SARS-CoV-2 Infection, Vaccination and Risk of Death in People with An Oncological Disease in Northeast Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Study Design
2.2. Data Sources and Linkage Procedures
2.3. Data Analyses
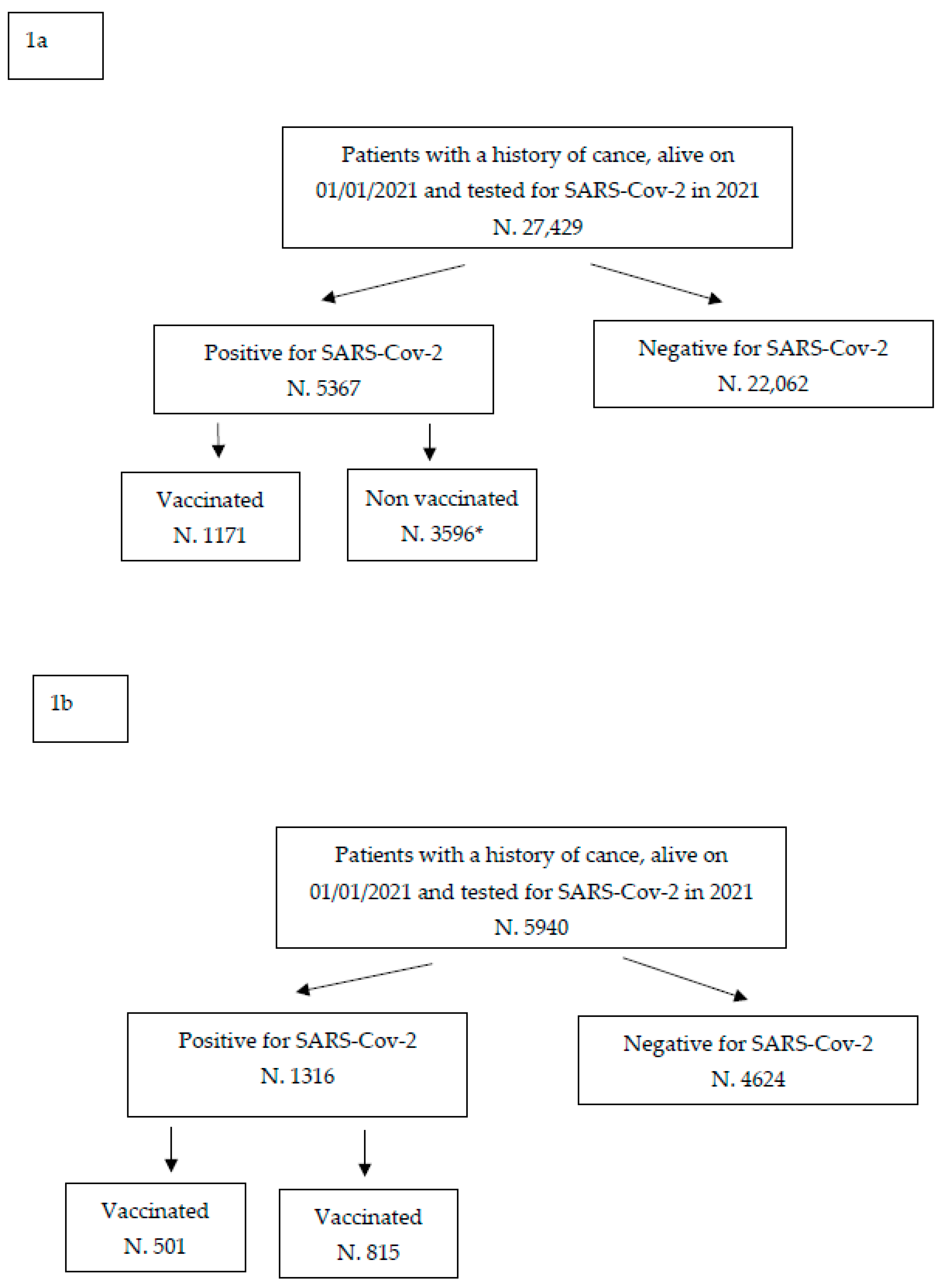
3. Results
3.1. Frequency of Deaths among Vaccinated and Unvaccinated
3.2. Multivariate Analysis of Deaths among Unvaccinated vs. Vaccinated Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.-Y.; Shyr, Y.; Li, X.; Choueiri, T.; Painter, C.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787–800. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918.e2. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Tabernero, J.; Bower, M.; Scotti, L.; Patel, M.; Colomba, E.; Dolly, S.; Loizidou, A.; Chester, P.J.; Mukherjee, U.; et al. Prevalence and impact of COVId-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: Evidence from the OnCovid retrospective, multicentre registry study. Lancet Oncol. 2021, 22, 1668–1680. [Google Scholar] [CrossRef]
- Pinato, D.J.; Aguilar-Company, J.; Ferrante, D.; Hanbury, G.; Bower, M.; Salazar, R.; Mirallas, O.; Sureda, A.; Plaja, A.; Cucurull, M.; et al. Outcomes of the SARS-CoV-2 omicron (B.1.1.529) variant outbreak among vaccinated and unvaccinated patients with cancer in Europe: Results from the retrospective, multicentre, OnCovid registry study. Lancet Oncol. 2022, 23, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Mangone, L.; Gioia, F.; Mancuso, P.; Bisceglia, I.; Ottone, M.; Vicentini, M.; Pinto, C.; Rossi, P.G. CumulativeCOVID-19 incidence, mortality and prognosis in cancer survivors: A population-based study in Reggio Emilia, Northern Italy. Int. J. Cancer 2021, 149, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Qiu, X.; Wang, C.; Zhao, J.; Jiang, X.; Niu, W.; Huang, J.; Zhang, F. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int. J. Cancer 2020, 148, 363–374. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Ferroni, E.; Rossi, P.G.; Alegiani, S.S.; Trifirò, G.; Pitter, G.; Leoni, O.; Cereda, D.; Marino, M.; Pellizzari, M.; Fabiani, M.; et al. Survival of Hospitalized COVID-19 Patients in Northern Italy: A Population-Based Cohort Study by the ITA-COVID-19 Network. Clin. Epidemiol. 