Intelligent Clinical Decision Support System for Managing COPD Patients
Abstract
:1. Introduction
1.1. COPD Introduction and Definition
1.2. Importance of COPD Management and Monitoring Systems
1.3. Effect of External Variables (Climate, Humidity, Particles) in COPD Patients
1.4. Research Questions and Problems
1.5. Purpose and Description of the Present Work
- Capability to categorize alerts.
- Capability to provide early warning scores and other relevant metrics of patients to healthcare professionals.
- Capability to obtain information about hospital visits internally or from other sources.
- Enabling the clinical team to have an overview of new alerts for each patient, including the client’s name, data type, and last measurement date.
- Allowing the clinical team to define what relevant health values to display on the dashboard.
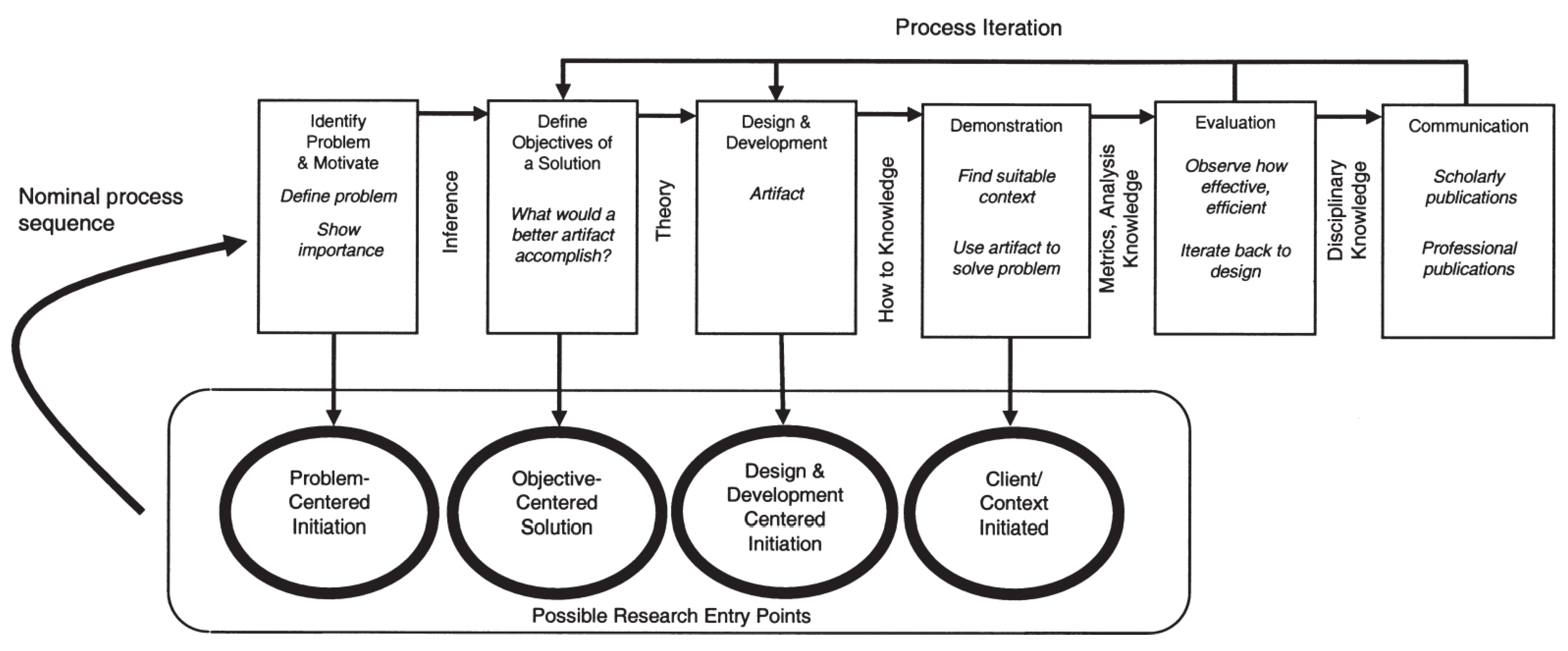
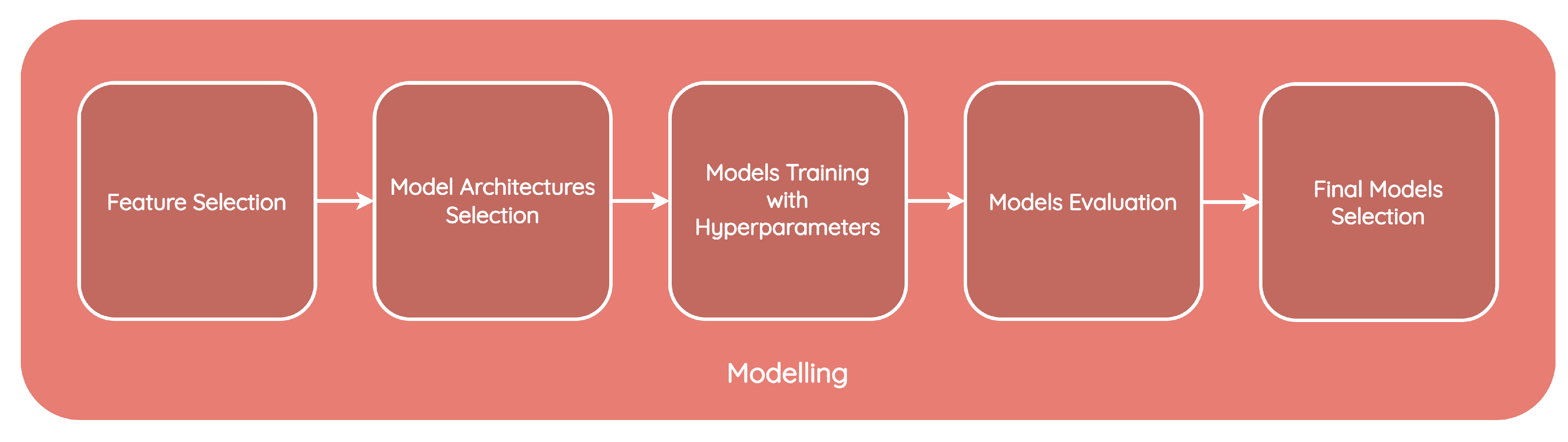
1.6. Methodology
2. Related Work
2.1. In-Home Healthcare for COPD
2.2. E-Healthcare Supported by Predictive Analytics
2.3. Factors Associated with COPD Exacerbation
2.4. Machine Learning for Early Identification of Deterioration
