Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction
Abstract
:1. Introduction
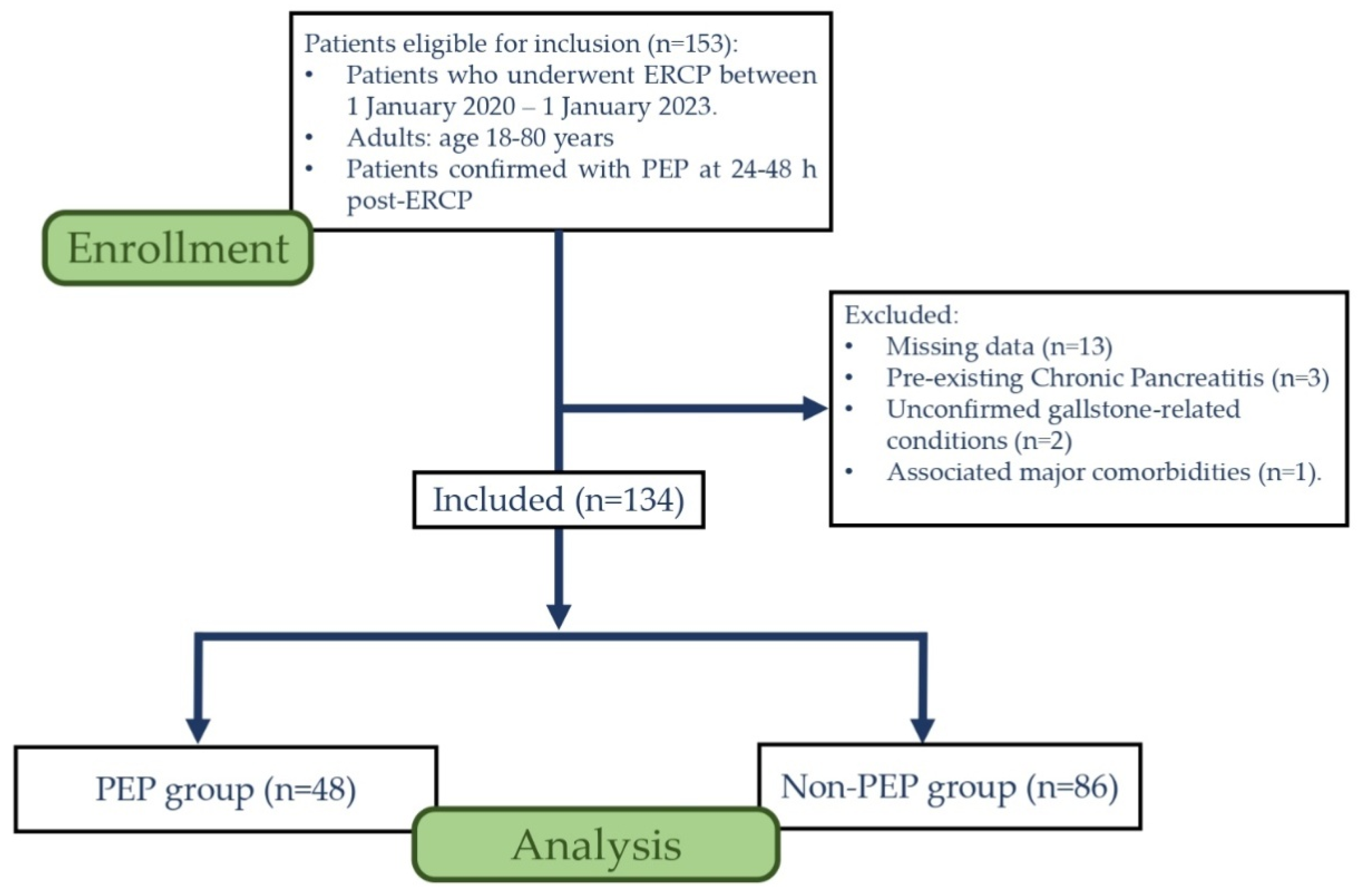
2. Materials and Methods
3. Results
4. Discussion
4.1. Clinical and Interventional Factors
4.1.1. Gender Disparities Regarding PEP Risk
4.1.2. Combined Dormia Basket with ERCP Balloon Dilation
4.2. Predictive Potential of Biological Markers
4.2.1. Pancreatic Enzymes
4.2.2. Dynamics of Inflammatory and Infectious Markers
4.2.3. Total Bilirubin Levels on Admission—Paramount Biological Marker in PEP Risk Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, D.J.; Bomman, S.; Krishnamoorthi, R.; Kozarek, R.A. Endoscopic retrograde cholangiopancreatography: Current practice and future research. World J. Gastrointest. Endosc. 2021, 13, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Tringali, A.; Loperfido, S. Patient Education: ERCP (Endoscopic Retrograde Cholangiopancreatography) (Beyond the Basics). UpToDate [Internet]. Available online: https://www.uptodate.com/contents/ercp-endoscopic-retrograde-cholangiopancreatography-beyond-the-basics/ (accessed on 15 May 2023).