2020, 12, 1337–1346. [Google Scholar] [CrossRef]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Byrne, F.; Cerrone, M.; Schmitt, A.M.; Joharatnam-Hogan, N.; Shum, B.; et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: The CAPTURE study. Nat. Cancer 2021, 2, 1305–1320. [Google Scholar] [CrossRef]
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutiérrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 160, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Carle, C.; Hughes, S.; Freeman, V.; Campbell, D.; Egger, S.; Caruana, M.; Hui, H.; Yap, S.; Deandrea, S.; Onyeka, T.C.; et al. The risk of contracting SARS-CoV-2 or developing COVID-19 for people with cancer: A systematic review of the early evidence. J. Cancer Policy 2022, 33, 100338. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Branco, L.; Cervantes, A.; Curigliano, G.; Garassino, M.C.; Giesen, N.; Grivas, P.; Haanen, J.; Jordan, K.; Gerd Liebert, U.; Lordick, F.; et al. ESMO Statements on Vaccination against COVID-19 in People with Cancer. Available online: https://www.esmo.org/covid-19-and-cancer/covid-19-vaccination (accessed on 7 August 2023).
- Mauri, D.; Kamposioras, K.; Tsali, L.; Dambrosio, M.; De Bari, B.; Hindi, N.; Salembier, C.; Nixon, J.; Dimitrios, T.; Alongi, F.; et al. COVID-19 Vaccinations: Summary Guidance for Cancer Patients in 28 Languages: Breaking Barriers to Cancer Patient Information. Rev. Recent Clin. Trials 2022, 17, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Serraino, D.; Zucchetto, A.; Maso, L.D.; Del Zotto, S.; Taboga, F.; Clagnan, E.; Fratino, L.; Tosolini, F.; Burba, I. Prevalence, determinants, and outcomes of SARS-COV-2 infection among cancer patients. A population-based study in northern Italy. Cancer Med. 2021, 10, 7781–7792. [Google Scholar] [CrossRef]
- Rugge, M.; Zorzi, M.; Guzzinati, S. SARS-CoV-2 infection in the Italian Veneto region: Adverse outcomes in patients with cancer. Nat. Cancer 2020, 1, 784–788. [Google Scholar] [CrossRef]
- Available online: https://www.aiom.it/documento-aiom-cipomo-comu-vaccinazione-covid-19-per-i-pazienti-oncologici/ (accessed on 7 August 2023).
