At-Home Blood Pressure Measurements Provide Better Assessments of Orthostatic Hypotension in Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Recruitment
2.2. Clinical Scales
2.3. Blood Pressure Recordings
- Attach the cuff to the arm, lie horizontal for 5 min, and then record BP;
- While still wearing the cuff, stand immediately and record the BP;
- Measure twice a day (on awakening and before arising and at night before retiring);
- Only perform measurements in the presence of a carer and sit or lie on the bed immediately if a risk of falling is perceived;
- After each reading, record the systolic and diastolic pressures on the provided chart. To avoid bias, PwPs were not informed about the meaning of the BP parameters they recorded.
2.4. Statistics
3. Results
3.1. Characteristics of Morning and Evening Systolic BP Readings
- Morning systolic lying pressures are higher than their evening pair in both PwP pairs (67%) and control pairs (75%). The difference between morning and evening systolic pressures was significant for both the PwPs (median difference = 6 mmHg, p < 0.0001—Wilcoxon matched pairs signed-rank test) and the controls (median difference = 4 mmHg, p < 0.01—Wilcoxon matched pairs signed-rank test);
- If the morning lying systolic BP was 20 mmHg higher than its evening pair, it was frequently hypertensive in PwPs (78%) but not controls (38%). On the other hand, when the evening lying systolic reading was the highest of the pair, the morning systolic was below 145 mmHg (80% of the PwPs and 98% of the controls).
3.2. Orthostatic Effects on Systolic BP
3.3. Measurement of ΔBP at Home Compared to the Clinic
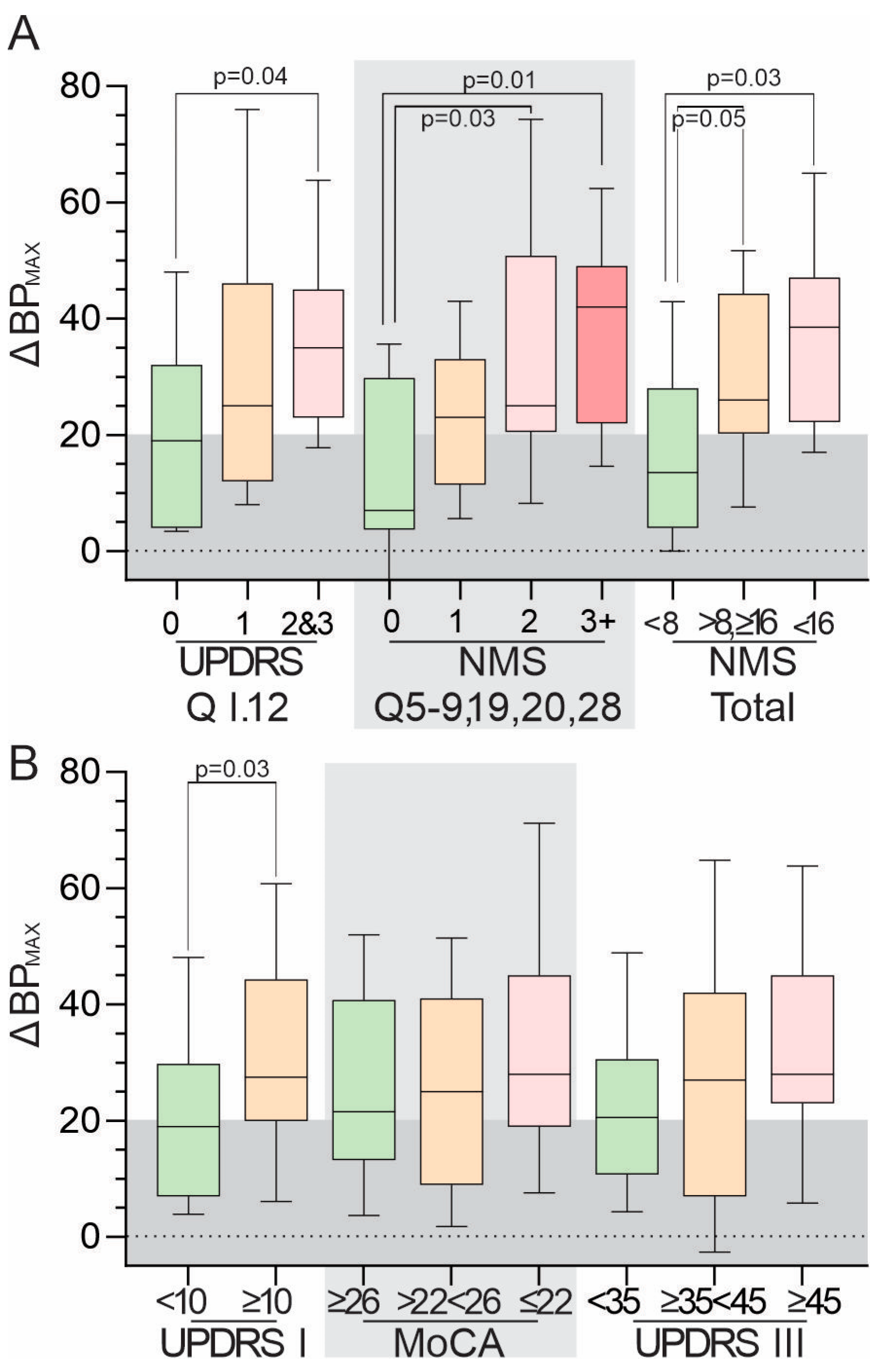
3.4. Relationship between ΔBPMAX and Scores from Clinical Scales
4. Discussion
Limitations of This Study
- Are 10 measures adequate or too few? Should measures at other times (e.g., postprandial) also be included?;
- What proportion of measures should be sufficient to identify OH: 50% (ΔBPMED), 33% (ΔBP75th), 10% (ΔBPMAX), 5%, or even less?;
- This study did not use the more stringent criteria for OH and systolic hypertension recommended by some authorities;
- This study excluded insulin-dependent diabetes and users of diuretics but not users of antihypertensive agents. However, because of the loss of ability to regulate vasodilation in the various vascular beds, it is these cases that introduce complexity to the management of OH in PD. Thus, future studies could examine the trade-off in treating hypertension in the presence of OH, especially when multiple measurements, such as those proposed here, are used;
- Although participants were trained to use the sphygmomanometers, we cannot exclude the possibility that some recordings were the result of poor technique or inaccurate recording. Poor technique might over-report hypotension and could also under-represent large postural drops. It is notable that very few systolic BP measures were less than 100 mmHg (Figure 1A);
