A Novel Technique for Autograft Preparation Using Patient-Specific Instrumentation (PSI) Assistance in Total Hip Arthroplasty in Developmental Dysplasia of Hip (DDH)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Data
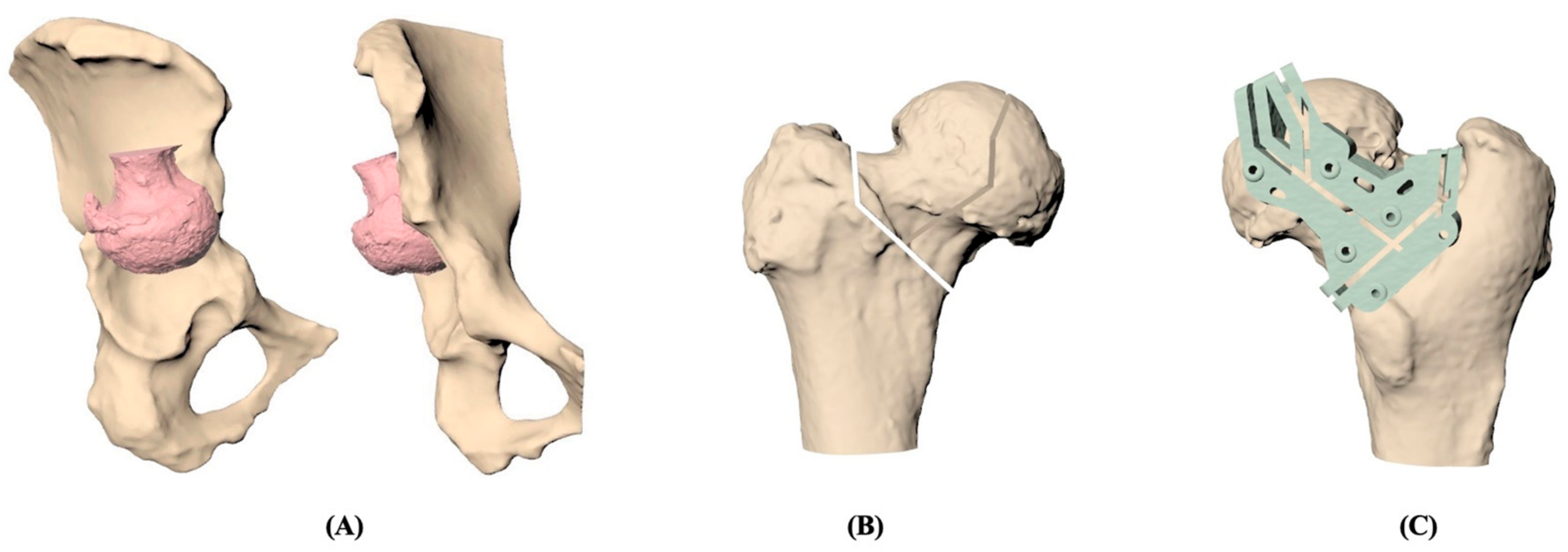
2.2. PSI Graft Preparation
2.3. Treatment Procedures
2.4. Postoperative Treatment
2.5. Outcome Assessment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaquero-Picado, A.; González-Morán, G.; Garay, E.G.; Moraleda, L. Developmental dysplasia of the hip: Update of management. EFORT Open Rev. 2019, 4, 548. [Google Scholar] [CrossRef] [PubMed]
- Swarup, I.; Penny, C.L.; Dodwell, E.R. Developmental dysplasia of the hip: An update on diagnosis and management from birth to 6 months. Curr. Opin. Pediatr. 2018, 30, 84–92. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Ren, X. Total hip arthroplasty for treatment of developmental dysplasia of the hip. Chin. J. Reparative Reconstr. Surg. 2006, 20, 647–650. [Google Scholar]
- Callanan, M.C.; Jarrett, B.; Bragdon, C.R.; Zurakowski, D.; Rubash, H.E.; Freiberg, A.A.; Malchau, H. The John Charnley Award: Risk factors for cup malpositioning: Quality improvement through a joint registry at a tertiary hospital. Clin. Orthop. Relat. Res. 2011, 469, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Thienpont, E.; Bellemans, J.; Delport, H.; Van Overschelde, P.; Stuyts, B.; Brabants, K.; Victor, J. Patient-specific instruments: Industry’s innovation with a surgeon’s interest. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 2227–2233. [Google Scholar] [CrossRef]
- Ast, M.P.; Nam, D.; Haas, S.B. Patient-specific instrumentation for total knee arthroplasty: A review. Orthop. Clin. 2012, 43, e17–e22. [Google Scholar] [CrossRef]
- Elnemr, M.A.; Hafez, M.A.; Aboelnasr, K.M.; Radwan, M.A. Patient-specific template shortens the operative time in total knee arthroplasty in comparison to the conventional technique. Curr. Orthop. Pract. 2016, 27, 187–191. [Google Scholar] [CrossRef]
- Thienpont, E.; Schwab, P.-E.; Fennema, P. Efficacy of patient-specific instruments in total knee arthroplasty: A systematic review and meta-analysis. JBJS 2017, 99, 521–530. [Google Scholar] [CrossRef]
- Stegman, J.; Casstevens, C.; Kelley, T.; Nistor, V. Patient-specific guides for total hip arthroplasty: A paired acetabular and femoral implantation approach. J. Med. Devices 2015, 9, 011006. [Google Scholar] [CrossRef]
- Sakai, T.; Hanada, T.; Murase, T.; Kitada, M.; Hamada, H.; Yoshikawa, H.; Sugano, N. Validation of patient specific surgical guides in total hip arthroplasty. Int. J. Med. Robot. Comput. Assist. Surg. 2014, 10, 113–120. [Google Scholar] [CrossRef]
- Hananouchi, T.; Giets, E.; Ex, J.; Delport, H. Patient-specific instrumentation for acetabular cup orientation: Accuracy analysis in a pre-clinical study. J. Contemp. Orthop. Res. 2014, 1, 35–47. [Google Scholar]
