Antibody–Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives
Abstract
:1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Selection of Sources of Evidence
2.4. Data Items and Synthesis of Results
3. Results
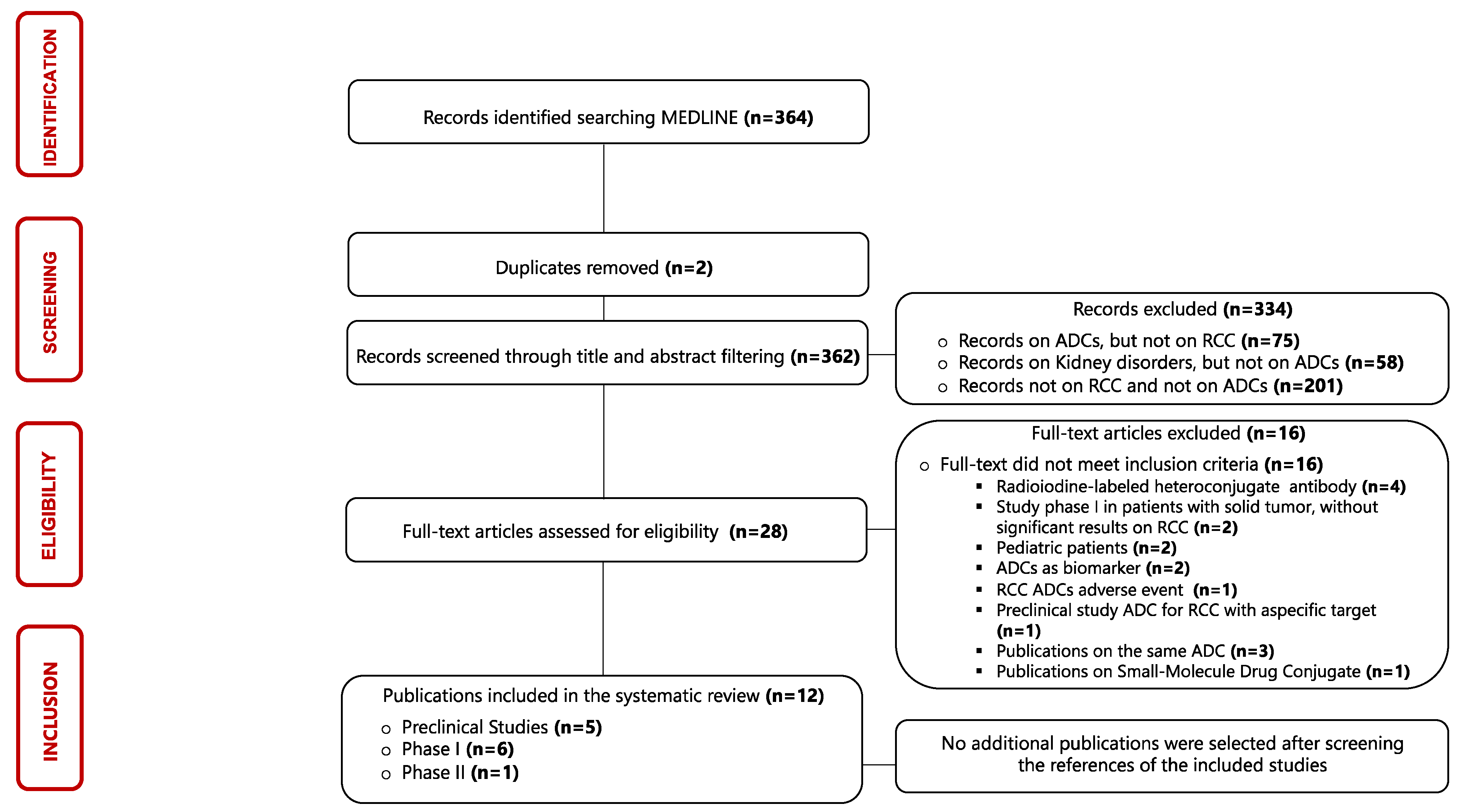
3.1. Selection of Sources of Evidence
3.2. Synthesis of Results
3.3. Preclinical Studies
3.3.1. L49 and L6
3.3.2. HKT288
3.3.3. 138H11
3.3.4. 1F6-vcAFP and 1F6-vcMMAF
3.3.5. h1F6
3.4. Phase I Clinical Trials
3.4.1. SGN-75 Anti-CD70
3.4.2. CDX-014 Anti-Immunoglobulin Mucin-1 TIM-1
3.4.3. SGN-CD70A Anti-CD70
3.4.4. MDX-1203 Anti-CD70
3.4.5. AMG 172 Anti-CD70
3.4.6. AGS-16M8F and AGS-16C3F Anti-Phosphodiesterases-Pyrophosphatase 3 (ENPP3)
3.5. Phase II Clinical Trials
AGS-16C3F versus Axitinib
4. Discussion
4.1. Summary of Evidence
4.2. Summary of Evidence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-Dose Interleukin-2 for the Treatment of Metastatic Renal Cell Carcinoma: A Retrospective Analysis of Response and Survival in Patients Treated in the Surgery Branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Amato, R. Modest Effect of Interferon Alfa on Metastatic Renal-Cell Carcinoma. Lancet 1999, 353, 6–7. [Google Scholar] [CrossRef]
- Rassy, E.; Flippot, R.; Albiges, L. Tyrosine Kinase Inhibitors and Immunotherapy Combinations in Renal Cell Carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907504. [Google Scholar] [CrossRef]
- Huang, J.J.; Hsieh, J.J. The Therapeutic Landscape of Renal Cell Carcinoma: From the Dark Age to the Golden Age. Semin. Nephrol. 2020, 40, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Demasure, S.; Spriet, I.; Debruyne, P.R.; Laenen, A.; Wynendaele, W.; Baldewijns, M.; Dumez, H.; Clement, P.M.; Wildiers, H.; Schöffski, P.; et al. Overall Survival Improvement in Patients with Metastatic Clear-Cell Renal Cell Carcinoma between 2000 and 2020: A Retrospective Cohort Study. Acta Oncol. 2022, 61, 22–29. [Google Scholar] [CrossRef]
- Chakiryan, N.H.; Jiang, D.D.; Gillis, K.A.; Green, E.; Hajiran, A.; Hugar, L.; Zemp, L.; Zhang, J.; Jain, R.K.; Chahoud, J.; et al. Real-World Survival Outcomes Associated With First-Line Immunotherapy, Targeted Therapy, and Combination Therapy for Metastatic Clear Cell Renal Cell Carcinoma. JAMA Netw. Open 2021, 4, e2111329. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Tarantino, P.; Carmagnani Pestana, R.; Corti, C.; Modi, S.; Bardia, A.; Tolaney, S.M.; Cortes, J.; Soria, J.-C.; Curigliano, G. Antibody-Drug Conjugates: Smart Chemotherapy Delivery across Tumor Histologies. CA Cancer J. Clin. 2022, 72, 165–182. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and Challenges for the next Generation of Antibody-Drug Conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the Potential of Antibody-Drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Chrétien, M.