- Chen, J.J.; Wang, X.M.; Liu, X.Q.; Li, W.; Dong, M.; Suo, Z.W.; Ding, P.; Li, Y. Risk factors for post-ERCP pancreatitis: A systematic review of clinical trials with a large sample size in the past 10 years. Eur. J. Med. Res. 2014, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Cahyadi, O.; Tehami, N.; de-Madaria, E.; Siau, K. Post-ERCP Pancreatitis: Prevention, Diagnosis and Management. Medicina 2022, 58, 1261. [Google Scholar] [CrossRef] [PubMed]
- Goyal, H.; Sachdeva, S.; Sherazi, S.A.A.; Gupta, S.; Perisetti, A.; Ali, A.; Chandan, S.; Tharian, B.; Sharma, N.; Thosani, N. Early prediction of post-ERCP pancreatitis by post-procedure amylase and lipase levels: A systematic review and meta-analysis. Endosc. Int. Open 2022, 10, E952–E970. [Google Scholar] [CrossRef]
- Lv, Z.H.; Kou, D.Q.; Guo, S.B. Three-hour post-ERCP amylase level: A useful indicator for early prediction of post-ERCP pancreatitis. BMC Gastroenterol. 2020, 20, 118. [Google Scholar] [CrossRef]
- Thomas, P.R.; Sengupta, S. Prediction of pancreatitis following endoscopic retrograde cholangiopancreatography by the 4-h post procedure amylase level. J. Gastroenterol. Hepatol. 2001, 16, 923–926. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, D.K.; Lee, S.H.; Jang, S.; Choi, J.H.; Kang, J.; Paik, W.H.; Lee, J.K.; Ryu, J.K.; Kim, Y.T. Utility of serum phosphate as a marker for predicting the severity of post-endoscopic retrograde cholangiopancreatography pancreatitis. United Eur. Gastroenterol. J. 2018, 6, 895–901. [Google Scholar] [CrossRef]
- Kapetanos, D.; Kokozidis, G.; Kinigopoulou, P.; Xiarchos, P.; Antonopoulos, Z.; Progia, E.; Kitis, G. The value of serum amylase and elastase measurements in the prediction of post-ERCP acute pancreatitis. Hepatogastroenterology 2007, 54, 556–560. [Google Scholar]
- Pieper-Bigelow, C.; Strocchi, A.; Levitt, M. Where does serum amylase come from and where does it go? Gastroenterol. Clin. N. Am. 1990, 19, 793–810. [Google Scholar] [CrossRef]
- Hormati, A.; Alemi, F.; Mohammadbeigi, A.; Sarkeshikian, S.S.; Saeidi, M. Prevalence of Endoscopic Retrograde Cholangiopancreatography Complications and Amylase Sensitivity for Predicting Pancreatitis in ERCP Patients. Gastroenterol. Nurs. 2020, 43, 350–354. [Google Scholar] [CrossRef]
- Tryliskyy, Y.; Bryce, G. Post-ERCP pancreatitis: Pathophysiology, early identification and risk stratification. Adv. Clin. Exp. Med. 2018, 27, 149–154. [Google Scholar] [CrossRef]