- Constantinescu, D.S.; Costello, J.P., II; Dalling, A.D.; Wagner, J.D.; Al-Hardan, W.; Carvajal, J.A. The efficacy of patient specific instrumentation (PSI) in total hip arthroplasty (THA): A systematic review and meta-analysis. J. Orthop. 2022, 34, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Sariali, E.; Mauprivez, R.; Khiami, F.; Pascal-Mousselard, H.; Catonné, Y. Accuracy of the preoperative planning for cementless total hip arthroplasty. A randomised comparison between three-dimensional computerised planning and conventional templating. Orthop. Traumatol. Surg. Res. 2012, 98, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Henckel, J.; Holme, T.J.; Radford, W.; Skinner, J.A.; Hart, A.J. 3D-printed patient-specific guides for hip arthroplasty. JAAOS-J. Am. Acad. Orthop. Surg. 2018, 26, e342–e348. [Google Scholar] [CrossRef]
- Spencer-Gardner, L.; Pierrepont, J.; Topham, M.; Baré, J.; McMahon, S.; Shimmin, A. Patient-specific instrumentation improves the accuracy of acetabular component placement in total hip arthroplasty. Bone Jt. J. 2016, 98, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Loder, R.T.; Skopelja, E.N. The epidemiology and demographics of hip dysplasia. Int. Sch. Res. Not. 2011, 2011, 1–46. [Google Scholar] [CrossRef]
- Den, H.; Ito, J.; Kokaze, A. Epidemiology of developmental dysplasia of the hip: Analysis of Japanese national database. J. Epidemiol. 2023, 33, 186–192. [Google Scholar] [CrossRef]
- Pulik, Ł.; Płoszka, K.; Romaniuk, K.; Sibilska, A.; Jedynak, A.; Tołwiński, I.; Kumięga, P.; Wojtyński, P.; Łęgosz, P. Impact of multiple factors on the incidence of developmental dysplasia of the hip: Risk assessment tool. Medicina 2022, 58, 1158. [Google Scholar] [CrossRef]
- Kremli, M.K.; Alshahid, A.H.; Khoshhal, K.I.; Zamzam, M.M. The pattern of developmental dysplasia of the hip. Saudi Med. J. 2003, 24, 1118–1120. [Google Scholar]
- Guille, J.T.; Pizzutillo, P.D.; MacEwen, G.D. Developmental dysplasia of the hip from birth to six months. JAAOS-J. Am. Acad. Orthop. Surg. 2000, 8, 232–242. [Google Scholar] [CrossRef]
- Ömerog˘lu, H.; Koparal, S. The role of clinical examination and risk factors in the diagnosis of developmental dysplasia of the hip: A prospective study in 188 referred young infants. Arch. Orthop. Trauma Surg. 2001, 121, 7–11. [Google Scholar] [CrossRef]
- Agostiniani, R.; Atti, G.; Bonforte, S.; Casini, C.; Cirillo, M.; De Pellegrin, M.; Di Bello, D.; Esposito, F.; Galla, A.; Marrè Brunenghi, G. Recommendations for early diagnosis of Developmental Dysplasia of the Hip (DDH): Working group intersociety consensus document. Ital. J. Pediatr. 2020, 46, 150. [Google Scholar] [CrossRef] [PubMed]
- Noordin, S.; Umer, M.; Hafeez, K.; Nawaz, H. Developmental dysplasia of the hip. Orthop. Rev. 2010, 2, e19. [Google Scholar]