-L.; Casasnovas, R.-O. Antibody-Drug Conjugates for the Treatment of Hematological Malignancies: A Comprehensive Review. Target. Oncol. 2018, 13, 287–308. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Brown, S. Antibody-Drug Conjugates as Novel Anti-Cancer Chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Staudacher, A.H.; Brown, M.P. Antibody Drug Conjugates and Bystander Killing: Is Antigen-Dependent Internalisation Required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel Anti-B-Cell Maturation Antigen Antibody-Drug Conjugate (GSK2857916) Selectively Induces Killing of Multiple Myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; González-Martín, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (InnovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Lorusso, D.; Oaknin, A.; Pignata, S.; Dean, A.; Denys, H.; Colombo, N.; Van Gorp, T.; Konner, J.A.; Marin, M.R.; et al. Efficacy and Safety of Mirvetuximab Soravtansine in Patients With Platinum-Resistant Ovarian Cancer With High Folate Receptor Alpha Expression: Results From the SORAYA Study. J. Clin. Oncol. 2023, 41, 2436–2445. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Svensson, H.P.; Frank, I.S.; Berry, K.K.; Senter, P.D. Therapeutic Effects of Monoclonal Antibody-Beta-Lactamase Conjugates in Combination with a Nitrogen Mustard Anticancer Prodrug in Models of Human Renal Cell Carcinoma. J. Med. Chem. 1998, 41, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Bialucha, C.U.; Collins, S.D.; Li, X.; Saxena, P.; Zhang, X.; Dürr, C.; Lafont, B.; Prieur, P.; Shim, Y.; Mosher, R.; et al. Discovery and Optimization of HKT288, a Cadherin-6-Targeting ADC for the Treatment of Ovarian and Renal Cancers. Cancer Discov. 2017, 7, 1030–1045. [Google Scholar] [CrossRef]
- Knoll, K.; Wrasidlo, W.; Scherberich, J.E.; Gaedicke, G.; Fischer, P. Targeted Therapy of Experimental Renal Cell Carcinoma with a Novel Conjugate of Monoclonal Antibody 138H11 and Calicheamicin ThetaI1. Cancer Res. 2000, 60, 6089–6094. [Google Scholar] [PubMed]
- Law, C.-L.; Gordon, K.A.; Toki, B.E.; Yamane, A.K.; Hering, M.A.; Cerveny, C.G.; Petroziello, J.M.; Ryan, M.C.; Smith, L.; Simon, R.; et al. Lymphocyte Activation Antigen CD70 Expressed by Renal Cell Carcinoma Is a Potential Therapeutic Target for Anti-CD70 Antibody-Drug Conjugates. Cancer Res. 2006, 66, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Oflazoglu, E.; Stone, I.J.; Gordon, K.; Wood, C.G.; Repasky, E.A.; Grewal, I.S.; Law, C.-L.; Gerber, H.-P. Potent Anticarcinoma Activity of the Humanized Anti-CD70 Antibody H1F6 Conjugated to the Tubulin Inhibitor Auristatin via an Uncleavable Linker. Clin. Cancer Res. 2008, 14, 6171–6180. [Google Scholar] [CrossRef]