- Calagnan, E.; Gobbato, M.; Burba, I.; Del Zotto, S.; Toffolutti, F.; Serraino, D.; Tonutti, G. COVID-19 infections in the Friuli Venezia Giulia Region (Northern Italy): A population-based retrospective analysis. Epidemiol. Prev. 2021, 44 (Suppl. S2), 323–329. [Google Scholar]
- Vicentini, M.; Venturelli, F.; Mancuso, P.; Bisaccia, E.; Zerbini, A.; Massari, M.; Cossarizza, A.; De Biasi, S.; Pezzotti, P.; Bedeschi, E.; et al. Risk of SARS-CoV-2 reinfection by vaccination status, predominant variant and time from prior infection: A cohort study, Reggio Emilia province, Italy, February 2020 to February 2022. Eurosurveillance 2023, 28, 2200494. [Google Scholar] [CrossRef]
- Gobbato, M.; Clagnan, E.; Toffolutti, F.; Del Zotto, S.; Burba, I.; Tosolini, F.; Polimeni, J.; Serraino, D.; Taborelli, M. Vaccination against SARS-CoV-2 and risk of hospital admission and death among infected cancer patients: A population-based study in northern Italy. Cancer Epidemiol. 2023, 82, 102318. [Google Scholar] [CrossRef]
- Li, H.-J.; Yang, Q.-C.; Yao, Y.-Y.; Huang, C.-Y.; Yin, F.-Q.; Xian-Yu, C.-Y.; Zhang, C.; Chen, S.-J. COVID-19 vaccination effectiveness and safety in vulnerable populations: A meta-analysis of 33 observational studies. Front. Pharmacol. 2023, 14, 1144824. [Google Scholar] [CrossRef]
- Shear, S.; Shams, K.; Weisberg, J.; Hamidi, N.; Scott, S. COVID-19 Vaccination Safety Profiles in Patients With Solid Tumour Cancers: A Systematic Review. Clin. Oncol. 2023, 35, e421–e433. [Google Scholar] [CrossRef]
- Oosting, S.F.; Van der Veldt, A.A.M.; GeurtsvanKessel, C.H.; Fehrmann, R.S.N.; van Binnendijk, R.S.; Dingemans, A.-M.C.; Smit, E.F.; Hiltermann, T.J.N.; Hartog, G.D.; Jalving, M.; et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: A prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021, 22, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Au, L.; Shepherd, S.T.C.; Byrne, F.; Cerrone, M.; Boos, L.A.; Rzeniewicz, K.; Gordon, W.; Shum, B.; Gerard, C.L.; et al. Functional antibody and T-cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: The CAPTURE study. Res Sq. 2021, 3, rs-916427, Update in: Nat. Cancer 2021, 2, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Puopolo, M.; Morciano, C.; Spuri, M.; Alegiani, S.S.; Filia, A.; D’ancona, F.; Del Manso, M.; Riccardo, F.; Tallon, M.; et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe COVID-19 during predominant circulation of the delta variant in Italy: Retrospective cohort study. BMJ 2022, 376, e069052. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Urdiales, A.; Alegiani, S.S.; Fabiani, M.; Pezzotti, P.; Filia, A.; Massari, M.; Riccardo, F.; Tallon, M.; Proietti, V.; Del Manso, M.; et al. Risk of SARS-CoV-2 infection and subsequent hospital admission and death at different time intervals since first dose of COVID-19 vaccine administration, Italy, 27 December 2020 to mid-April 2021. Eurosurveillance 2021, 26, 2100507. [Google Scholar] [CrossRef]