- The sample size was large enough to show that at least one elevated ΔBP in 10 measurements is more likely to be found in PD than the controls. Larger samples would be required to address the dot points outlined above.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velseboer, D.C.; de Haan, R.J.; Wieling, W.; Goldstein, D.S.; de Bie, R.M. Prevalence of orthostatic hypotension in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2011, 17, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.; Kaufmann, H. Epidemiology, Diagnosis, and Management of Neurogenic Orthostatic Hypotension. Mov. Disord. Clin. Pract. 2017, 4, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.; Gomez-Esteban, J.C.; Norcliffe-Kaufmann, L.; Martinez, J.; Tijero, B.; Berganzo, K.; Kaufmann, H. Orthostatic hypotension in Parkinson disease: How much you fall or how low you go? Mov. Disord. 2015, 30, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Tipton, P.W.; Cheshire, W.P. Mechanisms underlying unawareness of neurogenic orthostatic hypotension. Clin. Auton. Res. 2020, 30, 279–281. [Google Scholar] [CrossRef]
- Wieling, W.; Kaufmann, H.; Claydon, V.E.; van Wijnen, V.K.; Harms, M.P.M.; Juraschek, S.P.; Thijs, R.D. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022, 21, 735–746. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Daya, N.; Rawlings, A.M.; Appel, L.J.; Miller, E.R., 3rd; Windham, B.G.; Griswold, M.E.; Heiss, G.; Selvin, E. Association of History of Dizziness and Long-term Adverse Outcomes with Early vs Later Orthostatic Hypotension Assessment Times in Middle-aged Adults. JAMA Intern. Med. 2017, 177, 1316–1323. [Google Scholar] [CrossRef]
- Finucane, C.; O’Connell, M.D.; Donoghue, O.; Richardson, K.; Savva, G.M.; Kenny, R.A. Impaired Orthostatic Blood Pressure Recovery Is Associated with Unexplained and Injurious Falls. J. Am. Geriatr. Soc. 2017, 65, 474–482. [Google Scholar] [CrossRef]
- Shaw, B.H.; Claydon, V.E. The relationship between orthostatic hypotension and falling in older adults. Clin. Auton. Res. 2014, 24, 3–13. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Daya, N.; Appel, L.J.; Miller, E.R., 3rd; Windham, B.G.; Pompeii, L.; Griswold, M.E.; Kucharska-Newton, A.; Selvin, E. Orthostatic Hypotension in Middle-Age and Risk of Falls. Am. J. Hypertens. 2017, 30, 188–195. [Google Scholar] [CrossRef]
- Tanaka, R.; Hattori, N. Abnormal circadian blood pressure regulation and cognitive impairment in alpha-synucleinopathies. Hypertens. Res. 2022, 45, 1908–1917. [Google Scholar] [CrossRef]
- Frewen, J.; Savva, G.M.; Boyle, G.; Finucane, C.; Kenny, R.A. Cognitive performance in orthostatic hypotension: Findings from a nationally representative sample. J. Am. Geriatr. Soc. 2014, 62, 117–122. [Google Scholar] [CrossRef]
- Frewen, J.; Finucane, C.; Savva, G.M.; Boyle, G.; Kenny, R.A. Orthostatic hypotension is associated with lower cognitive performance in adults aged 50 plus with supine hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 878–885. [Google Scholar] [CrossRef]
- O’Hare, C.; Kenny, R.A.; Aizenstein, H.; Boudreau, R.; Newman, A.; Launer, L.; Satterfield, S.; Yaffe, K.; Rosano, C.; Health, A.B.C.S. Cognitive Status, Gray Matter Atrophy, and Lower Orthostatic Blood Pressure in Older Adults. J. Alzheimers Dis. 2017, 57, 1239–1250. [Google Scholar] [CrossRef]
