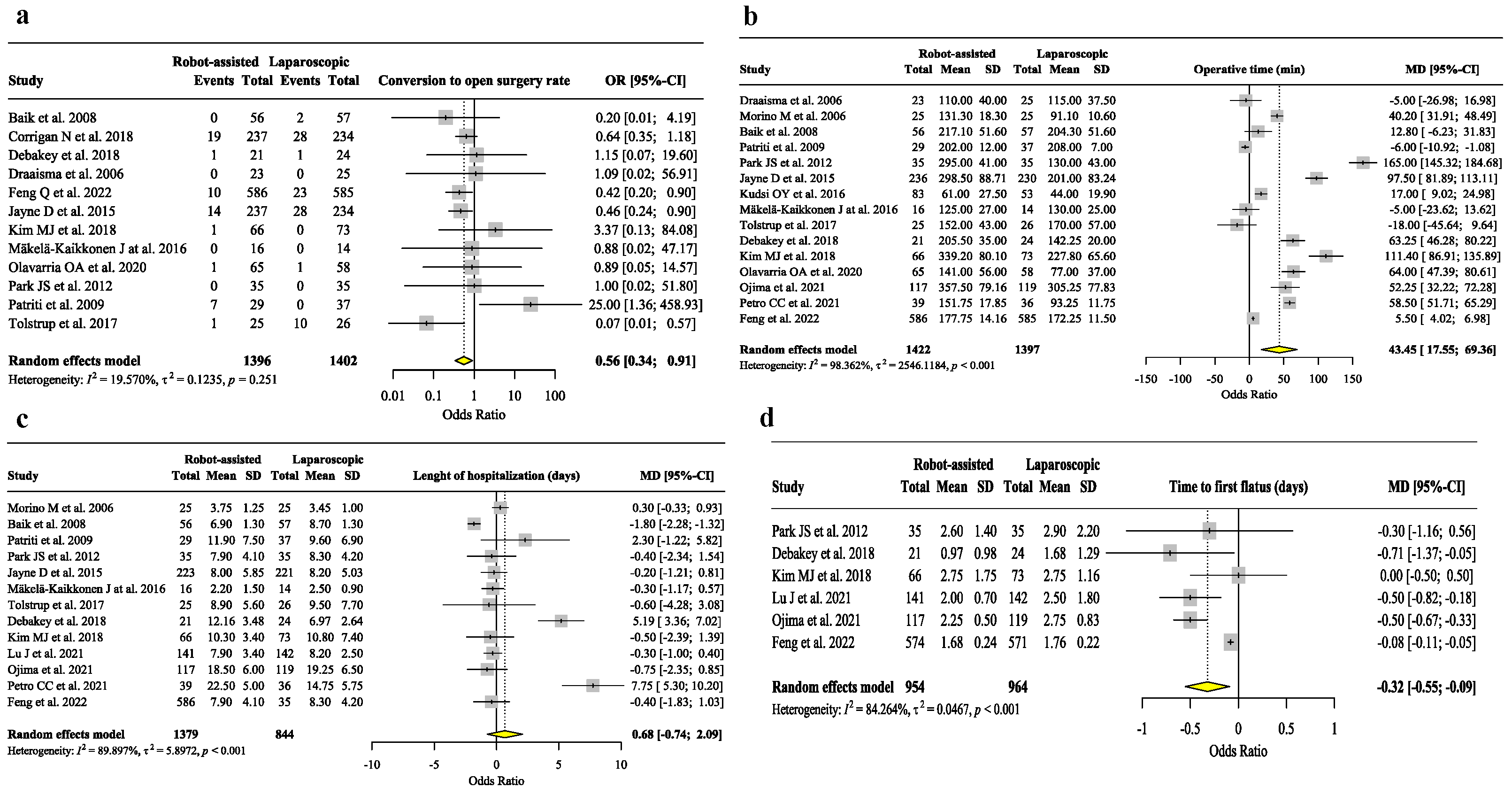
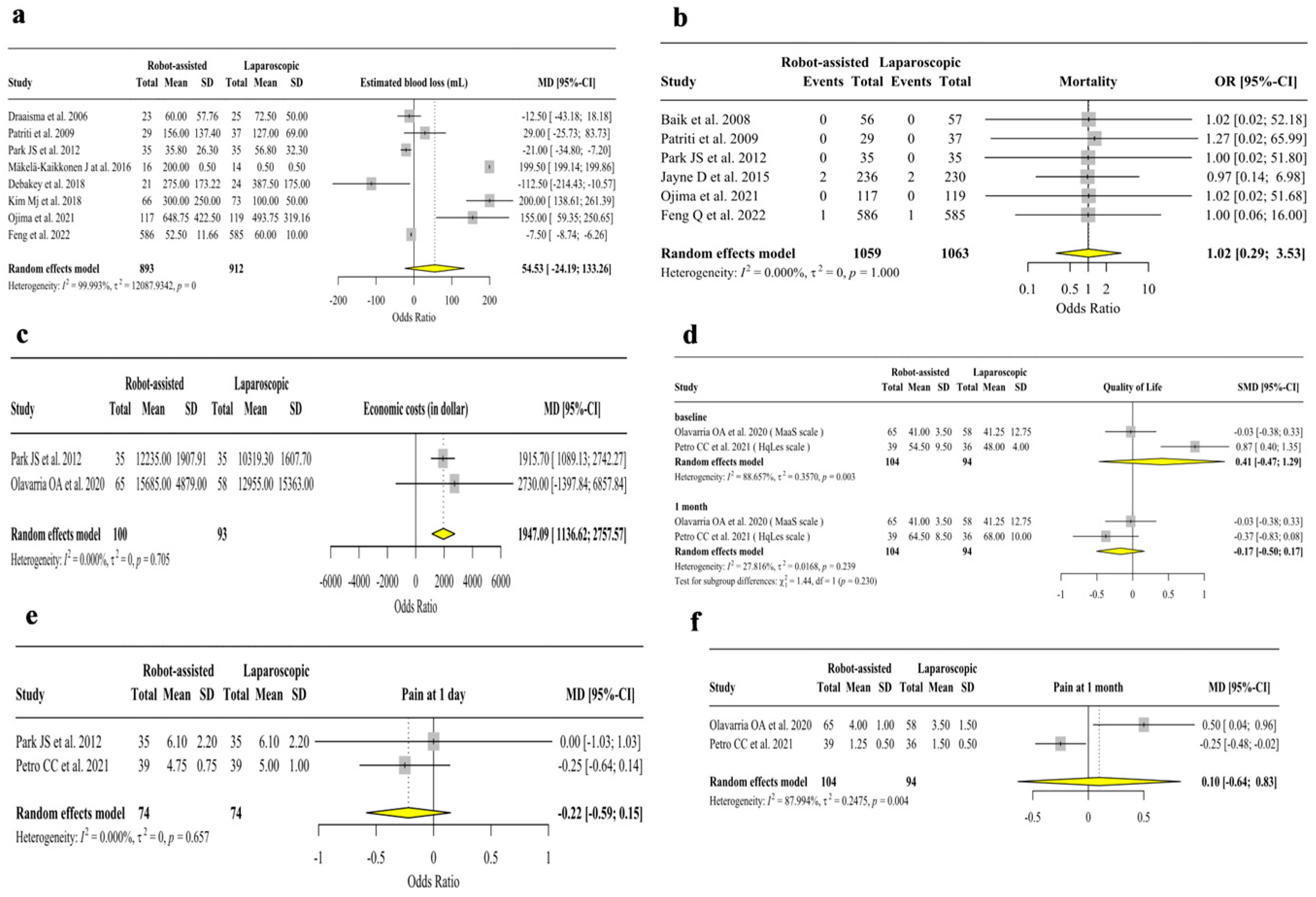
3. Results
3.1. Analysis of Intra- and Post-Operative Complications
3.2. Analysis of Secondary Outcomes
3.3. Trial Sequential Analysis
3.4. Fragility Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baukloh, J.-K.; Perez, D.; Reeh, M.; Biebl, M.; Izbicki, J.R.; Pratschke, J.; Aigner, F. Lower Gastrointestinal Surgery: Robotic Surgery versus Laparoscopic Procedures. Visc. Med. 2018, 34, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Palep, J. Robotic Assisted Minimally Invasive Surgery. J. Minim. Access Surg. 2009, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luca, F.; Valvo, M.; Ghezzi, T.L.; Zuccaro, M.; Cenciarelli, S.; Trovato, C.; Sonzogni, A.; Biffi, R. Impact of Robotic Surgery on Sexual and Urinary Functions after Fully Robotic Nerve-Sparing Total Mesorectal Excision for Rectal Cancer. Ann. Surg. 2013, 257, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Bencini, L.; Annecchiarico, M.; Di Marino, M.; Moraldi, L.; Perna, F.; Coratti, A. Gastrointestinal Robotic Surgery: Challenges and Developments. Robot. Surg. Res. Rev. 2015, 2015, 11–27. [Google Scholar] [CrossRef]
- El Dahdah, J.; Halabi, M.; Kamal, J.; Zenilman, M.E.; Moussa, H. Initial Experience with a Novel Robotic Surgical System in Abdominal Surgery. J. Robot. Surg. 2023, 17, 841–846. [Google Scholar] [CrossRef]
- Bingmer, K.; Ofshteyn, A.; Stein, S.L.; Marks, J.M.; Steinhagen, E. Decline of Open Surgical Experience for General Surgery Residents. Surg. Endosc. 2020, 34, 967–972. [Google Scholar] [CrossRef]
- Bhandare, M.; Thomas, M.; Joshi, R. Complications after Supramajor Gastrointestinal Surgery: Role of Enhanced Recovery after Surgery. Indian J. Crit. Care Med. 2020, 24, S205–S210. [Google Scholar] [CrossRef]
- Jakobson, T.; Karjagin, J.; Vipp, L.; Padar, M.; Parik, A.-H.; Starkopf, L.; Kern, H.; Tammik, O.; Starkopf, J. Postoperative Complications and Mortality after Major Gastrointestinal Surgery. Medicina 2014, 50, 111–117. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, C.-H.; Xu, B.-B.; Xie, J.-W.; Wang, J.-B.; Lin, J.-X.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; Tu, R.-H.; et al. Assessment of Robotic Versus Laparoscopic Distal Gastrectomy for Gastric Cancer. Ann. Surg. 2021, 273, 858–867. [Google Scholar] [CrossRef]