- Kochar, B.; Akshintala, V.S.; Afghani, E.; Elmunzer, B.J.; Kim, K.J.; Lennon, A.M.; Khashab, M.A.; Kalloo, A.N.; Singh, V.K. Incidence, severity, and mortality of post-ERCP pancreatitis: A systematic review by using randomized, controlled trials. Gastrointest. Endosc. 2015, 81, 143–149.e9. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; DiSario, J.A.; Nelson, D.B.; Fennerty, M.B.; Lee, J.G.; Bjorkman, D.J.; Overby, C.S.; Aas, J.; Ryan, M.E.; Bochna, G.S.; et al. Risk factors for post-ERCP pancreatitis: A prospective, multicenter study. Gastrointest. Endosc. 2001, 54, 425–434. [Google Scholar] [CrossRef]
- Ergin, E.; Oruç, N.; Ersöz, G.; Tekeşin, O.; Özütemiz, Ö. Prognosis and risk factors of ERCP pancreatitis in elderly. Sci. Rep. 2021, 11, 15930. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Yazumi, S.; Uza, N.; Kurita, A.; Asada, M.; Kodama, Y.; Goto, M.; Katayama, T.; Anami, T.; Watanabe, A.; et al. New practical scoring system to predict POST- endoscopic retrograde cholangiopancreatography pancreatitis: Development and validation. JGH Open 2021, 5, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Giussani, A.; Di Leo, M.; Testoni, S.; Testoni, P.A. Guidewire biliary cannulation does not reduce post-ERCP pancreatitis compared with the contrast injection technique in low-risk and high-risk patients. Gastrointest. Endosc. 2012, 75, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Tohda, G.; Dochin, M. Management of endoscopic biliary stenting for choledocholithiasis: Evaluation of stent-exchange intervals. World J. Gastrointest. Endosc. 2018, 10, 45–50. [Google Scholar] [CrossRef]
- Lella, F.; Bagnolo, F.; Colombo, E.; Bonassi, U. A simple way of avoiding post-ERCP pancreatitis. Gastrointest. Endosc. 2004, 59, 830–834. [Google Scholar] [CrossRef]
- Karjula, H.; Nordblad Schmidt, P.; Mäkelä, J.; Liisanantti, J.H.; Ohtonen, P.; Saarela, A. Prophylactic pancreatic duct stenting in severe acute necrotizing pancreatitis: A prospective randomized study. Endoscopy 2019, 51, 1027–1034. [Google Scholar] [CrossRef]
- DiSario, J.A.; Freeman, M.L.; Bjorkman, D.J.; MacMathuna, P.; Petersen, B.T.; Jaffe, P.E.; Morales, T.G.; Hixson, L.J.; Sherman, S.; Lehman, G.A.; et al. Endoscopic balloon dilation compared with sphincterotomy for extraction of bile duct stones. Gastroenterology 2004, 127, 1291–1299. [Google Scholar] [CrossRef]
- Bergman, J.; Huibregtse, K. What is the current status of endoscopic balloon dilation for stone removal? Endoscopy 1998, 30, 43–45. [Google Scholar] [CrossRef]
- Lam, R.; Muniraj, T. Hyperamylasemia [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2022; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559273/ (accessed on 10 June 2023).
- Sutton, V.R.; Hong, M.K.; Thomas, P.R. Using the 4-hour Post-ERCP Amylase Level to Predict Post-ERCP Pancreatitis. JOP J. Pancreas 2011, 12, 372–376. [Google Scholar]