- Holm, H.; Nagga, K.; Nilsson, E.D.; Melander, O.; Minthon, L.; Bachus, E.; Fedorowski, A.; Magnusson, M. Longitudinal and postural changes of blood pressure predict dementia: The Malmo Preventive Project. Eur. J. Epidemiol. 2017, 32, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; McGarrigle, C.A.; Coen, R.F.; Soraghan, C.J.; Foran, T.; Lawlor, B.A.; Kenny, R.A. Orthostatic Blood Pressure Behavior in People with Mild Cognitive Impairment Predicts Conversion to Dementia. J. Am. Geriatr. Soc. 2015, 63, 1868–1873. [Google Scholar] [CrossRef]
- Kang, S.H.; Chung, S.J.; Lee, J.; Koh, S.B. Independent effect of neurogenic orthostatic hypotension on mild cognitive impairment in Parkinson’s disease. Clin. Auton. Res. 2022, 32, 43–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Ke, S.J.; Qiu, X.P.; Liu, L.B. Prevalence, risk factors, and prognosis of orthostatic hypotension in diabetic patients: A systematic review and meta-analysis. Medicine 2017, 96, e8004. [Google Scholar] [CrossRef] [PubMed]
- Lagro, J.; Schoon, Y.; Heerts, I.; Meel-van den Abeelen, A.S.; Schalk, B.; Wieling, W.; Olde Rikkert, M.G.; Claassen, J.A. Impaired systolic blood pressure recovery directly after standing predicts mortality in older falls clinic patients. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 471–478. [Google Scholar] [CrossRef]
- Fedorowski, A.; Stavenow, L.; Hedblad, B.; Berglund, G.; Nilsson, P.M.; Melander, O. Orthostatic hypotension predicts all-cause mortality and coronary events in middle-aged individuals (The Malmo Preventive Project). Eur. Heart J. 2010, 31, 85–91. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Newton, J.L.; Burn, D.J. Orthostatic hypotension and cognitive impairment in Parkinson’s disease: Causation or association? Mov. Disord. 2016, 31, 937–946. [Google Scholar] [CrossRef]
- Groothuis, J.T.; Esselink, R.A.; Seeger, J.P.; van Aalst, M.J.; Hopman, M.T.; Bloem, B.R. Lower vascular tone and larger plasma volume in Parkinson’s disease with orthostatic hypotension. J. Appl. Physiol. 2011, 111, 443–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacob, G.; Ertl, A.C.; Shannon, J.R.; Furlan, R.; Robertson, R.M.; Robertson, D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J. Appl. Physiol. 1998, 84, 914–921. [Google Scholar] [CrossRef]
- Sulzer, D.; Surmeier, D.J. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov. Disord. 2013, 28, 715–724. [Google Scholar] [CrossRef]
- Papapetropoulos, S.; Mash, D.C. Insular pathology in Parkinson’s disease patients with orthostatic hypotension. Park. Relat. Disord. 2007, 13, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Pavelic, A.; Krbot Skoric, M.; Crnosija, L.; Habek, M. Postprandial hypotension in neurological disorders: Systematic review and meta-analysis. Clin. Auton. Res. 2017, 27, 263–271. [Google Scholar] [CrossRef]
- Claydon, V.E.; Steeves, J.D.; Krassioukov, A. Orthostatic hypotension following spinal cord injury: Understanding clinical pathophysiology. Spinal Cord. 2006, 44, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Eldadah, B.A.; Holmes, C.; Pechnik, S.; Moak, J.; Saleem, A.; Sharabi, Y. Neurocirculatory abnormalities in Parkinson disease with orthostatic hypotension: Independence from levodopa treatment. Hypertension 2005, 46, 1333–1339. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Sharabi, Y. Baroreflex-sympathoneural dysfunction characterizes at-risk individuals with preclinical central Lewy body diseases. Clin. Auton. Res. 2023, 33, 41–49. [Google Scholar] [CrossRef]