- Tannir, N.M.; Forero-Torres, A.; Ramchandren, R.; Pal, S.K.; Ansell, S.M.; Infante, J.R.; de Vos, S.; Hamlin, P.A.; Kim, S.K.; Whiting, N.C.; et al. Phase I Dose-Escalation Study of SGN-75 in Patients with CD70-Positive Relapsed/Refractory Non-Hodgkin Lymphoma or Metastatic Renal Cell Carcinoma. Investig. New Drugs 2014, 32, 1246–1257. [Google Scholar] [CrossRef]
- McGregor, B.A.; Gordon, M.; Flippot, R.; Agarwal, N.; George, S.; Quinn, D.I.; Rogalski, M.; Hawthorne, T.; Keler, T.; Choueiri, T.K. Safety and Efficacy of CDX-014, an Antibody-Drug Conjugate Directed against T Cell Immunoglobulin Mucin-1 in Advanced Renal Cell Carcinoma. Investig. New Drugs 2020, 38, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Forero-Torres, A.; Thompson, J.A.; Morris, J.C.; Chhabra, S.; Hoimes, C.J.; Vogelzang, N.J.; Boyd, T.; Bergerot, P.G.; Adashek, J.J.; et al. A Phase 1 Trial of SGN-CD70A in Patients with CD70-Positive, Metastatic Renal Cell Carcinoma. Cancer 2019, 125, 1124–1132. [Google Scholar] [CrossRef]
- Owonikoko, T.K.; Hussain, A.; Stadler, W.M.; Smith, D.C.; Kluger, H.; Molina, A.M.; Gulati, P.; Shah, A.; Ahlers, C.M.; Cardarelli, P.M.; et al. First-in-Human Multicenter Phase I Study of BMS-936561 (MDX-1203), an Antibody-Drug Conjugate Targeting CD70. Cancer Chemother. Pharmacol. 2016, 77, 155–162. [Google Scholar] [CrossRef]
- Massard, C.; Soria, J.-C.; Krauss, J.; Gordon, M.; Lockhart, A.C.; Rasmussen, E.; Upreti, V.V.; Patel, S.; Ngarmchamnanrith, G.; Henary, H. First-in-Human Study to Assess Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the Anti-CD27L Antibody-Drug Conjugate AMG 172 in Patients with Relapsed/Refractory Renal Cell Carcinoma. Cancer Chemother. Pharmacol. 2019, 83, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Motzer, R.J.; Molina, A.M.; Choueiri, T.K.; Heath, E.I.; Redman, B.G.; Sangha, R.S.; Ernst, D.S.; Pili, R.; Kim, S.K.; et al. Phase I Trials of Anti-ENPP3 Antibody-Drug Conjugates in Advanced Refractory Renal Cell Carcinomas. Clin. Cancer Res. 2018, 24, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Kollmannsberger, C.; Choueiri, T.K.; Heng, D.Y.C.; George, S.; Jie, F.; Croitoru, R.; Poondru, S.; Thompson, J.A. A Randomized Phase II Study of AGS-16C3F Versus Axitinib in Previously Treated Patients with Metastatic Renal Cell Carcinoma. Oncologist 2021, 26, 182-e361. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, R.G.; Brown, J.P.; Yeh, M.Y.; Hellström, I.; Hellström, K.E. Identification of a Cell Surface Protein, P97, in Human Melanomas and Certain Other Neoplasms. Proc. Natl. Acad. Sci. USA 1980, 77, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Mazahreh, R.; Mason, M.L.; Gosink, J.J.; Olson, D.J.; Thurman, R.; Hale, C.; Westendorf, L.; Pires, T.A.; Leiske, C.I.; Carlson, M.; et al. SGN-CD228A Is an Investigational CD228-Directed Antibody-Drug Conjugate with Potent Antitumor Activity across a Wide Spectrum of Preclinical Solid Tumor Models. Mol. Cancer Ther. 2023, 22, 421–434. [Google Scholar] [CrossRef]