- Testoni, P.A. Serum Amylase Measured Four Hours After Endoscopic Sphincterotomy Is a Reliable Predictor of Postprocedure Pancreatitis. Am. J. Gastroenterol. 1999, 94, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, A.; Messerer, D.A.C.; Scharffetter-Kochanek, K.; Huber-Lang, M.; Ignatius, A. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J. Immunol Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P.; Dickson, G.R. Serum c-reactive protein in patients with serious trauma. Injury 1992, 23, 483–486. [Google Scholar] [CrossRef]
- Funari, M.P.; Ribeiro, I.B.; de Moura, D.T.H.; Bernardo, W.M.; Brunaldi, V.O.; Rezende, D.T.; Resende, R.H.; de Marco, M.O.; Franzini, T.A.P.; de Moura, E.G.H. Adverse events after biliary sphincterotomy: Does the electric current mode make a difference? A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 739–752. [Google Scholar] [CrossRef]
- Sherman, S.; Hawes, R.H.; Troiano, F.P.; Lehman, G.A. Pancreatitis following bile duct sphincter of Oddi manometry: Utility of the aspirating catheter. Gastrointest. Endosc. 1992, 38, 347–350. [Google Scholar] [CrossRef]
- Funatsu, E.; Masuda, A.; Takenaka, M.; Nakagawa, T.; Shiomi, H.; Yoshinaka, H.; Kobayashi, T.; Sakai, A.; Yagi, Y.; Yoshida, M.; et al. History of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis and Acute Pancreatitis as Risk Factors for Post-ERCP Pancreatitis. Kobe J. Med. Sci. 2017, 63, E1. [Google Scholar]
- Arendt, T.; Nizze, H.; Mönig, H.; Kloehn, S.; Stüber, E.; Fölsch, U. Biliary pancreatic reflux-induced acute pancreatitis-myth or possibility? Eur. J. Gastroenterol. Hepato. 1999, 11, 329–335. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, T.Y.; Cheon, Y.K. The Neutrophil-Lymphocyte Ratio as an Early Predictive Marker of the Severity of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Medicina 2021, 58, 13. [Google Scholar] [CrossRef]
- Wang, Y.; Fuentes, H.E.; Attar, B.M.; Jaiswal, P.; Demetria, M. Evaluation of the prognostic value of neutrophil to lymphocyte ratio in patients with hypertriglyceridemia-induced acute pancreatitis. Pancreatology 2017, 17, 893–897. [Google Scholar] [CrossRef]
- Jeon, T.J.; Park, J.Y. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J. Gastroenterol. 2017, 23, 3883. [Google Scholar] [CrossRef]
- Simadibrata, D.M.; Calvin, J.; Wijaya, A.D.; Ibrahim, N.A.A. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Todor, S.B.; Bîrluțiu, V.; Topîrcean, D.; Mihăilă, R.G. Role of biological markers and CT severity score in predicting mortality in patients with COVID-19: An observational retrospective study. Exp. Ther. Med. 2022, 24, 698. [Google Scholar] [CrossRef] [PubMed]
