The Relationship of Cognitive Dysfunction with Inflammatory Markers and Carotid Intima Media Thickness in Schizophrenia
Abstract
:1. Introduction
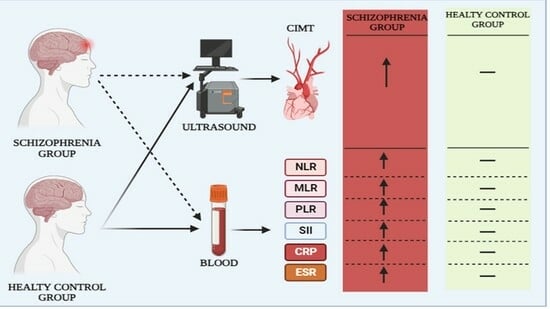
2. Material and Methods
2.1. Ethical Approval and Participants
2.2. Systemic Immune-Inflammation Index (SII)
2.3. The Positive and Negative Syndrome Scale (PANSS)
2.4. Montreal Cognitive Assessment Scale (MoCA)
2.5. Carotid Intima Media Thickness (CIMT) Measurement
2.6. Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chong, H.Y.; Teoh, S.L.; Wu, D.B.-C.; Kotirum, S.; Chiou, C.-F.; Chaiyakunapruk, N. Global economic burden of schizophrenia: A systematic review. Neuropsychiatr. Dis. Treat. 2016, 12, 357–373. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia—An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Yücel, M.; Pantelis, C. Cognitive Impairment in Schizophrenia and Affective Psychoses: Implications for DSM-V Criteria and Beyond. Schizophr. Bull. 2010, 36, 36–42. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive impairment in schizophrenia: Aetiology, pathophysiology, and treatment. Mol. Psychiatry 2023. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Levander, S.; Kjaersdam Telléus, G.; Jensen, S.O.W.; Østergaard Christensen, T.; Leucht, S. Second-generation antipsychotic effect on cognition in patients with schizophrenia—A meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015, 131, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.A.; Eissa, M.A.-E.; Kolkaila, E.A.; Amer, R.A.R.; Kotait, M.A. Mismatch negativity as an early biomarker of cognitive impairment in schizophrenia. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 24. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Wang, L.; Wang, R.; Yang, X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: A meta-analysis. Medicine 2019, 98, e13788. [Google Scholar] [CrossRef]
- Mazza, M.G.; Lucchi, S.; Rossetti, A.; Clerici, M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: A meta-analysis and systematic review. World J. Biol. Psychiatry 2020, 21, 326–338. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Saadeh, C. The erythrocyte sedimentation rate: Old and new clinical applications. South. Med. J. 1998, 91, 219–226. [Google Scholar] [CrossRef]
- Ortega, E.; Gilabert, R.; Nuñez, I.; Cofán, M.; Sala-Vila, A.; de Groot, E.; Ros, E. White blood cell count is associated with carotid and femoral atherosclerosis. Atherosclerosis 2012, 221, 275–281. [Google Scholar] [CrossRef]
- Bauer, M.; Caviezel, S.; Teynor, A.; Erbel, R.; Mahabadi, A.A.; Schmidt-Trucksäss, A. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med. Wkly. 2012, 142, w13705. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.; Abdelati, T.; Rizk, M.; Youssef, E.; Moghazy, K.; Gaber, N.; Yafei, S. Association of lipid peroxidation and interleukin-6 with carotid atherosclerosis in type 2 diabetes. Cardiovasc. Endocrinol. Metab. 2019, 8, 73–76. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM 4 TR (Text Revision); American Psychiatric Association: Washington, DC, USA, 2003. [Google Scholar]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Rosca, E.C.; Cornea, A.; Simu, M. Montreal Cognitive Assessment for evaluating the cognitive impairment in patients with schizophrenia: A systematic review. Gen. Hosp. Psychiatry 2020, 65, 64–73. [Google Scholar] [CrossRef]