- Kotlarsky, P.; Haber, R.; Bialik, V.; Eidelman, M. Developmental dysplasia of the hip: What has changed in the last 20 years? World J. Orthop. 2015, 6, 886. [Google Scholar] [CrossRef] [PubMed]
- Nunley, R.M.; Prather, H.; Hunt, D.; Schoenecker, P.L.; Clohisy, J.C. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. JBJS 2011, 93 (Suppl. 2), 17–21. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.R.; Murtha, A.S.; Clohisy, J.C.; Group, A.S. Developmental dysplasia of the hip in adolescents and young adults. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J.; Trousdale, R.T.; Berry, D.J.; Cabanela, M.E. Surgical treatment of developmental dysplasia of the hip in adults: I. Nonarthroplasty options. JAAOS-J. Am. Acad. Orthop. Surg. 2002, 10, 321–333. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J.; Berry, D.J.; Trousdale, R.T.; Cabanela, M.E. Surgical treatment of developmental dysplasia of the hip in adults: II. Arthroplasty options. JAAOS-J. Am. Acad. Orthop. Surg. 2002, 10, 334–344. [Google Scholar] [CrossRef]
- Kosuge, D.; Yamada, N.; Azegami, S.; Achan, P.; Ramachandran, M. Management of developmental dysplasia of the hip in young adults: Current concepts. Bone Jt. J. 2013, 95, 732–737. [Google Scholar] [CrossRef]
- Thawrani, D.; Sucato, D.J.; Podeszwa, D.A.; DeLaRocha, A. Complications associated with the Bernese periacetabular osteotomy for hip dysplasia in adolescents. JBJS 2010, 92, 1707–1714. [Google Scholar] [CrossRef]
- Rosenstein, A.D.; Diaz, R.J. Challenges and solutions for total hip arthroplasty in treatment of patients with symptomatic sequelae of developmental dysplasia of the hip. Am. J. Orthop. 2011, 40, 87–91. [Google Scholar]
- Bradley, C.S.; Perry, D.C.; Wedge, J.H.; Murnaghan, M.; Kelley, S.P. Avascular necrosis following closed reduction for treatment of developmental dysplasia of the hip: A systematic review. J. Child. Orthop. 2016, 10, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Scheerlinck, T. Cup positioning in total hip arthroplasty. Acta Orthop. Belg. 2014, 80, 336–347. [Google Scholar] [PubMed]
- Kunutsor, S.K.; Barrett, M.C.; Beswick, A.D.; Judge, A.; Blom, A.W.; Wylde, V.; Whitehouse, M.R. Risk factors for dislocation after primary total hip replacement: A systematic review and meta-analysis of 125 studies involving approximately five million hip replacements. Lancet Rheumatol. 2019, 1, e111–e121. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, D.; Klinkenbijl, M.N.; Somford, M.P.; Albers, G.R.; van der Vis, H.M. Obesity in total hip arthroplasty—Does it really matter? A meta-analysis. Acta Orthop. 2011, 82, 417–422. [Google Scholar] [CrossRef]