- Bedel, C.; Korkut, M.; Armağan, H.H. NLR, d-NLR and PLR can be affected by many factors. Int. Immunopharmacol. 2021, 90, 107154. [Google Scholar] [CrossRef]
- Loperfido, S.; Angelini, G.; Benedetti, G.; Chilovi, F.; Costan, F.; De Berardinis, F.; De Bernardin, M.; Ederle, A.; Fina, P.; Fratton, A. Major early complications from diagnostic and therapeutic ERCP: A prospective multicenter study. Gastrointest. Endosc. 1998, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.; Ruffolo, T.A.; Hawes, R.H.; Lehman, G.A. Complications of endoscopic sphincterotomy. Gastroenterology 1991, 101, 1068–1075. [Google Scholar] [CrossRef]
- Freeman, M.L.; Herman, M.E.; Ryan, M.E. Complications of Endoscopic Biliary Sphincterotomy. N. Engl. J. Med. 1996, 335, 909–991. [Google Scholar] [CrossRef]
- Chen, Y.K.; Foliente, R.L.; Santoro, M.J.; Walter, M.H.; Collen, M.J. Endoscopic sphincterotomy-induced pancreatitis: Increased risk associated with nondilated bile ducts and sphincter of Oddi dysfunction. Am. J. Gastroenterol. 1994, 89, 327–333. [Google Scholar]
- Wang, L.; Mirzaie, S.; Dunnsiri, T.; Chen, F.; Wilhalme, H.; MacQueen, I.T.; Cryer, H.; Eastoak-Siletz, A.; Guan, M.; Cuff, C.; et al. Systematic review and meta-analysis of the 2010 ASGE non-invasive predictors of choledocholithiasis and comparison to the 2019 ASGE predictors. Clin. J. Gastroenterol. 2022, 15, 286–300. [Google Scholar] [CrossRef]
- Wang, M.; He, X.; Tian, C.; Li, J.; Min, F.; Li, H.Y. The Diagnostic Accuracy of Linear Endoscopic Ultrasound for Evaluating Symptoms Suggestive of Common Bile Duct Stones. Gastroenterol. Res. Pract. 2016, 2016, 6957235. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y. Optimal Evaluation of Suspected Choledocholithiasis: Does This Patient Really Have Choledocholithiasis? Clin. Endosc. 2017, 50, 415–416. [Google Scholar] [CrossRef] [PubMed]

| Variable | TOTAL | NON-PEP | PEP | p-VALUE |
|---|---|---|---|---|
| Demographic characteristics | 86 (64.1%) | 48 (35.8%) | ||
| Age (IQR) | 67 (57–73) | 67 (61–73) | 65.5 (47–76.75) | 0.492 |
| Sex | 0.006 | |||
| Male | 66 (49.2%) | 50 (75.8%) | 16 (24.2%) | |
| Female | 68 (50.7%) | 36 (52.9%) | 32 (47.1%) | |
| ---------------------- | ||||
| History of Cholecystectomy ---------------------- Comorbidities | 70 (52.2%) | 44 (51.2%) | 26 (54.2%) | 0.739 |
| Diabetes | 22 (16.4%) | 14 (16.3%) | 8 (16.7%) | 0.954 |
| Hypertension | 84 (62.6%) | 56 (65.1%) | 28 (58.3%) | 0.436 |
| Atrial fibrillation | 21 (15.6%) | 13 (15.1%) | 8 (16.7%) | 0.813 |
| ERCP procedure type | ||||
| DB combined with ERCPBD | 86 (64.2%) | 62 (72.1%) | 24 (50%) | 0.011 |
| DB combined with BSP | 88 (65.7%) | 58 (67.4%) | 24 (50%) | 0.563 |