- Ramsay, C.; Pickard, R.; Robertson, C.; Close, A.; Vale, L.; Armstrong, N.; Barocas, D.; Eden, C.; Fraser, C.; Gurung, T.; et al. Systematic Review and Economic Modelling of the Relative Clinical Benefit and Cost-Effectiveness of Laparoscopic Surgery and Robotic Surgery for Removal of the Prostate in Men with Localised Prostate Cancer. Health Technol. Assess. 2012, 16, 1–313. [Google Scholar] [CrossRef]
- Chang, K.; Raheem, A.; Rha, K. Novel Robotic Systems and Future Directions. Indian J. Urol. 2018, 34, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Tekkis, P.P.; Senagore, A.J.; Delaney, C.P.; Fazio, V.W. Evaluation of the Learning Curve in Laparoscopic Colorectal Surgery. Ann. Surg. 2005, 242, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Maynou, L.; Pearson, G.; McGuire, A.; Serra-Sastre, V. The Diffusion of Robotic Surgery: Examining Technology Use in the English NHS. Health Policy 2022, 126, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Grosek, J.; Ales Kosir, J.; Sever, P.; Erculj, V.; Tomazic, A. Robotic versus Laparoscopic Surgery for Colorectal Cancer: A Case-Control Study. Radiol. Oncol. 2021, 55, 433–438. [Google Scholar] [CrossRef]
- Katayama, S.; Mori, K.; Pradere, B.; Yanagisawa, T.; Mostafaei, H.; Quhal, F.; Motlagh, R.S.; Laukhtina, E.; Grossmann, N.C.; Rajwa, P.; et al. Influence of Steep Trendelenburg Position on Postoperative Complications: A Systematic Review and Meta-Analysis. J. Robot. Surg. 2021, 16, 1233–1247. [Google Scholar] [CrossRef]
- Gao, D.; Sun, L.; Wang, N.; Shi, Y.; Song, J.; Liu, X.; Yang, Q.; Su, Z. Impact of 30° Reserve Trendelenburg Position on Lung Function in Morbidly Obese Patients Undergoing Laparoscopic Sleeve Gastrectomy. Front. Surg. 2022, 9, 792697. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Jaramillo, S.; Montane-Muntane, M.; Gambus, P.L.; Capitan, D.; Navarro-Ripoll, R.; Blasi, A. Perioperative Blood Loss: Estimation of Blood Volume Loss or Haemoglobin Mass Loss? Blood Transfus. 2020, 18, 20–29. [Google Scholar] [CrossRef]