- Woolson, S.T.; Rahimtoola, Z.O. Risk factors for dislocation during the first 3 months after primary total hip replacement. J. Arthroplast. 1999, 14, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.J.; Ghadimi, E.; Ardakani, M.V.; Razzaghof, M.; Ghasemi, M.A.; Nili, A.; Vafaei, A.; Moharrami, A.; Rasta, S. Risk factors of dislocation after total hip arthroplasty in patients with developmental dysplasia of the hip. Int. Orthop. 2022, 46, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Lewinnek, G.E.; Lewis, J.; Tarr, R.; Compere, C.; Zimmerman, J. Dislocations after total hip-replacement arthroplasties. JBJS 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, H.; Yang, W.; Wang, L.; Hu, Y.; Liu, H.; Zhong, D. Accuracy and practicability of a patient-specific guide using acetabular superolateral rim during THA in Crowe II/III DDH patients: A retrospective study. J. Orthop. Surg. Res. 2019, 14, 19. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Hu, Y.; Liu, H.; Wang, L.; Xie, J.; Xiao, H.; Su, S.; Gao, F.; Zhong, D. Patient-specific total hip arthroplasty is superior to conventional methods for Crowe III and IV adult developmental hip dysplasia: A randomized controlled trial. Ann. Transl. Med. 2021, 9, 212. [Google Scholar] [CrossRef]
- Toner, R.W.; Pizzi, L.; Leas, B.; Ballas, S.K.; Quigley, A.; Goldfarb, N.I. Costs to hospitals of acquiring and processing blood in the US: A survey of hospital-based blood banks and transfusion services. Appl. Health Econ. Health Policy 2011, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kuldanek, S.A.; Kelher, M.; Silliman, C.C. Risk factors, management and prevention of transfusion-related acute lung injury: A comprehensive update. Expert Rev. Hematol. 2019, 12, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sihler, K.C.; Napolitano, L.M. Complications of Massive Transfusion. Chest 2010, 137, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Rosencher, N.; Kerkkamp, H.E.; Macheras, G.; Munuera, L.; Menichella, G.; Barton, D.M.; Cremers, S.; Abraham, I.L. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: Blood management in elective knee and hip arthroplasty in Europe. Transfusion 2003, 43, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Ampuero, J.; de Dios, M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties? Clin. Orthop. Relat. Res. 2010, 468, 3268–3277. [Google Scholar] [CrossRef]
- Rudy, M.D.; Bentley, J.; Ahuja, N.; Rohatgi, N. Determinants of cost variation in total hip and knee arthroplasty: Implications for alternative payment models. JAAOS-J. Am. Acad. Orthop. Surg. 2020, 28, e245–e254. [Google Scholar] [CrossRef]
- Garbarino, L.J.; Gold, P.A.; Sodhi, N.; Anis, H.K.; Ehiorobo, J.O.; Boraiah, S.; Danoff, J.R.; Rasquinha, V.J.; Higuera-Rueda, C.A.; Mont, M.A. The effect of operative time on in-hospital length of stay in revision total knee arthroplasty. Ann. Transl. Med. 2019, 7, 66. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, C.; Zhong, D.; Lei, P.; Hu, Y.; Su, S. Effect of patient-specific instrument on lowering threshold for junior physicians to perform total hip arthroplasty on developmental dysplasia of the hip patients. Int. Orthop. 2020, 44, 1281–1286. [Google Scholar] [CrossRef]