- Kramer, H.H.; Lautenschlager, G.; de Azevedo, M.; Doppler, K.; Schanzer, A.; Best, C.; Oertel, W.H.; Reuter, I.; Sommer, C.; Birklein, F. Reduced central sympathetic activity in Parkinson’s disease. Brain Behav. 2019, 9, e01463. [Google Scholar] [CrossRef]
- Barbic, F.; Perego, F.; Canesi, M.; Gianni, M.; Biagiotti, S.; Costantino, G.; Pezzoli, G.; Porta, A.; Malliani, A.; Furlan, R. Early abnormalities of vascular and cardiac autonomic control in Parkinson’s disease without orthostatic hypotension. Hypertension 2007, 49, 120–126. [Google Scholar] [CrossRef]
- Senard, J.M.; Valet, P.; Durrieu, G.; Berlan, M.; Tran, M.A.; Montastruc, J.L.; Rascol, A.; Montastruc, P. Adrenergic supersensitivity in parkinsonians with orthostatic hypotension. Eur. J. Clin. Invest. 1990, 20, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, Y.; Goldstein, D.S. Mechanisms of orthostatic hypotension and supine hypertension in Parkinson disease. J. Neurol. Sci. 2011, 310, 123–128. [Google Scholar] [CrossRef]
- Cuenca-Bermejo, L.; Almela, P.; Navarro-Zaragoza, J.; Fernandez Villalba, E.; Gonzalez-Cuello, A.M.; Laorden, M.L.; Herrero, M.T. Cardiac Changes in Parkinson’s Disease: Lessons from Clinical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 13488. [Google Scholar] [CrossRef]
- Zhu, S.; Li, H.; Xu, X.; Luo, Y.; Deng, B.; Guo, X.; Guo, Y.; Yang, W.; Wei, X.; Wang, Q. The Pathogenesis and Treatment of Cardiovascular Autonomic Dysfunction in Parkinson’s Disease: What We Know and Where to Go. Aging Dis. 2021, 12, 1675–1692. [Google Scholar] [CrossRef]
- Mehagnoul-Schipper, D.J.; Boerman, R.H.; Hoefnagels, W.H.; Jansen, R.W. Effect of levodopa on orthostatic and postprandial hypotension in elderly Parkinsonian patients. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M749–M755. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Q.; Xu, E.; Zeng, J.; Li, Q.; Mei, S.; Hua, Y. Impaired Cerebral Autoregulation in Parkinson’s Disease: An Orthostatic Hypotension Analysis. Front. Neurol. 2022, 13, 811698. [Google Scholar] [CrossRef] [PubMed]
- Polverino, P.; Ajcevic, M.; Catalan, M.; Bertolotti, C.; Furlanis, G.; Marsich, A.; Buoite Stella, A.; Accardo, A.; Manganotti, P. Comprehensive telemedicine solution for remote monitoring of Parkinson’s disease patients with orthostatic hypotension during COVID-19 pandemic. Neurol. Sci. 2022, 43, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Vallelonga, F.; Di Stefano, C.; Merola, A.; Romagnolo, A.; Sobrero, G.; Milazzo, V.; Burrello, A.; Burrello, J.; Zibetti, M.; Veglio, F.; et al. Blood pressure circadian rhythm alterations in alpha-synucleinopathies. J. Neurol. 2019, 266, 1141–1152. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, H.A.; Peri-Okonny, P.A.; Schesing, K.; Phelps, K.; Ngo, C.; Evans, H.; Arbique, D.; Price, A.L.; Vernino, S.; Phillips, L.; et al. Usefulness of Blood Pressure Variability Indices Derived From 24-Hour Ambulatory Blood Pressure Monitoring in Detecting Autonomic Failure. J. Am. Heart Assoc. 2019, 8, e010161. [Google Scholar] [CrossRef]