- Sun, H.-L.; Bai, W.; Li, X.-H.; Huang, H.; Cui, X.-L.; Cheung, T.; Su, Z.-H.; Yuan, Z.; Ng, C.H.; Xiang, Y.-T. Schizophrenia and Inflammation Research: A Bibliometric Analysis. Front. Immunol. 2022, 13, 907851. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Borges, M.C.; Horta, B.L.; Bowden, J.; Davey Smith, G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2017, 74, 1226–1233. [Google Scholar] [CrossRef]
- Delaney, S.; Fallon, B.; Alaedini, A.; Yolken, R.; Indart, A.; Feng, T.; Wang, Y.; Javitt, D. Inflammatory biomarkers in psychosis and clinical high risk populations. Schizophr. Res. 2019, 206, 440–443. [Google Scholar] [CrossRef]
- Zhu, X.; Li, R.; Zhu, Y.; Zhou, J.; Huang, J.; Zhou, Y.; Tong, J.; Zhang, P.; Luo, X.; Chen, S.; et al. Changes in Inflammatory Biomarkers in Patients with Schizophrenia: A 3-Year Retrospective Study. Neuropsychiatr. Dis. Treat. 2023, 19, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Inaltekin, A.; Yağci, İ. Evaluation of Simple Markers of Inflammation and Systemic Immune Inflammation Index in Schizophrenia, Bipolar Disorder Patients and Healthy Controls. Turk Psikiyatr. Derg. 2023, 34, 11–15. [Google Scholar]
- Fond, G.; Lançon, C.; Auquier, P.; Boyer, L. C-Reactive Protein as a Peripheral Biomarker in Schizophrenia. An Updated Systematic Review. Front. Psychiatry 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Lestra, V.; Romeo, B.; Martelli, C.; Benyamina, A.; Hamdani, N. Could CRP be a differential biomarker of illness stages in schizophrenia? A systematic review and meta-analysis. Schizophr. Res. 2022, 246, 175–186. [Google Scholar] [CrossRef]
- Kappelmann, N.; Khandaker, G.M.; Dal, H.; Stochl, J.; Kosidou, K.; Jones, P.B.; Dalman, C.; Karlsson, H. Systemic inflammation and intelligence in early adulthood and subsequent risk of schizophrenia and other non-affective psychoses: A longitudinal cohort and co-relative study. Psychol. Med. 2019, 49, 295–302. [Google Scholar] [CrossRef]
- Misiak, B.; Stańczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2018, 192, 16–29. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Mantini, A.M.; Fridberg, D.J.; Buckley, P.F. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: A meta-analysis. Psychiatry Res. 2015, 226, 1–13. [Google Scholar] [CrossRef]
- Bulzacka, E.; Boyer, L.; Schürhoff, F.; Godin, O.; Berna, F.; Brunel, L.; Andrianarisoa, M.; Aouizerate, B.; Capdevielle, D.; Chéreau-Boudet, I.; et al. Chronic Peripheral Inflammation is Associated With Cognitive Impairment in Schizophrenia: Results From the Multicentric FACE-SZ Dataset. Schizophr. Bull. 2016, 42, 1290–1302. [Google Scholar] [CrossRef]
- Bora, E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: A meta-analysis. Psychol. Med. 2019, 49, 1971–1979. [Google Scholar] [CrossRef]
- Leung, K.K.; Wong, Y.C.; Shea, K.S.; Chan, S.C.; Chang, W.C.; Mo, Y.M.F.; Chan, S.M.S. Altered neutrophil-to-lymphocyte ratio in patients with non-affective first episode psychosis and its relationship with symptom severity and cognitive impairment. Sci. Rep. 2023, 13, 11453. [Google Scholar] [CrossRef]