- Kahn, F.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Björk, J. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities—Surveillance results from southern Sweden, July 2021 to January 2022. Eurosurveillance 2022, 27, 2200121. [Google Scholar] [CrossRef] [PubMed]
- Coviello, V.; Buzzoni, C.; Fusco, M.; Barchielli, A.; Cuccaro, F.; De Angelis, R.; Giacomin, A.; Luminari, S.; Randi, G.; Mangone, L.; et al. Survival of cancer patients in Italy. Epidemiol. Prev. 2017, 41 (Suppl. S1), 1–244. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, E.; Dorrucci, M.; Minelli, G.; Lasinio, G.J.; Prati, S.; Battaglini, M.; Corsetti, G.; Bella, A.; Boros, S.; Petrone, D.; et al. Assessing COVID-19-Related Excess Mortality Using Multiple Approaches—Italy, 2020–2021. Int. J. Environ. Res. Public Health 2022, 19, 16998. [Google Scholar] [CrossRef]
- Tagliamento, M.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Saini, K.S.; de Azambuja, E.; Punie, K.; Westphalen, C.B.; Morgan, G.; Pronzato, P.; et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast cancers: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103365. [Google Scholar] [CrossRef]
| Positive for SARS-CoV-2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Friuli Venezia Giulia | Reggio Emilia | |||||||
| Vaccinated | Not Vaccinated | Vaccinated | Not Vaccinated | |||||
| Total | Deaths | Total | Deaths | Total | Deaths | Total | Deaths | |
| N | N (Row %) | N | N (Row %) | N | N (Row %) | N | N (Row %) | |
| All | 1771 | 102 (5.8) | 3596 | 595 (16.6) | 501 | 24 (4.8) | 815 | 79 (9.7) |
| Sex | ||||||||
| Male | 858 | 64 (7.5) | 1665 | 346 (20.8) | 237 | 9 (3.8) | 341 | 40 (11.7) |
| Female | 913 | 38 (4.2) | 1931 | 249 (12.9) | 264 | 15 (5.7) | 474 | 39 (8.2) |
| Age at infection (years) | ||||||||
| <40 | 56 | 0 (0.0) | 145 | 1 (0.7) | 43 | 0 (0.0) | 67 | 0 (0.0) |
| 40–59 | 364 | 3 (0.8) | 869 | 25 (2.9) | 124 | 1 (0.8) | 289 | 6 (2.1) |
| 60–69 | 285 | 6 (2.1) | 710 | 61 (8.6) | 88 | 2 (2.3) | 149 | 4 (2.7) |
| 70–79 | 522 | 19 (3.6) | 1017 | 187 (18.4) | 134 | 3 (2.2) | 178 | 22 (12.4) |
| ≥80 | 544 | 74 (13.6) | 855 | 321 (37.5) | 112 | 18 (16.1) | 131 | 47 (35.9) |
| Time since cancer diagnosis | ||||||||
| <1 year | 168 | 14 (8.3) | 354 | 102 (28.8) | 40 | 2 (5.0) | 75 | 16 (21.3) |
| 1–2 years | 128 | 5 (3.9) | 298 | 59 (19.8) | 41 | 0 (0.0) | 87 | 8 (9.2) |
| 2–5 years | 365 | 21 (5.8) | 735 | 95 (12.9) | 136 | 7 (5.2) | 221 | 9 (4.1) |