- Xing, Q.Q.; Zhong, D.; Pan, Y.X.; An, S.B.; Wang, C.G.; Su, S.L.; Wang, L.; Hu, Y.H. A Comparative Study of Patients’ Subjective Feelings Toward Total Hip Arthroplasty with Patient-Specific Instruments and Traditional Total Hip Arthroplasty. Orthop. Surg. 2020, 12, 269–276. [Google Scholar] [CrossRef]
- Lai, K.-A. Developmental Dysplasia and Dislocation of the Hip in Adults; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]


| No. | Sex | Age (Years Old) | Height (cm) | Body Weight (Kg) | BMI | Primary Complaint | Diagnosis | Crowe’s Classification | Comorbidity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | 161 | 56 | 21.6 | Pain, weakness | Left DDH * with advanced OA | Crowe I | Leiomyoma, leiomyoma-related anemia |
| 2 | F | 59 | 147.8 | 59 | 27 | Pain, weakness | Right DDH * with advanced OA | Crowe I | Left leg poliomyelitis |
| 3 | F | 43 | 160 | 53 | 20.7 | Pain, weakness, limited ROM | Left DDH * with advanced OA | Crowe I | Eczema |
| 4 | F | 40 | 160 | 62 | 24.2 | Pain, limping gait | Left DDH * with advanced OA | Crowe I | None |
| 5 | F | 35 | 166 | 59 | 21.4 | Pain, weakness, limited ROM | Right DDH * with advanced OA | Crowe I | None |
| 6 | F | 69 | 154 | 52 | 21.9 | Pain, limping, | Left DDH * with advanced OA | Crowe I | None |
| No. | Sex | Operation Time (min) | Blood Loss (mL) | Anesthesia | Length of Hospitalization (Day) | Blood Transfusion (U) | Pre- Operation Hb (g/dL) | Post- Operation Hb (g/dL) | MD * (g/dL) | Complication |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 220 | 1250 | General anesthesia | 5 | 2U | 9.6 | 8.9 | 0.7 | No complication |
| 2 | 190 | 350 | 7 | X | 15.1 | 11.8 | 3.3 | |||
| 3 | 225 | 450 | 5 | X | 13.1 | 10.5 | 2.6 | |||
| 4 | 237 | 500 | 6 | X | 13.5 | 10.6 | 2.9 | |||
| 5 | 245 | 850 | 7 | 2U | 12.4 | 11.1 | 1.3 | |||
| 6 | 210 | 1000 | 6 | 2U | 13.6 | 11.5 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-R.; Chou, H.; Luo, C.-A.; Chang, S.-H. A Novel Technique for Autograft Preparation Using Patient-Specific Instrumentation (PSI) Assistance in Total Hip Arthroplasty in Developmental Dysplasia of Hip (DDH). J. Pers. Med. 2023, 13, 1331. https://doi.org/10.3390/jpm13091331
Lin C-R, Chou H, Luo C-A, Chang S-H. A Novel Technique for Autograft Preparation Using Patient-Specific Instrumentation (PSI) Assistance in Total Hip Arthroplasty in Developmental Dysplasia of Hip (DDH). Journal of Personalized Medicine. 2023; 13(9):1331. https://doi.org/10.3390/jpm13091331
Chicago/Turabian StyleLin, Chun-Ru, Hsuan Chou, Chu-An Luo, and Shu-Hao Chang. 2023. "A Novel Technique for Autograft Preparation Using Patient-Specific Instrumentation (PSI) Assistance in Total Hip Arthroplasty in Developmental Dysplasia of Hip (DDH)" Journal of Personalized Medicine 13, no. 9: 1331. https://doi.org/10.3390/jpm13091331