- Murad, M.H.; Wang, Z.; Chu, H.; Lin, L. When Continuous Outcomes Are Measured Using Different Scales: Guide for Meta-Analysis and Interpretation. BMJ 2019, 364, k4817. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available online: www.handbook.cochrane.org (accessed on 28 June 2023).
- Vargas, M.; Buonanno, P.; Marra, A.; Iacovazzo, C.; Servillo, G. Fragility Index in Multicenter Randomized Controlled Trials in Critical Care Medicine That Have Shown Reduced Mortality. Crit. Care Med. 2020, 48, e250–e251. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Lan, K.K.G.; DeMets, D.L. Discrete Sequential Boundaries for Clinical Trials. Biometrika 1983, 70, 659–663. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, G.-S.; Park, S.Y.; Kim, H.J.; Ryuk, J.P. Randomized Clinical Trial of Robot-Assisted versus Standard Laparoscopic Right Colectomy. Br. J. Surg. 2012, 99, 1219–1226. [Google Scholar] [CrossRef]
- Dhanani, N.H.; Olavarria, O.A.; Holihan, J.L.; Shah, S.K.; Wilson, T.D.; Loor, M.M.; Ko, T.C.; Kao, L.S.; Liang, M.K. Robotic Versus Laparoscopic Ventral Hernia Repair. Ann. Surg. 2021, 273, 1076–1080. [Google Scholar] [CrossRef]
- Olavarria, O.A.; Bernardi, K.; Shah, S.K.; Wilson, T.D.; Wei, S.; Pedroza, C.; Avritscher, E.B.; Loor, M.M.; Ko, T.C.; Kao, L.S.; et al. Robotic versus Laparoscopic Ventral Hernia Repair: Multicenter, Blinded Randomized Controlled Trial. BMJ 2020, 370, m2457. [Google Scholar] [CrossRef]
- Petro, C.C.; Zolin, S.; Krpata, D.; Alkhatib, H.; Tu, C.; Rosen, M.J.; Prabhu, A.S. Patient-Reported Outcomes of Robotic vs Laparoscopic Ventral Hernia Repair With Intraperitoneal Mesh. JAMA Surg. 2020, 156, 22–29. [Google Scholar] [CrossRef]
- Baik, S.H.; Ko, Y.T.; Kang, C.M.; Lee, W.J.; Kim, N.K.; Sohn, S.K.; Chi, H.S.; Cho, C.H. Robotic Tumor-Specific Mesorectal Excison of Rectal Cancer: Short-Term Outcome of a Pilot Randomized Trial. Surg. Endosc. 2008, 22, 1601–1608. [Google Scholar] [CrossRef]
- Patriti, A.; Ceccarelli, G.; Bartoli, A.; Spaziani, A.; Biancafarina, A.; Casciola, L. Short- and Medium-Term Outcome of Robot-Assisted and Traditional Laparoscopic Rectal Resection. JSLS 2009, 13, 176–183. [Google Scholar]