- Patlola, S.R.; Donohoe, G.; McKernan, D.P. The relationship between inflammatory biomarkers and cognitive dysfunction in patients with schizophrenia: A systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 121, 110668. [Google Scholar] [CrossRef] [PubMed]
- Upthegrove, R.; Khandaker, G.M. Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia. In Neuroinflammation and Schizophrenia; Khandaker, G.M., Meyer, U., Jones, P.B., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 49–66. [Google Scholar]
- Fond, G.; Lançon, C.; Korchia, T.; Auquier, P.; Boyer, L. The Role of Inflammation in the Treatment of Schizophrenia. Front. Psychiatry 2020, 11, 518291. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Lee, T.Y.; Kwak, Y.B.; Yoon, Y.B.; Kim, M.; Kwon, J.S. Adjunctive use of anti-inflammatory drugs for schizophrenia: A meta-analytic investigation of randomized controlled trials. Aust. N. Zeal. J. Psychiatry 2019, 53, 742–759. [Google Scholar] [CrossRef] [PubMed]
- Baykara, S.; Gündoğan Bozdağ, P.; Baykara, M.; Namlı, M.N. Evaluation of arterial stiffness in patients with schizophrenia. J. Clin. Neurosci. 2020, 79, 149–153. [Google Scholar] [CrossRef]
- Ünsal, C.; Oran, M.; Tureli, H.O.; Alpsoy, S.; Yeşilyurt, S.; Arslan, M.; Topcu, B.; Karakaya, O.; Kurt, E. Detection of subclinical atherosclerosis and diastolic dysfunction in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2013, 9, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess Early Mortality in Schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef]
- Pillinger, T.; Osimo, E.F.; de Marvao, A.; Shah, M.; Francis, C.; Huang, J.; D’Ambrosio, E.; Firth, J.; Nour, M.M.; McCutcheon, R.A.; et al. Effect of polygenic risk for schizophrenia on cardiac structure and function: A UK Biobank observational study. Lancet Psychiatry 2023, 10, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Ihle-Hansen, H.; Ihle-Hansen, H.; Sandset, E.C.; Hagberg, G. Subclinical Carotid Artery Atherosclerosis and Cognitive Function: A Mini-Review. Front. Neurol. 2021, 12, 705043. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Norby, F.L.; Alonso, A.; Gottesman, R.F.; Jack, C.R.; Meyer, M.L.; Knopman, D.S.; Sullivan, K.J.; Hughes, T.M.; Lakshminarayan, K.; et al. Association of Carotid Intima-Media Thickness with Brain MRI Markers in the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J. Stroke Cerebrovasc. Dis. 2022, 31, 106388. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.L.; Woodard, T.; Sigurdsson, S.; van Buchem, M.A.; Torjesen, A.A.; Inker, L.A.; Aspelund, T.; Eiriksdottir, G.; Harris, T.B.; Gudnason, V.; et al. Cerebrovascular Damage Mediates Relations Between Aortic Stiffness and Memory. Hypertension 2016, 67, 176–182. [Google Scholar] [CrossRef]
- Moroni, F.; Ammirati, E.; Magnoni, M.; D’Ascenzo, F.; Anselmino, M.; Anzalone, N.; Rocca, M.A.; Falini, A.; Filippi, M.; Camici, P.G. Carotid atherosclerosis, silent ischemic brain damage and brain atrophy: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 223, 681–687. [Google Scholar] [CrossRef]