| ERCP BD combined with BSP | 80 (59.7%) | 56 (65.1%) | 24 (50%) | 0.087 |
| All three procedures | 68 (50.7%) | 48 (55.8%) | 20 (41.7%) | 0.116 |
| Multiple ERCP’s | 26 (19.4%) | 20 (23.3%) | 6 (12.5%) | 0.271 |
| Laboratory Findings | On Admission | After ERCP | |||||
|---|---|---|---|---|---|---|---|
| Normal Range | Non-PEP | PEP | p-Value | Non-PEP | PEP | p-Value | |
| Hematological markers | |||||||
| Hemoglobin g/dL | 12–15 | 13,1 (12.3–13.9) | 13.5 (11.9–14.4) | 0.745 | 12.5 (11.5–13.3) | 12.9 (11.4–14) | 0.146 |
| WBC × 109/L | 4–10 | 8.1 (6.6–10.6) | 7.2 (6.5–10.5) | 0.441 | 8.0 (6.7–10.2) | 8.6 (7.7–10.9) | 0.073 |
| Neut. Count × 109/L | 2–7.5 | 5.6 (4.1–8.9) | 4.7 (3.6–8.5) | 0.492 | 5.2 (4.2–6.3) | 6.4 (5.2–8.6) | 0.003 |
| NLR | 3.1 (1.8–7.3) | 3.1 (2.1–6.6) | 0.824 | 3.1 (2.4–4.5) | 4.1 (3.0–6.1) | 0.006 | |
| Tr. Count × 109/L | 150–400 | 250 (196–327) | 271 (232–322) | 0.419 | 247 (200–329) | 250 (217–309) | 0.726 |
| Blood biochemistry | |||||||
| Amylase U/L | 25–125 | 61 (44–166) | 48 (42–76) | 0.334 | 94 (79–138) | 881 (363–1443) | <0.001 |
| Lipase U/L | 8–78 | 72 (28–211) | 24 (13–103) | 0.001 | 41 (34–64) | 1717 (363–2648) | <0.001 |
| AST U/L | 9–39 | 64 (61–121) | 82 (49–85) | 0.841 | 69 (48–78) | 56 (37–64) | 0.553 |
| ALT U/L | 3–43 | 190 (100–213) | 80 (63–124) | 0.884 | 169 (76–188) | 69 (41–84) | 0.985 |
| Creatinine mg/dL | 0.7–1.3 | 0.75 (0.72–0.85) | 0.78 (0.61–0.83) | 0.114 | 0.72 (0.71–0.86) | 0.75 (0.57–0.92) | 0.355 |
| TB mg/dL | 0.2–1.2 | 5.7 (5.4–9.3) | 2.8 (1.9–3.8) | 0.003 | 3.6 (2.9–4.8) | 2.3 (1.4–4.8) | 0.027 |
| CB mg/dL | 0–0.5 | 3.1 (1.4–5.0) | 1.1 (0.9–1.9) | 0.010 | 2.7(2.0–3.7) | 1.3 (1.0–3.3) | 0.006 |
| UB mg/dL | 0–0.7 | 3.9 (2.1–5.0) | 1.6 (0.8–3.0) | 0.280 | 0.9 (0.8–1.2) | 1.0 (0.4–1.6) | 0.248 |
| Total Cholesterol (mg/dL) | 109–200 | 195 (144–239) | 188(162–232) | 0.478 | |||
| Triglycerides (mg/dL) | 40–150 | 145 (87–210) | 121 (72–171) | 0.103 | |||
| Acute phase reactants | |||||||
| CRP mg/L | 0–5 | 90 (28–160) | 25 (10–46) | 0.083 | 42 (35–85) | 54 (35–90) | 0.626 |
| Fibrinogen mg/dL | 170–420 | 550 (488–620) | 530 (323–573) | 0.662 | 510 (481–555) | 574 (417–681) | 0.710 |
| Coagulation markers | |||||||
| INR | 0.86–1.1 | 1.1 (1.0–1.3) | 1.0 (0.9–1.1) | 0.339 | 1.1 (1.0–1.2) | 1.0 (0.9–1.1) | 0.705 |
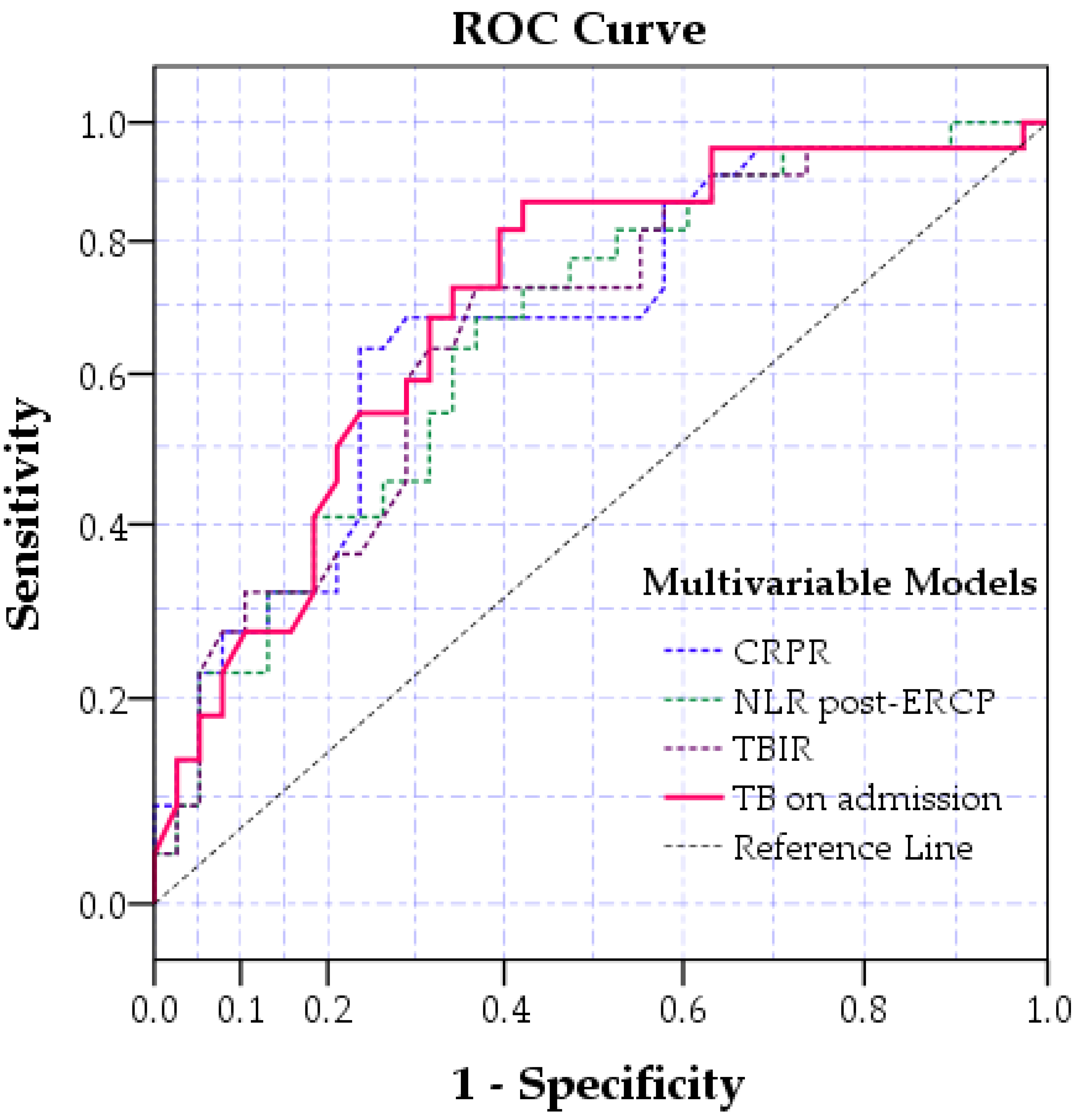
| Variable | AUC | p-Value | CI 95% | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| CRPR | 0.677 | 0.001 | 0.574 | 0.786 |
| NLR after ERCP | 0.646 | 0.006 | 0.548 | 0.744 |
| TB on admission | 0.654 | 0.003 | 0.560 | 0.748 |
| TBIR | 0.623 | 0.019 | 0.521 | 0.728 |
| Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| C.I. 95% for O.R | C.I. 95% for O.R | |||||||
| p-Value | Odd Ratio | Lower | Upper | p Value | Odds Ratio | Lower | Upper | |
| CRPR | <0.001 | 4.451 | 2.023 | 9.789 | <0.001 | 4.337 | 1.945 | 9.668 |
| TBIR NLR post-ERCP | 0.003 0.004 | 3.455 3.047 | 1.525 1.440 | 7.825 6.448 | 0.002 0.003 | 4.004 3.281 | 1.664 1.490 | 9.634 7.221 |
| TB on admission | <0.001 | 4.773 | 2.002 | 11.380 | <0.001 | 5.262 | 2.111 | 13.113 |
| Gender female | 0.009 | 2.890 | 1.307 | 6.398 | 0.005 | 2.893 | 1.377 | 6.105 |
| Age | 0.134 | 0.977 | 0.947 | 1.007 | ||||
| Combined DB with ERCP BD | 0.012 | 2.583 | 1.237 | 5.395 | 0.009 | 2.890 | 1.307 | 6.393 |
| History of Cholecystectomy | 0.426 | 1.382 | 0.623 | 3.065 | ||||
| Hypertension | 0.704 | 0.836 | 0.333 | 2.100 | ||||
| Variable | Normal Level of Pancreatic Enzymes | Elevated Pancreatic Enzymes | Post-ERCP Pancreatitis | Kruskal-Wallis Test (p-Value) |
|---|---|---|---|---|
| TB on admission (mg/dL) | 2.1 (0.8–4.3) | 2.5 (1.0–6.4) | 1.5 (0.8–2.3) | 0.079 |
| NLR post-ERCP | 3.0 (2.4–4.1) * | 4.0 (2.9–5.6) | 3.9 (3.0–5.9) * | 0.014 |
| TBIR | 0.8 (0.6–1.2) | 0.8 (0.5–1.2) | 0.9 (0.6–1.3) | 0.342 |
| CRPR | 1.0 (0.7–2.4) | 0.8 (0.6–1.9) * | 1.4 (0.9–6.2) * | 0.013 |
| Variable | Area | p-Value | Confidence Interval 95% | |
|---|---|---|---|---|
| Lower | Upper | |||
| TB on admission | 0.73 | <0.001 | 0.632 | 0.829 |
| TBIR | 0.698 | 0.001 | 0.595 | 0.798 |
| CRPR | 0.710 | <0.001 | 0.612 | 0.810 |
| NLRpost-ERCP | 0.690 | 0.001 | 0.588 | 0.793 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boicean, A.; Birlutiu, V.; Ichim, C.; Todor, S.B.; Hasegan, A.; Bacila, C.; Solomon, A.; Cristian, A.; Dura, H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. J. Pers. Med. 2023, 13, 1356. https://doi.org/10.3390/jpm13091356
Boicean A, Birlutiu V, Ichim C, Todor SB, Hasegan A, Bacila C, Solomon A, Cristian A, Dura H. Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. Journal of Personalized Medicine. 2023; 13(9):1356. https://doi.org/10.3390/jpm13091356
Chicago/Turabian StyleBoicean, Adrian, Victoria Birlutiu, Cristian Ichim, Samuel B. Todor, Adrian Hasegan, Ciprian Bacila, Adelaida Solomon, Adrian Cristian, and Horatiu Dura. 2023. "Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction" Journal of Personalized Medicine 13, no. 9: 1356. https://doi.org/10.3390/jpm13091356
APA StyleBoicean, A., Birlutiu, V., Ichim, C., Todor, S. B., Hasegan, A., Bacila, C., Solomon, A., Cristian, A., & Dura, H. (2023). Predictors of Post-ERCP Pancreatitis (P.E.P.) in Choledochal Lithiasis Extraction. Journal of Personalized Medicine, 13(9), 1356. https://doi.org/10.3390/jpm13091356