- Dunn, L.L.; Sekyere, E.O.; Suryo Rahmanto, Y.; Richardson, D.R. The Function of Melanotransferrin: A Role in Melanoma Cell Proliferation and Tumorigenesis. Carcinogenesis 2006, 27, 2157–2169. [Google Scholar] [CrossRef]
- Inoue, Y.U.; Asami, J.; Inoue, T. Cadherin-6 Gene Regulatory Patterns in the Postnatal Mouse Brain. Mol. Cell Neurosci. 2008, 39, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Halbleib, J.M.; Nelson, W.J. Cadherins in Development: Cell Adhesion, Sorting, and Tissue Morphogenesis. Genes. Dev. 2006, 20, 3199–3214. [Google Scholar] [CrossRef]
- Köbel, M.; Kalloger, S.E.; Boyd, N.; McKinney, S.; Mehl, E.; Palmer, C.; Leung, S.; Bowen, N.J.; Ionescu, D.N.; Rajput, A.; et al. Ovarian Carcinoma Subtypes Are Different Diseases: Implications for Biomarker Studies. PLoS Med. 2008, 5, e232. [Google Scholar] [CrossRef]
- Paul, R.; Ewing, C.M.; Robinson, J.C.; Marshall, F.F.; Johnson, K.R.; Wheelock, M.J.; Isaacs, W.B. Cadherin-6, a Cell Adhesion Molecule Specifically Expressed in the Proximal Renal Tubule and Renal Cell Carcinoma. Cancer Res. 1997, 57, 2741–2748. [Google Scholar] [CrossRef]
- Schöffski, P.; Concin, N.; Suarez, C.; Subbiah, V.; Ando, Y.; Ruan, S.; Wagner, J.P.; Mansfield, K.; Zhu, X.; Origuchi, S.; et al. A Phase 1 Study of a CDH6-Targeting Antibody-Drug Conjugate in Patients with Advanced Solid Tumors with Evaluation of Inflammatory and Neurological Adverse Events. Oncol. Res. Treat. 2021, 44, 547–556. [Google Scholar] [CrossRef]
- Bansal, A.; Sanchez, D.J.; Nimgaonkar, V.; Sanchez, D.; Riscal, R.; Skuli, N.; Simon, M.C. Gamma-Glutamyltransferase 1 Promotes Clear Cell Renal Cell Carcinoma Initiation and Progression. Mol. Cancer Res. 2019, 17, 1881–1892. [Google Scholar] [CrossRef]
- Brugnoni, D.; Airò, P.; Marino, R.; Notarangelo, L.D.; van Lier, R.A.; Cattaneo, R. CD70 Expression on T-Cell Subpopulations: Study of Normal Individuals and Patients with Chronic Immune Activation. Immunol. Lett. 1997, 55, 99–104. [Google Scholar] [CrossRef]
- Junker, K.; Hindermann, W.; von Eggeling, F.; Diegmann, J.; Haessler, K.; Schubert, J. CD70: A New Tumor Specific Biomarker for Renal Cell Carcinoma. J. Urol. 2005, 173, 2150–2153. [Google Scholar] [CrossRef]
- McEarchern, J.A.; Oflazoglu, E.; Francisco, L.; McDonagh, C.F.; Gordon, K.A.; Stone, I.; Klussman, K.; Turcott, E.; van Rooijen, N.; Carter, P.; et al. Engineered Anti-CD70 Antibody with Multiple Effector Functions Exhibits in Vitro and in Vivo Antitumor Activities. Blood 2007, 109, 1185–1192. [Google Scholar] [CrossRef]
- Claus, C.; Riether, C.; Schürch, C.; Matter, M.S.; Hilmenyuk, T.; Ochsenbein, A.F. CD27 Signaling Increases the Frequency of Regulatory T Cells and Promotes Tumor Growth. Cancer Res. 2012, 72, 3664–3676. [Google Scholar] [CrossRef]