- Freeman, R.; Illigens, B.M.W.; Lapusca, R.; Campagnolo, M.; Abuzinadah, A.R.; Bonyhay, I.; Sinn, D.I.; Miglis, M.; White, J.; Gibbons, C.H. Symptom Recognition Is Impaired in Patients With Orthostatic Hypotension. Hypertension 2020, 75, 1325–1332. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Martinez-Martin, P.; Schapira, A.H.; Stocchi, F.; Sethi, K.; Odin, P.; Brown, R.G.; Koller, W.; Barone, P.; MacPhee, G.; et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov. Disord. 2006, 21, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Fanciulli, A.; Jordan, J.; Biaggioni, I.; Calandra-Buonaura, G.; Cheshire, W.P.; Cortelli, P.; Eschlboeck, S.; Grassi, G.; Hilz, M.J.; Kaufmann, H.; et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin. Auton. Res. 2018, 28, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, H.; Palma, J.A. White Matter Hyperintensities in the Synucleinopathies: Orthostatic Hypotension, Supine Hypertension, or Both? Mov. Disord. Clin. Pract. 2020, 7, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Merola, A.; Romagnolo, A.; Rosso, M.; Lopez-Castellanos, J.R.; Wissel, B.D.; Larkin, S.; Bernardini, A.; Zibetti, M.; Maule, S.; Lopiano, L.; et al. Orthostatic hypotension in Parkinson’s disease: Does it matter if asymptomatic? Park. Relat. Disord. 2016, 33, 65–71. [Google Scholar] [CrossRef]

| Parameter | Control | PwD |
|---|---|---|
| Age | 69 (9) | 72 (8) |
| MoCA | 26 (3) | 24 (5) |
| Systolic BP | 128 (22) | 131 (25) |
| Diastolic BP | 74 (12) | 77 (14) |
| Disease Duration | 10 (6) | |
| UPDRS I | 11 (7) | |
| UPDRS II | 15 (12) | |
| UPDRS III | 40 (20) | |
| UPDRS IV | 6 (6) | |
| UPDRS Total | 60 (29) | |
| MDS_H&Y | 2 (1) | |
| OHSA TOTAL | 0 (3) | |
| OHDAS TOTAL | 0 (0) | |
| PDQ 39 | 21 (44.5) | |
| NMS TOTAL | 12 (10) | |
| Prior Diagnosis of OH | 1/16 (6%) | 12/44 (27%) |
| MDS-UPDRS Q1.12 Response | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Number (%) | 25 (57%) | 9 (21%) | 7 (16%) | 3 (7%) |
| Median ΔBPMAX | 21 (20.5) | 29 (35) | 35 (36) | 42 (41) |
| Median ΔBP75th | 9 (20) | 19 (25) | 26 (39) | 19 (26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, C.V.; Osborn, S.; Horne, M. At-Home Blood Pressure Measurements Provide Better Assessments of Orthostatic Hypotension in Parkinson’s Disease. J. Pers. Med. 2023, 13, 1324. https://doi.org/10.3390/jpm13091324
Fernando CV, Osborn S, Horne M. At-Home Blood Pressure Measurements Provide Better Assessments of Orthostatic Hypotension in Parkinson’s Disease. Journal of Personalized Medicine. 2023; 13(9):1324. https://doi.org/10.3390/jpm13091324
Chicago/Turabian StyleFernando, Chathurini V, Sarah Osborn, and Malcolm Horne. 2023. "At-Home Blood Pressure Measurements Provide Better Assessments of Orthostatic Hypotension in Parkinson’s Disease" Journal of Personalized Medicine 13, no. 9: 1324. https://doi.org/10.3390/jpm13091324
APA StyleFernando, C. V., Osborn, S., & Horne, M. (2023). At-Home Blood Pressure Measurements Provide Better Assessments of Orthostatic Hypotension in Parkinson’s Disease. Journal of Personalized Medicine, 13(9), 1324. https://doi.org/10.3390/jpm13091324