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Edlin, R.; Hulme, C.; et al. Robotic-Assisted Surgery Compared with Laparoscopic Resection Surgery for Rectal Cancer: The ROLARR RCT. Effic. Mech. Eval. 2019, 6, 1–140. [Google Scholar] [CrossRef]
- Tolstrup, R.; Funder, J.A.; Lundbech, L.; Thomassen, N.; Iversen, L.H. Perioperative Pain after Robot-Assisted versus Laparoscopic Rectal Resection. Int. J. Colorectal Dis. 2018, 33, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Debakey, Y.; Zaghloul, A.; Farag, A.; Mahmoud, A.; Elattar, I. Robotic-Assisted versus Conventional Laparoscopic Approach for Rectal Cancer Surgery, First Egyptian Academic Center Experience, RCT. Minim. Invasive Surg. 2018, 2018, 5836562. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, S.C.; Park, J.W.; Chang, H.J.; Kim, D.Y.; Nam, B.-H.; Sohn, D.K.; Oh, J.H. Robot-Assisted Versus Laparoscopic Surgery for Rectal Cancer. Ann. Surg. 2018, 267, 243–251. [Google Scholar] [CrossRef]
- Corrigan, N.; Marshall, H.; Croft, J.; Copeland, J.; Jayne, D.; Brown, J. Exploring and Adjusting for Potential Learning Effects in ROLARR: A Randomised Controlled Trial Comparing Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection. Trials 2018, 19, 339. [Google Scholar] [CrossRef]
- Mäkelä-Kaikkonen, J.; Rautio, T.; Pääkkö, E.; Biancari, F.; Ohtonen, P.; Mäkelä, J. Robot-Assisted vs Laparoscopic Ventral Rectopexy for External or Internal Rectal Prolapse and Enterocele: A Randomized Controlled Trial. Color. Dis. 2016, 18, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yuan, W.; Li, T.; Tang, B.; Jia, B.; Zhou, Y.; Zhang, W.; Zhao, R.; Zhang, C.; Cheng, L.; et al. Robotic versus Laparoscopic Surgery for Middle and Low Rectal Cancer (REAL): Short-Term Outcomes of a Multicentre Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 991–1004. [Google Scholar] [CrossRef]
- Draaisma, W.A.; Ruurda, J.P.; Scheffer, R.C.H.; Simmermacher, R.K.J.; Gooszen, H.G.; Rijnhart-de Jong, H.G.; Buskens, E.; Broeders, I.A.M.J. Randomized Clinical Trial of Standard Laparoscopic versus Robot-Assisted Laparoscopic Nissen Fundoplication for Gastro-Oesophageal Reflux Disease. Br. J. Surg. 2006, 93, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Morino, M.; Pellegrino, L.; Giaccone, C.; Garrone, C.; Rebecchi, F. Randomized