| >5 years | 1110 | 62 (5.6) | 2209 | 339 (15.4) | 284 | 15 (5.3) | 432 | 46 (10.6) |
| Tumor site | ||||||||
| Solid tumors | 1614 | 89 (5.5) | 3275 | 535 (16.3) | 440 | 19 (4.3) | 847 | 130 (15.3) |
| Breast | 415 | 13 (3.1) | 882 | 84 (9.5) | 118 | 4 (3.4) | 222 | 14 (6.3) |
| Prostate | 284 | 20 (7.0) | 539 | 109 (20.2) | 54 | 3 (5.6) | 77 | 9 (11.7) |
| Colorectal | 236 | 21 (8.9) | 434 | 85 (19.6) | 53 | 2 (3.8) | 67 | 10 (14.9) |
| Skin Melanoma | 148 | 6 (4.1) | 287 | 18 (6.3) | 42 | 2 (4.8) | 53 | 2 (3.8) |
| Lung and larynx | 73 | 4 (5.5) | 180 | 67 (37.2) | 12 | 0 (0.0) | 34 | 9 (26.5) |
| Thyroid | 91 | 1 (1.1) | 155 | 10 (6.5) | 43 | 0 (0.0) | 74 | 1 (1.4) |
| Kidney | 63 | 3 (4.8) | 141 | 19 (13.5) | 11 | 2 (18.2) | 29 | 1 (3.4) |
| Bladder | 48 | 5 (10.4) | 93 | 25 (26.9) | 34 | 3 (8.8) | 44 | 5 (11.4) |
| Endometrium | 47 | 1 (2.1) | 107 | 13 (12.2) | 15 | 0 (0.0) | 27 | 8 (29.6) |
| Other solid tumours | 209 | 15 (7.2) | 457 | 105 (23.0) | 58 | 3 (5.2) | 109 | 19 (17.4) |
| Hematological malignancies | 157 | 13 (8.3) | 321 | 60 (18.7) | 61 | 5 (8.2) | 79 | 1 (1.3) |
| Non-Hodgkin lymphoma | 73 | 5 (6.9) | 165 | 38 (23.0) | 24 | 2 (8.3) | 38 | 1 (2.6) |
| Leukaemia | 42 | 4 (9.5) | 65 | 12 (18.5) | 20 | 2 (10.0) | 28 | 0 (0.0) |
| Hodgkin’s lymphoma | 21 | 2 (9.5) | 51 | 2 (3.9) | 7 | 0 (0.0) | 7 | 0 (0.0) |
| Multiple myeloma | 21 | 2 (9.5) | 40 | 8 (20.0) | 10 | 1 (10.0) | 6 | 0 (0.0) |
| Friuli Venezia | Reggio Emilia | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Giulia | |||||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||||
| All | 2.7 | 2.2 | 3.4 | 8.2 | 5.1 | 13.2 | 3.4 | 2.9 | 4.1 |
| Sex | |||||||||
| Male | 2.7 | 2.1 | 3.6 | 12.1 | 6 | 24.5 | 3.4 | 2.8 | 4.3 |
| Female | 2.7 | 1.9 | 3.8 | 3.1 | 1.7 | 5.4 | 2.8 | 2.0 | 3.9 |
| Age at infection (years) | |||||||||
| <40 | - | - | - | - | - | - | - | - | - |
| 40–59 | 1.8 | 0.52 | 6 | 4.2 | 0.46 | 37.9 | 2.1 | 0.8 | 6.3 |
| 60–69 | 2.4 | 1 | 5.6 | 5.9 | 1 | 33.4 | 2.8 | 1.4 | 6.0 |
| 70–79 | 3.7 | 2.3 | 5.9 | 19.3 | 5.7 | 65.7 | 4.6 | 3.2 | 6.8 |
| ≥80 | 2.5 | 2 | 3.3 | 6.5 | 3.6 | 11.6 | 3.1 | 2.6 | 3.9 |
| Time since cancer diagnosis | |||||||||
| <1 year | 2.7 | 1.5 | 4.7 | 20.7 | 4 | 10.7 | 8.5 | 7.3 | 10.5 |
| 1–2 years | 4.5 | 1.8 | 11.3 | Infinite | - | - | 5.3 | 2.6 | 12.1 |
| 2–5 years | 2.4 | 1.5 | 3.8 | 5 | 1.9 | 13.6 | 2.8 | 1.9 | 4.2 |
| >5 years | 2.7 | 2.1 | 3.6 | 6.6 | 3.6 | 12.1 | 3.3 | 2.7 | 4.2 |
| Tumor site | |||||||||
| Solid tumors | 2.6 | 2.2 | 3.5 | 9.1 | 5.4 | 15.2 | 3.4 | 3.0 | 4.3 |
| Breast | 3 | 1.6 | 5.3 | 7.5 | 2.1 | 26.6 | 3.6 | 2.2 | 5.9 |
| Prostate | 2.8 | 1.7 | 4.6 | 8.2 | 2 | 34.1 | 3.2 | 2.1 | 5.0 |