- Sadekova, N.; Vallerand, D.; Guevara, E.; Lesage, F.; Girouard, H. Carotid Calcification in Mice: A New Model to Study the Effects of Arterial Stiffness on the Brain. J. Am. Heart Assoc. 2013, 2, e000224. [Google Scholar] [CrossRef]
- Piegza, M.; Więckiewicz, G.; Wierzba, D.; Piegza, J. Cognitive Functions in Patients after Carotid Artery Revascularization—A Narrative Review. Brain Sci. 2021, 11, 1307. [Google Scholar] [CrossRef]
- Lin, H.-F.; Huang, L.-C.; Chen, C.-K.; Juo, S.-H.H.; Chen, C.-S. Carotid atherosclerosis among middle-aged individuals predicts cognition: A 10-year follow-up study. Atherosclerosis 2020, 314, 27–32. [Google Scholar] [CrossRef]
| n | % | ||
|---|---|---|---|
| Gender | Female | 11 | 21.6 |
| Male | 40 | 78.4 | |
| Marriage Status | Single | 34 | 66.7 |
| Married | 5 | 9.8 | |
| Divorced | 12 | 23.5 | |
| Smoker | Yes | 22 | 43.1 |
| No | 29 | 56.9 | |
| Psychiatric disorder in the family | Yes | 21 | 41.2 |
| No | 30 | 58.8 | |
| Depot antipsychotic | Yes | 22 | 43.1 |
| No | 29 | 56.9 | |
| Depot antipsychotic type | Paliperidone | 11 | 50.0 |
| Zuclopenthixol | 6 | 27.3 | |
| Risperidone | 4 | 18.2 | |
| Aripiprazole | 1 | 4.5 | |
| Antidepressant | Yes | 25 | 49.0 |
| No | 26 | 51.0 | |
| Mood stabilizer | Yes | 12 | 23.5 |
| No | 39 | 76.5 | |
| Mood stabilizer type | Valproic acid | 8 | 61.5 |
| Lithium | 3 | 23.1 | |
| Lamotrigine | 1 | 7.7 | |
| Carbamazepine | 1 | 7.7 | |
| Antipsychotic type | Olanzapine | 28 | 38.9 |
| Quetiapine | 12 | 16.7 | |
| Clozapine | 10 | 13.9 | |
| Aripiprazole | 7 | 9.7 | |
| Amisulpride | 7 | 9.7 | |
| Risperidone | 4 | 5.6 | |
| Haloperidol | 2 | 2.8 | |
| Paliperidone | 1 | 1.4 | |
| Zuclopenthixol | 1 | 1.4 | |
| Schizophrenia | HC | Total | x2 | * p | |||
|---|---|---|---|---|---|---|---|
| Gender | Female | n (%) | 11 (21.6) | 17 (29.8) | 28 (25.9) | 0.955 | 0.328 |
| Male | n (%) | 40 (78.4) | 40 (70.2) | 80 (74.1) | |||
| Smoker | Yes | n (%) | 22 (43.1) | 24 (42.1) | 46 (42.6) | 0.012 | 0.914 |
| No | n (%) | 29 (56.9) | 33 (57.9) | 62 (57.4) |
| 95% CI of the Difference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Sd | t | Md | Lowest Limit | Md | * p | ||||
| MoCA | SCH | 51 | 18.06 | 6.41 | −10.91 | −9.91 | −11.73 | −8.08 | <0.001 | |
| HC | 57 | 27.96 | 1.07 | |||||||
| Age | SCH | 51 | 47.16 | 10.23 | 0.47 | 0.84 | −2.72 | 4.40 | 0.640 | |
| HC | 57 | 46.31 | 8.14 | |||||||
| Education years | SCH | 51 | 8.24 | 4.24 | −9.68 | −6.33 | −7.63 | −5.02 | <0.001 | |
| HC | 57 | 14.56 | 2.06 | |||||||
| BMI | SCH | 51 | 28.18 | 6.95 | 1.56 | 1.78 | −0.48 | 4.04 | 0.122 | |
| HC | 57 | 26.41 | 4.29 | |||||||
| WBC | SCH | 51 | 7.38 | 2.03 | −0.31 | −0.11 | −0.81 | 0.59 | 0.756 | |
| HC | 57 | 7.49 | 1.62 | |||||||
| Neutrophil | SCH | 51 | 4.81 | 1.66 | 1.45 | 0.40 | −0.15 | 0.94 | 0.152 | |
| HC | 57 | 4.41 | 1.11 | |||||||
| Lymphocyte | SCH | 51 | 1.94 | 0.78 | −2.99 | −0.42 | −0.69 | −0.14 | 0.004 | |
| HC | 57 | 2.35 | 0.67 | |||||||
| Monocyte | SCH | 51 | 0.45 | 0.17 | −1.43 | −0.04 | −0.10 | 0.02 | 0.156 | |
| HC | 57 | 0.49 | 0.14 | |||||||
| Platelet | SCH | 51 | 233.35 | 60.25 | −1.39 | −13.95 | −33.89 | 5.99 | 0.168 | |
| HC | 57 | 247.30 | 40.98 | |||||||