Clinical Trial of Robot-Assisted versus Laparoscopic Nissen Fundoplication. Br. J. Surg. 2006, 93, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Ojima, T.; Nakamura, M.; Hayata, K.; Kitadani, J.; Katsuda, M.; Takeuchi, A.; Tominaga, S.; Nakai, T.; Nakamori, M.; Ohi, M.; et al. Short-Term Outcomes of Robotic Gastrectomy vs Laparoscopic Gastrectomy for Patients With Gastric Cancer. JAMA Surg. 2021, 156, 954–963. [Google Scholar] [CrossRef]
- Kudsi, O.Y.; Castellanos, A.; Kaza, S.; McCarty, J.; Dickens, E.; Martin, D.; Tiesenga, F.M.; Konstantinidis, K.; Hirides, P.; Mehendale, S.; et al. Cosmesis, Patient Satisfaction, and Quality of Life after Da Vinci Single-Site Cholecystectomy and Multiport Laparoscopic Cholecystectomy: Short-Term Results from a Prospective, Multicenter, Randomized, Controlled Trial. Surg. Endosc. 2017, 31, 3242–3250. [Google Scholar] [CrossRef]
- Ntutumu, R.; Liu, H.; Zhen, L.; Hu, Y.-F.; Mou, T.-Y.; Lin, T.; Balde, A.I.; Yu, J.; Li, G.-X. Risk Factors for Pulmonary Complications Following Laparoscopic Gastrectomy. Medicine 2016, 95, e4567. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.; Gaudiosi, C.; Mazzella, F.; Scognamiglio, A.; Mattucci, I.; Carone, M.; Ferrara, N.; Abete, P. Pneumonia and Hospitalizations in the Elderly. Geriatr. Care 2017, 3, 6377. [Google Scholar] [CrossRef][Green Version]
- Chughtai, M.; Gwam, C.U.; Mohamed, N.; Khlopas, A.; Newman, J.M.; Khan, R.; Nadhim, A.; Shaffiy, S.; Mont, M.A. The Epidemiology and Risk Factors for Postoperative Pneumonia. J. Clin. Med. Res. 2017, 9, 466–475. [Google Scholar] [CrossRef]
- Zhai, T.; Zhang, L.; Sun, J.; Li, Y.; Hou, J.; Du, F. Study on the Risk Factors of Pulmonary Infection after Laparoscopic Surgery and Analysis of the Detection Results of Drug-Resistant Bacteria. J. Healthc. Eng. 2022, 2022, 6510068. [Google Scholar] [CrossRef] [PubMed]
- Lanzillo, R.; Cennamo, G.; Moccia, M.; Criscuolo, C.; Carotenuto, A.; Frattaruolo, N.; Sparnelli, F.; Melenzane, A.; Lamberti, A.; Servillo, G.; et al. Retinal Vascular Density in Multiple Sclerosis: A 1-Year Follow-Up. Eur. J. Neurol. 2019, 26, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Buonanno, P.; Marra, A.; Iacovazzo, C.; Merola, R.; De Siena, A.U.; Servillo, G.; Vargas, M. Electric Impedance Tomography and Protective Mechanical Ventilation in Elective Robotic-Assisted Laparoscopy Surgery with Steep Trendelenburg Position: A Randomized Controlled Study. Sci. Rep. 2023, 13, 2753. [Google Scholar] [CrossRef]