- Han, W.K.; Alinani, A.; Wu, C.-L.; Michaelson, D.; Loda, M.; McGovern, F.J.; Thadhani, R.; Bonventre, J.V. Human Kidney Injury Molecule-1 Is a Tissue and Urinary Tumor Marker of Renal Cell Carcinoma. J. Am. Soc. Nephrol. 2005, 16, 1126–1134. [Google Scholar] [CrossRef]
- Tiberghien, A.C.; Vijayakrishnan, B.; Esfandiari, A.; Ahmed, M.; Pardo, R.; Bingham, J.; Adams, L.; Santos, K.; Kang, G.-D.; Pugh, K.M.; et al. Comparison of Pyrrolobenzodiazepine Dimer Bis-Imine versus Mono-Imine: DNA Interstrand Cross-Linking, Cytotoxicity, Antibody-Drug Conjugate Efficacy and Toxicity. Mol. Cancer Ther. 2023, 22, 254–263. [Google Scholar] [CrossRef]
- Doñate, F.; Raitano, A.; Morrison, K.; An, Z.; Capo, L.; Aviña, H.; Karki, S.; Morrison, K.; Yang, P.; Ou, J.; et al. AGS16F Is a Novel Antibody Drug Conjugate Directed against ENPP3 for the Treatment of Renal Cell Carcinoma. Clin. Cancer Res. 2016, 22, 1989–1999. [Google Scholar] [CrossRef]
- Tsai, S.H.; Kinoshita, M.; Kusu, T.; Kayama, H.; Okumura, R.; Ikeda, K.; Shimada, Y.; Takeda, A.; Yoshikawa, S.; Obata-Ninomiya, K.; et al. The Ectoenzyme E-NPP3 Negatively Regulates ATP-Dependent Chronic Allergic Responses by Basophils and Mast Cells. Immunity 2015, 42, 279–293. [Google Scholar] [CrossRef]
- Korekane, H.; Park, J.Y.; Matsumoto, A.; Nakajima, K.; Takamatsu, S.; Ohtsubo, K.; Miyamoto, Y.; Hanashima, S.; Kanekiyo, K.; Kitazume, S.; et al. Identification of Ectonucleotide Pyrophosphatase/Phosphodiesterase 3 (ENPP3) as a Regulator of N-Acetylglucosaminyltransferase GnT-IX (GnT-Vb). J. Biol. Chem. 2013, 288, 27912–27926. [Google Scholar] [CrossRef]
- Navani, V.; Heng, D.Y.C. Treatment Selection in First-Line Metastatic Renal Cell Carcinoma-The Contemporary Treatment Paradigm in the Age of Combination Therapy: A Review. JAMA Oncol. 2022, 8, 292–299. [Google Scholar] [CrossRef]
- Tran, J.; Ornstein, M.C. Clinical Review on the Management of Metastatic Renal Cell Carcinoma. JCO Oncol. Pract. 2022, 18, 187–196. [Google Scholar] [CrossRef]
- Iannantuono, G.M.; Riondino, S.; Sganga, S.; Roselli, M.; Torino, F. Activity of ALK Inhibitors in Renal Cancer with ALK Alterations: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 3995. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; de Velasco, G.; ESMO Guidelines Committee. ESMO Clinical Practice Guideline Update on the Use of Immunotherapy in Early Stage and Advanced Renal Cell Carcinoma. Ann. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef]
- Kim, I.-H.; Lee, H.J. The Frontline Immunotherapy-Based Treatment of Advanced Clear Cell Renal Cell Carcinoma: Current Evidence and Clinical Perspective. Biomedicines 2022, 10, 251. [Google Scholar] [CrossRef]