| NLR | SCH | 51 | 2.86 | 1.40 | 4.16 | 0.88 | 0.46 | 1.31 | <0.001 | |
| HC | 57 | 1.98 | 0.60 | |||||||
| MLR | SCH | 51 | 0.26 | 0.13 | 2.15 | 0.04 | 0.00 | 0.08 | 0.035 | |
| HC | 57 | 0.22 | 0.06 | |||||||
| PLR | SCH | 51 | 135.78 | 52.16 | 2.74 | 23.29 | 6.38 | 40.20 | 0.008 | |
| HC | 57 | 112.49 | 32.87 | |||||||
| SII | SCH | 51 | 653.97 | 348.80 | 3.19 | 168.94 | 63.31 | 274.56 | 0.002 | |
| HC | 57 | 485.03 | 153.78 | |||||||
| CRP | SCH | 51 | 6.43 | 5.32 | 6.60 | 5.05 | 3.51 | 6.59 | <0.001 | |
| HC | 57 | 1.38 | 1.06 | |||||||
| ESR | SCH | 51 | 9.94 | 10.52 | 3.35 | 5.18 | 2.08 | 8.29 | <0.001 | |
| HC | 57 | 4.76 | 3.22 | |||||||
| CIMT | SCH | 51 | 0.98 | 0.19 | 8.08 | 0.23 | 0.18 | 0.29 | <0.001 | |
| HC | 57 | 0.75 | 0.09278 | |||||||
| SAPS | SANS | Age | İllnessduration | BMİ | WBC | Neutrophil | Lymphocyte | Monocyte | Platelet | CRP | ESR | NLR | MLR | PLR | SII | CIMT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MoCA | r | −0.349 | −0.377 | −0.391 | −0.274 | 0.154 | 0.171 | 0.092 | 0.259 | −0.033 | 0.018 | 0.009 | −0.121 | −0.122 | −0.232 | −0.265 | −0.107 | −0.549 |
| p | 0.013 | 0.007 | 0.005 | 0.052 | <0.001 | 0.286 | 0.230 | 0.521 | 0.066 | 0.816 | 0.951 | 0.401 | 0.395 | 0.102 | 0.060 | 0.454 | <0.001 | |
| SAPS | r | −0.383 | 0.040 | −0.187 | −0.205 | −0.166 | −0.118 | −0.147 | −0.081 | 0.014 | 0.061 | 0.040 | 0.128 | 0.168 | 0.201 | 0.089 | 0.017 | |
| p | 0.006 | 0.783 | 0.193 | 0.158 | 0.250 | 0.413 | 0.307 | 0.576 | 0.923 | 0.680 | 0.787 | 0.377 | 0.245 | 0.161 | 0.539 | 0.906 | ||
| SANS | r | 0.103 | 0.305 | −0.309 | −0.001 | 0.092 | −0.240 | 0.151 | −0.128 | −0.296 | −0.036 | 0.208 | 0.248 | 0.222 | 0.155 | 0.467 | ||
| p | 0.476 | 0.031 | 0.031 | 0.993 | 0.526 | 0.093 | 0.295 | 0.374 | 0.039 | 0.808 | 0.148 | 0.082 | 0.121 | 0.281 | <0.001 |
| 95% CI of the Difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Sd | t | Md | Lowest Limit | Highest Limit | * p | |||
| Smoker | Yes | 22 | 19.05 | 5.51 | 0.96 | 1.74 | −1.91 | 5.38 | 0.343 |
| No | 29 | 17.31 | 7.02 | ||||||
| Depot_antipsychotic | Yes | 22 | 17.77 | 6.14 | −0.28 | −0.50 | −4.18 | 3.17 | 0.784 |
| No | 29 | 18.28 | 6.70 | ||||||
| Antidepressant | Yes | 25 | 18.52 | 6.52 | 0.50 | 0.90 | −2.73 | 4.54 | 0.619 |
| No | 26 | 17.62 | 6.39 | ||||||
| Mood_stabilizer | Yes | 12 | 18.25 | 6.73 | 0.12 | 0.25 | −4.04 | 4.54 | 0.907 |
| No | 39 | 18.00 | 6.39 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İmre, O.; Caglayan, C.; Muştu, M. The Relationship of Cognitive Dysfunction with Inflammatory Markers and Carotid Intima Media Thickness in Schizophrenia. J. Pers. Med. 2023, 13, 1342. https://doi.org/10.3390/jpm13091342
İmre O, Caglayan C, Muştu M. The Relationship of Cognitive Dysfunction with Inflammatory Markers and Carotid Intima Media Thickness in Schizophrenia. Journal of Personalized Medicine. 2023; 13(9):1342. https://doi.org/10.3390/jpm13091342
Chicago/Turabian Styleİmre, Okan, Cuneyt Caglayan, and Mehmet Muştu. 2023. "The Relationship of Cognitive Dysfunction with Inflammatory Markers and Carotid Intima Media Thickness in Schizophrenia" Journal of Personalized Medicine 13, no. 9: 1342. https://doi.org/10.3390/jpm13091342