- De Robertis, E.; Zito Marinosci, G.; Romano, G.M.; Piazza, O.; Iannuzzi, M.; Cirillo, F.; De Simone, S.; Servillo, G. The Use of Sugammadex for Bariatric Surgery: Analysis of Recovery Time from Neuromuscular Blockade and Possible Economic Impact. Clin. Outcomes Res. 2016, 8, 317–322. [Google Scholar] [CrossRef]
- Vargas, M.; Servillo, G.; Tessitore, G.; Aloj, F.; Brunetti, I.; Arditi, E.; Salami, D.; Kacmarek, R.M.; Pelosi, P. Double Lumen Endotracheal Tube for Percutaneous Tracheostomy. Respir. Care 2014, 59, 1652–1659. [Google Scholar] [CrossRef]
- Vargas, M.; Sutherasan, Y.; Brunetti, I.; Micalizzi, C.; Insorsi, A.; Ball, L.; Folentino, M.; Sileo, R.; De Lucia, A.; Cerana, M.; et al. Mortality and Long-Term Quality of Life after Percutaneous Tracheotomy in Intensive Care Unit: A Prospective Observational Study. Minerva Anestesiol. 2018, 84, 1024–1031. [Google Scholar] [CrossRef]
- Prete, F.P.; Pezzolla, A.; Prete, F.; Testini, M.; Marzaioli, R.; Patriti, A.; Jimenez-Rodriguez, R.M.; Gurrado, A.; Strippoli, G.F.M. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer. Ann. Surg. 2018, 267, 1034–1046. [Google Scholar] [CrossRef]
- Solaini, L.; Cavaliere, D.; Avanzolini, A.; Rocco, G.; Ercolani, G. Robotic versus Laparoscopic Inguinal Hernia Repair: An Updated Systematic Review and Meta-Analysis. J. Robot. Surg. 2022, 16, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Asklid, D.; Ljungqvist, O.; Xu, Y.; Gustafsson, U.O. Short-Term Outcome in Robotic vs Laparoscopic and Open Rectal Tumor Surgery within an ERAS Protocol: A Retrospective Cohort Study from the Swedish ERAS Database. Surg. Endosc. 2022, 36, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Deretti, S.; Mongelli, F.; Staccini, G.; Murgante, N.; Majno-Hurst, P.; Christoforidis, D. Laparoscopic Versus Open Re-Operations Within 30 Days After Lower Gastrointestinal Tract Surgery: A Retrospective Comparative Study. World J. Surg. 2021, 45, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J. Poorly Controlled Postoperative Pain: Prevalence, Consequences, and Prevention. J. Pain Res. 2017, 10, 2287–2298. [Google Scholar] [CrossRef]