- Iacovelli, R.; Ciccarese, C.; Procopio, G.; Astore, S.; Cannella, M.A.; Maratta, M.G.; Rizzo, M.; Verzoni, E.; Porta, C.; Tortora, G. Current Evidence for Second-Line Treatment in Metastatic Renal Cell Carcinoma after Progression to Immune-Based Combinations. Cancer Treat. Rev. 2022, 105, 102379. [Google Scholar] [CrossRef]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-Drug Conjugates Come of Age in Oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef]
- Tarantino, P.; Ricciuti, B.; Pradhan, S.M.; Tolaney, S.M. Optimizing the Safety of Antibody-Drug Conjugates for Patients with Solid Tumours. Nat. Rev. Clin. Oncol. 2023, 20, 558–576. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef]
- Heist, R.S.; Guarino, M.J.; Masters, G.; Purcell, W.T.; Starodub, A.N.; Horn, L.; Scheff, R.J.; Bardia, A.; Messersmith, W.A.; Berlin, J.; et al. Therapy of Advanced Non-Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; VanderWeele, D.J. Antibody-Drug Conjugates and Predictive Biomarkers in Advanced Urothelial Carcinoma. Front. Oncol. 2022, 12, 1069356. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Hayashi, T.; Hinata, N. Current Status and Future Prospects of Antibody-Drug Conjugates in Urological Malignancies. Int. J. Urol. 2022, 29, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Scribner, J.A.; Brown, J.G.; Son, T.; Chiechi, M.; Li, P.; Sharma, S.; Li, H.; De Costa, A.; Li, Y.; Chen, Y.; et al. Preclinical Development of MGC018, a Duocarmycin-Based Antibody-Drug Conjugate Targeting B7-H3 for Solid Cancer. Mol. Cancer Ther. 2020, 19, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Khera, E.; Cilliers, C.; Smith, M.D.; Ganno, M.L.; Lai, K.C.; Keating, T.A.; Kopp, A.; Nessler, I.; Abu-Yousif, A.O.; Thurber, G.M. Quantifying ADC Bystander Payload Penetration with Cellular Resolution Using Pharmacodynamic Mapping. Neoplasia 2021, 23, 210–221. [Google Scholar] [CrossRef]
- Schettini, F.; Barbao, P.; Brasó-Maristany, F.; Galván, P.; Martínez, D.; Paré, L.; De Placido, S.; Prat, A.; Guedan, S. Identification of Cell Surface Targets for CAR-T Cell Therapies and Antibody-Drug Conjugates in Breast Cancer. ESMO Open 2021, 6, 100102. [Google Scholar] [CrossRef]
- García-Alonso, S.; Ocaña, A.; Pandiella, A. Resistance to Antibody-Drug Conjugates. Cancer Res. 2018, 78, 2159–2165. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Sapra, P.; Loganzo, F.; May, C. Combining Antibody-Drug Conjugates and Immune-Mediated Cancer Therapy: What to Expect? Biochem. Pharmacol. 2016, 102, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maruani, A. Bispecifics and Antibody-Drug Conjugates: A Positive Synergy. Drug Discov. Today Technol. 2018, 30, 55–61. [Google Scholar] [CrossRef]