| Colorectal | 2.3 | 1.4 | 3.7 | 18.2 | 3.5 | 95.5 | 2.7 | 1.8 | 4.1 |
| Skin Melanoma | 1.4 | 0.54 | 3.8 | 1.5 | 0.17 | 13.5 | 1.4 | 0.6 | 3.8 |
| Lung and larynx | 5.1 | 1.8 | 14.1 | Infinite | - | - | 5.8 | 2.5 | 14.8 |
| Thyiroid | 6.5 | 0.8 | 51 | - | - | - | 7.0 | 1.3 | 51.5 |
| Kidney | 2.2 | 0.6 | 7.5 | 0.46 | 0.04 | 5.6 | 1.2 | 0.1 | 6.5 |
| Bladder | 1.2 | 0.43 | 3.3 | 20.6 | 1.5 | 275 | 1.4 | 0.63 | 3.5 |
| Endometrium | 6.1 | 0.77 | 48.6 | Infinite | - | - | 10.0 | 4.67 | 52.5 |
| Other solid cancers | 3 | 1.7 | 5.2 | 12.4 | 3.2 | 48.8 | 3.7 | 2.4 | 5.9 |
| Haematologic Malignancies | 2.4 | 1.3 | 4.4 | - | - | - | 2.2 | 1.1 | 4.2 |
| Friuli Venezia | Reggio Emilia | |||
|---|---|---|---|---|
| Giulia | ||||
| 1-Year NS | 5-Year NS | 1-Year NS | 5-Year NS | |
| Tumor site | ||||
| Breast | 97 | 90 | 99 | 92 |
| Prostate | 98 | 94 | 98 | 88 |
| Colorectal | 82 | 64 | 84 | 64 |
| Skin Melanoma | 97 | 91 | 99 | 91 |
| Lung | 44 | 17 | 41 | 16 |
| Thyroid | 96 | 94 | 94 | 92 |
| Kidney | 83 | 72 | 84 | 65 |
| Bladder | 90 | 77 | 90 | 76 |
| Corpus Uteri | 93 | 79 | 91 | 78 |
| Non-Hodgkin lymphomas | 82 | 70 | 88 | 72 |
| Leukemias | 66 | 42 | 76 | 57 |
| All sites, but skin non-melanoma | 77 | 62 | 78 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangone, L.; Giorgi Rossi, P.; Taborelli, M.; Toffolutti, F.; Mancuso, P.; Dal Maso, L.; Gobbato, M.; Clagnan, E.; Del Zotto, S.; Ottone, M.; et al. SARS-CoV-2 Infection, Vaccination and Risk of Death in People with An Oncological Disease in Northeast Italy. J. Pers. Med. 2023, 13, 1333. https://doi.org/10.3390/jpm13091333
Mangone L, Giorgi Rossi P, Taborelli M, Toffolutti F, Mancuso P, Dal Maso L, Gobbato M, Clagnan E, Del Zotto S, Ottone M, et al. SARS-CoV-2 Infection, Vaccination and Risk of Death in People with An Oncological Disease in Northeast Italy. Journal of Personalized Medicine. 2023; 13(9):1333. https://doi.org/10.3390/jpm13091333
Chicago/Turabian StyleMangone, Lucia, Paolo Giorgi Rossi, Martina Taborelli, Federica Toffolutti, Pamela Mancuso, Luigino Dal Maso, Michele Gobbato, Elena Clagnan, Stefania Del Zotto, Marta Ottone, and et al. 2023. "SARS-CoV-2 Infection, Vaccination and Risk of Death in People with An Oncological Disease in Northeast Italy" Journal of Personalized Medicine 13, no. 9: 1333. https://doi.org/10.3390/jpm13091333
APA StyleMangone, L., Giorgi Rossi, P., Taborelli, M., Toffolutti, F., Mancuso, P., Dal Maso, L., Gobbato, M., Clagnan, E., Del Zotto, S., Ottone, M., Bisceglia, I., Neri, A., & Serraino, D. (2023). SARS-CoV-2 Infection, Vaccination and Risk of Death in People with An Oncological Disease in Northeast Italy. Journal of Personalized Medicine, 13(9), 1333. https://doi.org/10.3390/jpm13091333