- Ruurda, J.P.; Visser, P.L.; Broeders, I.A.M.J. Analysis of Procedure Time in Robot-Assisted Surgery: Comparative Study in Laparoscopic Cholecystectomy. Comput. Aided Surg. 2003, 8, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Luckey, A. Mechanisms and Treatment of Postoperative Ileus. Arch. Surg. 2003, 138, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Forester, B.; Attaar, M.; Donovan, K.; Kuchta, K.; Ujiki, M.; Denham, W.; Haggerty, S.P.; Carbray, J.; Linn, J. Short-Term Quality of Life Comparison of Laparoscopic, Open, and Robotic Incisional Hernia Repairs. Surg. Endosc. 2021, 35, 2781–2788. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovazzo, C.; Buonanno, P.; Massaro, M.; Ianniello, M.; de Siena, A.U.; Vargas, M.; Marra, A. Robot-Assisted versus Laparoscopic Gastrointestinal Surgery: A Systematic Review and Metanalysis of Intra- and Post-Operative Complications. J. Pers. Med. 2023, 13, 1297. https://doi.org/10.3390/jpm13091297
Iacovazzo C, Buonanno P, Massaro M, Ianniello M, de Siena AU, Vargas M, Marra A. Robot-Assisted versus Laparoscopic Gastrointestinal Surgery: A Systematic Review and Metanalysis of Intra- and Post-Operative Complications. Journal of Personalized Medicine. 2023; 13(9):1297. https://doi.org/10.3390/jpm13091297
Chicago/Turabian StyleIacovazzo, Carmine, Pasquale Buonanno, Maria Massaro, Marilena Ianniello, Andrea Uriel de Siena, Maria Vargas, and Annachiara Marra. 2023. "Robot-Assisted versus Laparoscopic Gastrointestinal Surgery: A Systematic Review and Metanalysis of Intra- and Post-Operative Complications" Journal of Personalized Medicine 13, no. 9: 1297. https://doi.org/10.3390/jpm13091297
APA StyleIacovazzo, C., Buonanno, P., Massaro, M., Ianniello, M., de Siena, A. U., Vargas, M., & Marra, A. (2023). Robot-Assisted versus Laparoscopic Gastrointestinal Surgery: A Systematic Review and Metanalysis of Intra- and Post-Operative Complications. Journal of Personalized Medicine, 13(9), 1297. https://doi.org/10.3390/jpm13091297