- Zhou, Q. Site-Specific Antibody Conjugation for ADC and Beyond. Biomedicines 2017, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.E.; Gonzalez, J.C.; Pearson, C.I.; Gregorio, J.D.; Hartmann, F.J.; Kenkel, J.A.; Luo, A.; Ho, P.Y.; LeBlanc, H.; Kimmey, S.C.; et al. Immune-Stimulating Antibody Conjugates Elicit Robust Myeloid Activation and Durable Anti-Tumor Immunity. Nat. Cancer 2021, 2, 18–33. [Google Scholar] [CrossRef]
- Nicolò, E.; Giugliano, F.; Ascione, L.; Tarantino, P.; Corti, C.; Tolaney, S.M.; Cristofanilli, M.; Curigliano, G. Combining Antibody-Drug Conjugates with Immunotherapy in Solid Tumors: Current Landscape and Future Perspectives. Cancer Treat. Rev. 2022, 106, 102395. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 Pathway Blockade for Cancer Therapy: Mechanisms, Response Biomarkers, and Combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Friedlander, T.W.; Milowsky, M.I.; Bilen, M.A.; Srinivas, S.; McKay, R.R.; Flaig, T.W.; Hoimes, C.J.; Balar, A.V.; Henry, E.; Petrylak, D.P.; et al. Study EV-103: Update on Durability Results and Long Term Outcome of Enfortumab Vedotin + Pembrolizumab in First Line Locally Advanced or Metastatic Urothelial Carcinoma (La/MUC). JCO 2021, 39, 4528. [Google Scholar] [CrossRef]
- Schwach, J.; Abdellatif, M.; Stengl, A. More than Toxins-Current Prospects in Designing the Next Generation of Antibody Drug Conjugates. Front. Biosci. 2022, 27, 240. [Google Scholar] [CrossRef] [PubMed]
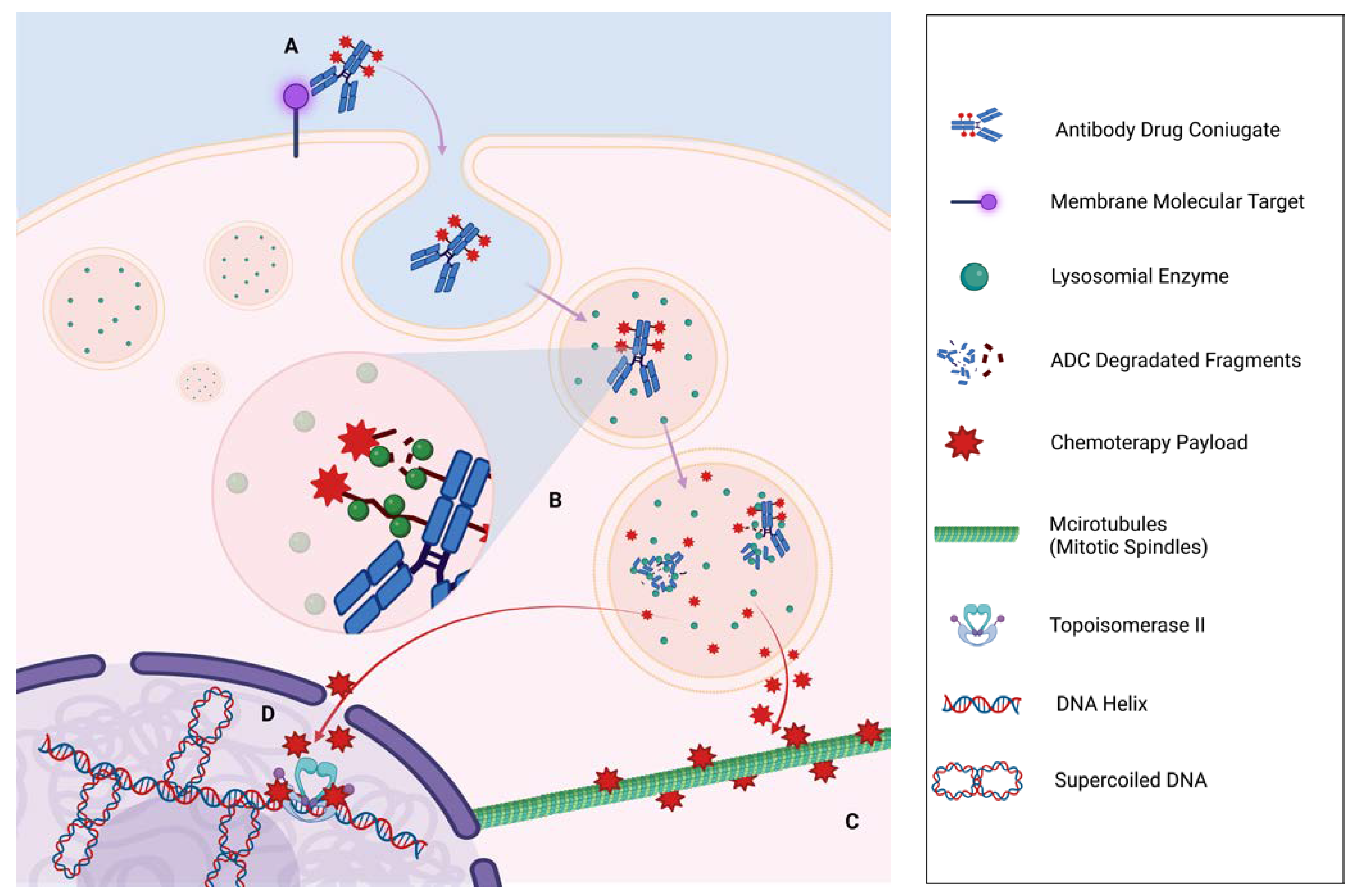
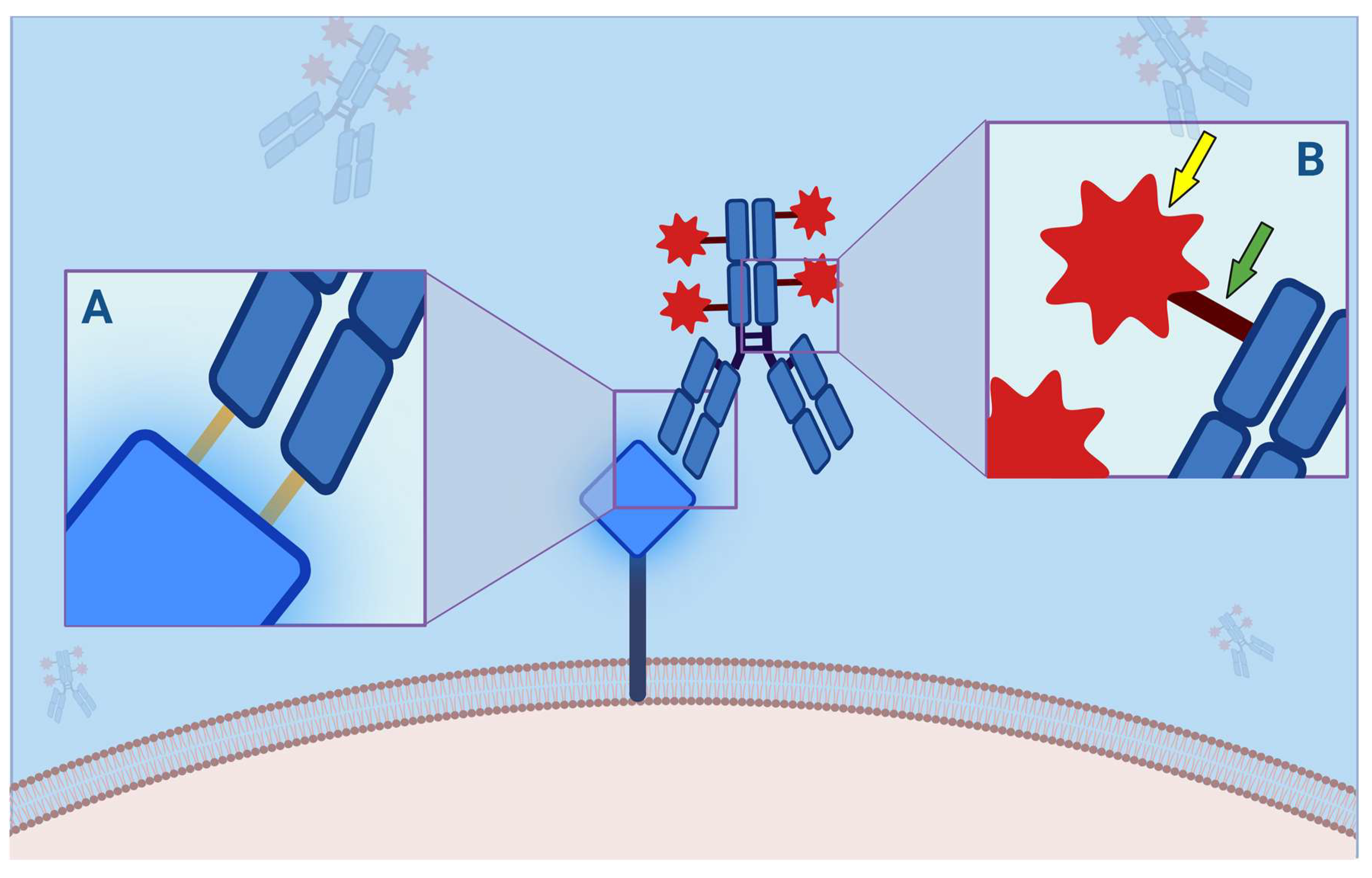
| Approval | ||||||||
|---|---|---|---|---|---|---|---|---|
| ADC | Trade Name (Pharma. Industry) | Target Antigen | Linkers | Payloads | FDA | EMA | Indications | Clinical Trial (NCT) |
| Ado-trastuzumab Emtansine [21,22,23] | Kadcyla (Roche) | HER-2 | SMCC | DM1 | X | X | Adjuvant treatment for HER-2 positive eBC patients with residual invasive disease after neoadjuvant taxane and trastuzumab-based treatment | KATHERINE Trial (NCT01772472) |
| X | X | Locally advanced or metastatic HER-2 positive BC previously treated with taxane and trastuzumab | EMILIA Trial (NCT00829166) | |||||
| X | - | Unresectable or metastatic HER2-positive BC | DESTINY-Breast03 (NCT03529110) | |||||
| Enfortumab vedotin [24] | Padcev (Seagen) | Nectin-4 | mc-VC-PABC | MMAE | X | X | Locally advanced or mUC that have been previously treated with platinum chemotherapy and a PD-L1/PD-1 inhibitor | EV-301 Trial (NCT03474107) |
| Fam-trastuzumab Deruxtecan [25,26] | Enhertu (Daiichi Sankyo) | HER-2 | Tetrapeptide | DXd | X | X | Unresectable or HER-2 positive mBC previously treated with two or more anti-HER2-based regimens | DESTINY-Breast01 (NCT03248492) |
| X | - | Locally advanced or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma who have received a prior trastuzumab-based regimen | DESTINY-Gastric01 (NCT03329690) | |||||
| Sacituzumab govitecan [27,28] | Trodelvy (Immunomedics) | Trop-2 | CL2A | SN38 | X | X | Unresectable, locally advanced, or metastatic TNBC that have received two or more prior systemic therapies, at least one of them for metastatic disease | ASCENT (NCT02574455) |
| X | - | Locally advanced or mUC that previously received a platinum-containing chemotherapy and either a PD-1 or a PD-L1 inhibitor | TROPHY-U-01 (NCT03547973) | |||||
| Tisotumab vedotin [29] | Tivdak (Genmab/Seagen) | TF | mc-VC-PABC | MMAE | X | - | Recurrent or metastatic cervical cancer progressed on or after chemotherapy | innovaTV 204/GOG-3023/ENGOT-cx6 (NCT03438396) |
| Mirvetuximab soravtansine-gynx [30] | Elahere (ImmunoGen) | folate receptor alpha | sulfo-SPDB | DM4 | X | - | Metastatic ovarian cancer that previously received platinum-based chemotherapy and have received 1 to 3 prior types of chemotherapy | MIRASOL trial (NCT04209855) |
| ADC | Phase | mAb | Linker (Cleavable/Non-Cleavable) | Payload | Target | NCT |
|---|---|---|---|---|---|---|
| L49 L6 [32] | Preclinical | IgG2a IgG1 | Valine-citrulline (CL) | CCM/CCM | p97 | - |
| HKT288 [33] | Preclinical | IgG1 | Sulfo-SPDB (CL) | DM4 | CDH6 | - |
| 138H11 [34] | Preclinical | IgG1 | SPDP (CL) | Camtheta | GGT | - |
| 1F6 [35] | Preclinical | IgG1 | Vc/pab (NCL) | vcAFP/vcMMAF | CD 70 | - |
| h1F6 [36] | Preclinical | IgG1 | MC (NCL) | MMAF | CD 70 | - |
| SGN-75 [37] | Phase I | IgG1 | MC (NCL) | MMAF | CD 70 | NCT01015911 |
| CDX-014 [38] | Phase I | IgG1 | Valine-citrulline (CL) | MMAE | TIM-1 | NCT02837991 |
| SGN-CD70A [39] | Phase I | IgG1 | Maleimidocaproic Valine-alanine (CL) | PBD | CD 70 | NCT02216890 |
| MDX-1203 [40] | Phase I | IgG1 | Valine-citrulline (CL) | Duocarmycin | CD 70 | NCT00944905 |
| AMG 172 [41] | Phase I | IgG1 | MCC (NCL) | DM1 | CD 70 | NCT01497821 |
| AGS-16M8F AGS-16C3F [42] | Phase I | IgG2a/IgG2a | MC (NCL)/MC (NCL) | MMAF/MMAF | ENPP3 | NCT01114230/ NCT01672775 |
| AGS-16C3F [43] | Phase II | IgG2a | MC (NCL) | MMAF | ENPP3 | NCT02639182 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sganga, S.; Riondino, S.; Iannantuono, G.M.; Rosenfeld, R.; Roselli, M.; Torino, F. Antibody–Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives. J. Pers. Med. 2023, 13, 1339. https://doi.org/10.3390/jpm13091339
Sganga S, Riondino S, Iannantuono GM, Rosenfeld R, Roselli M, Torino F. Antibody–Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives. Journal of Personalized Medicine. 2023; 13(9):1339. https://doi.org/10.3390/jpm13091339
Chicago/Turabian StyleSganga, Stefano, Silvia Riondino, Giovanni Maria Iannantuono, Roberto Rosenfeld, Mario Roselli, and Francesco Torino. 2023. "Antibody–Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives" Journal of Personalized Medicine 13, no. 9: 1339. https://doi.org/10.3390/jpm13091339