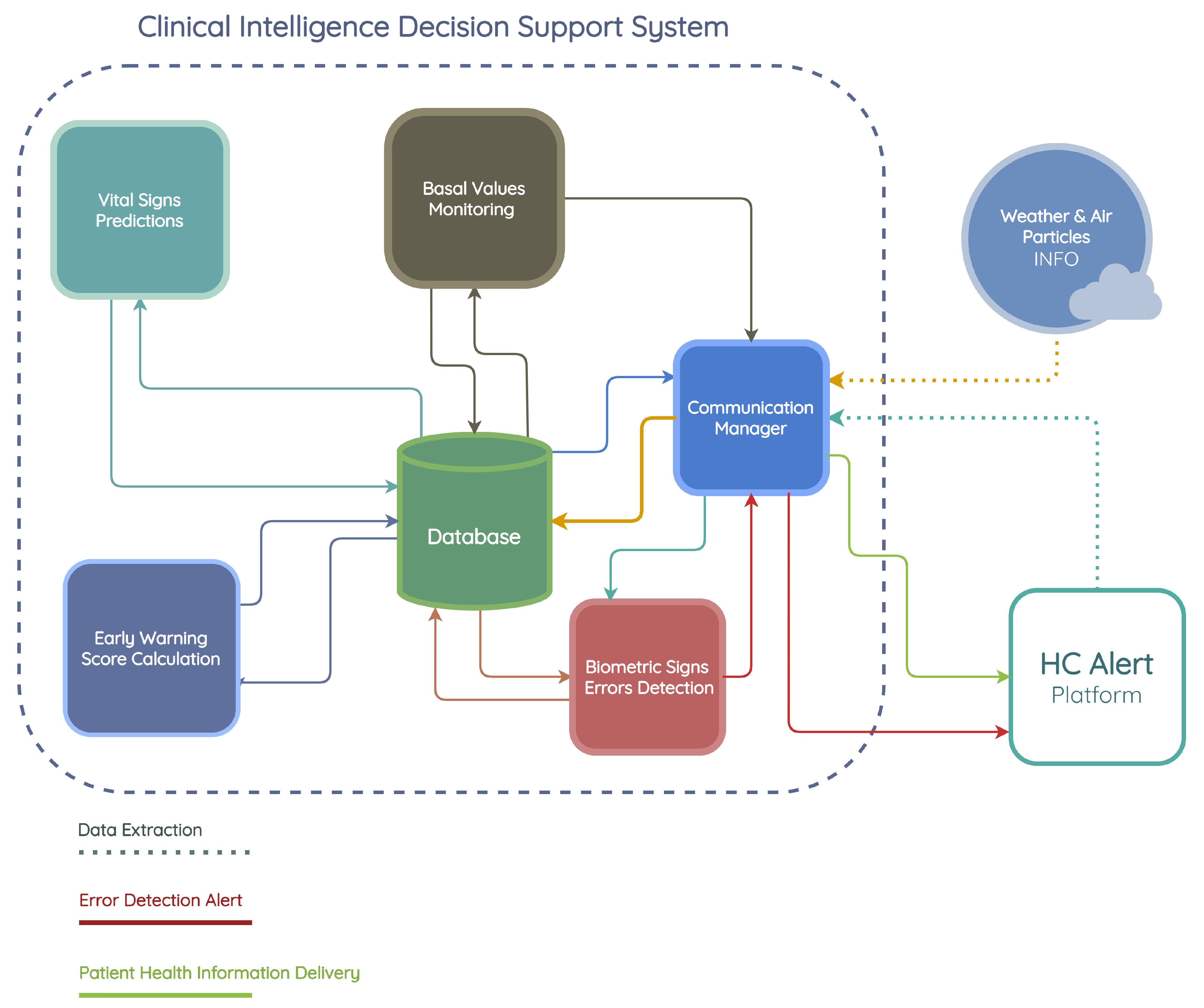
3. CIDSS Design
3.1. Requirements
3.2. Communication Manager
3.2.1. Data Extraction
3.2.2. Measurement Error Alert
3.2.3. Basal Value Monitoring Notification
3.2.4. Patient Risk Information Delivery
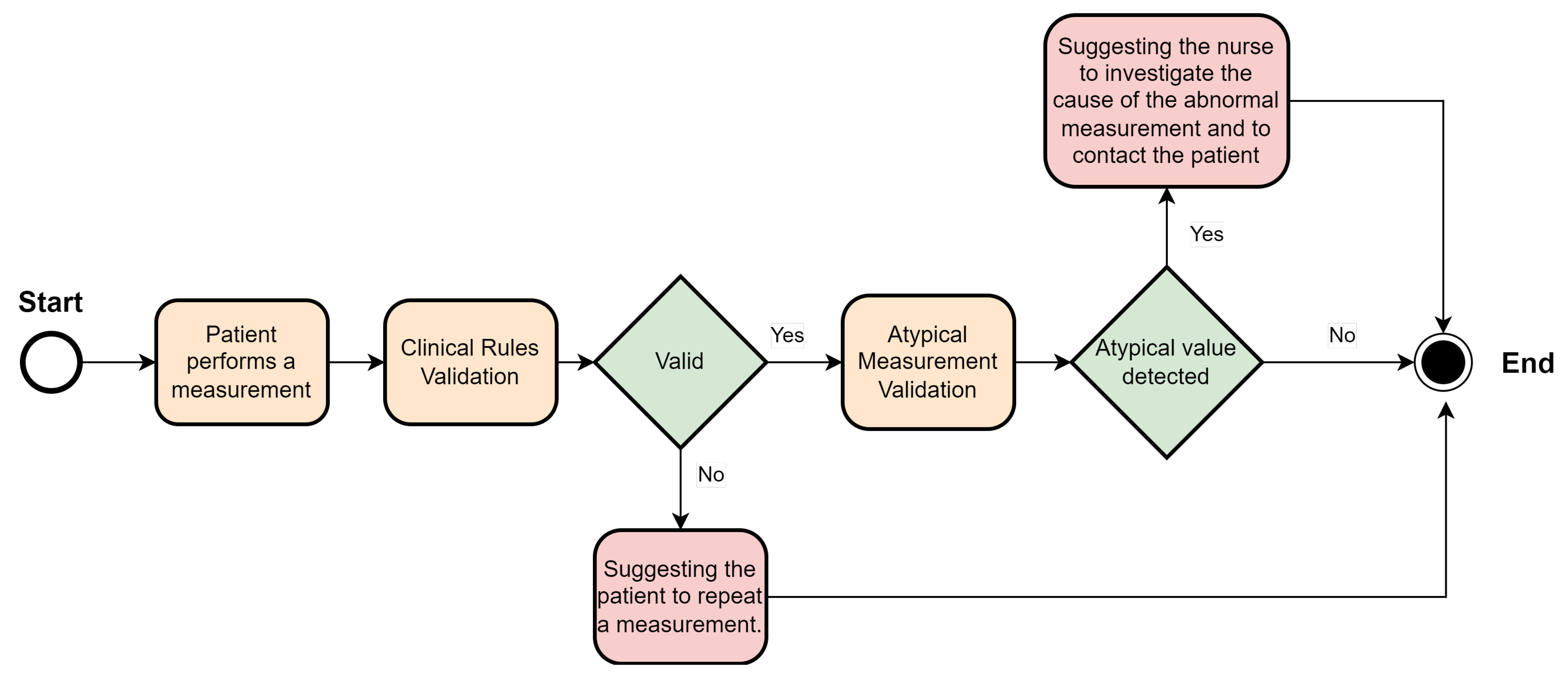
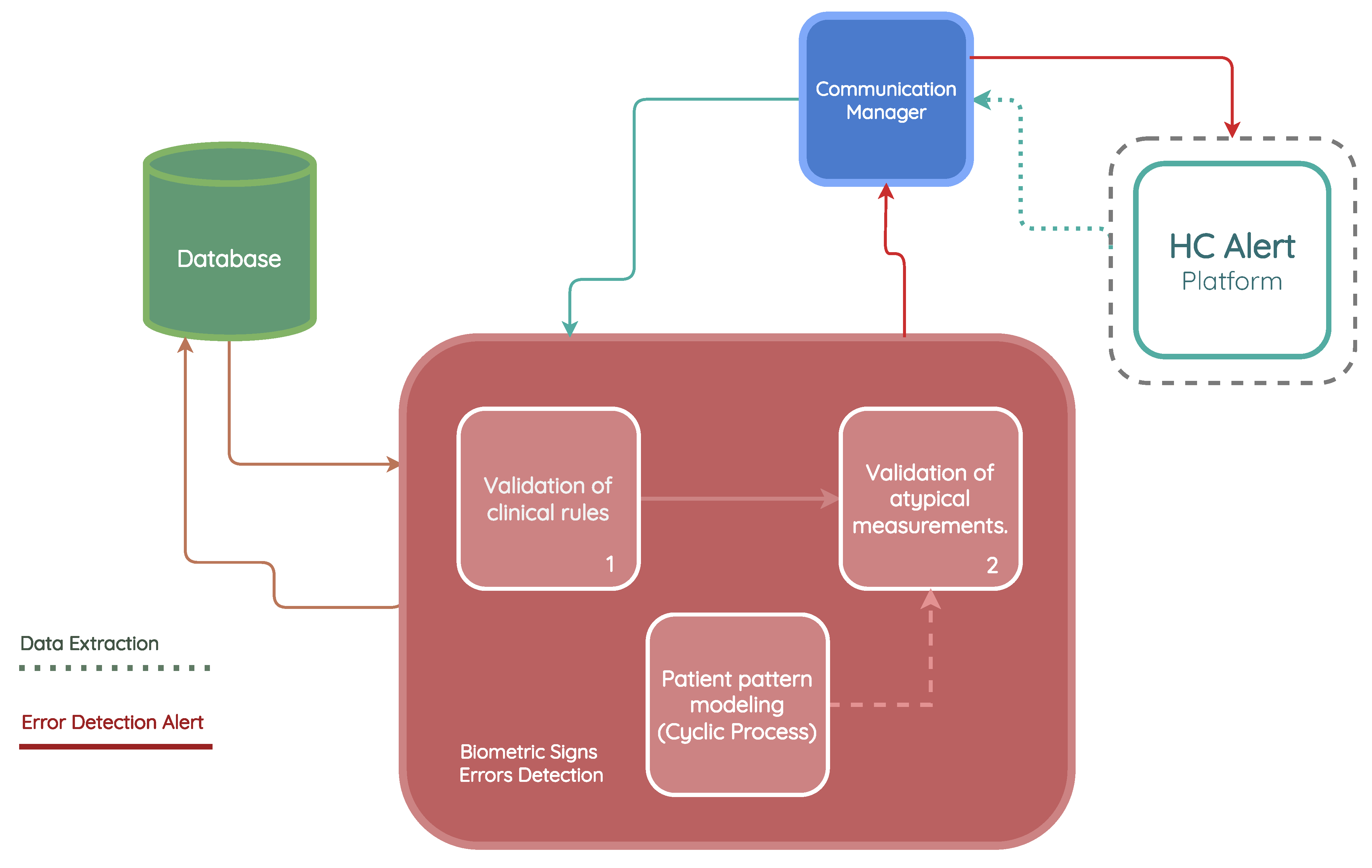
3.3. Biometric Sign Error Detection
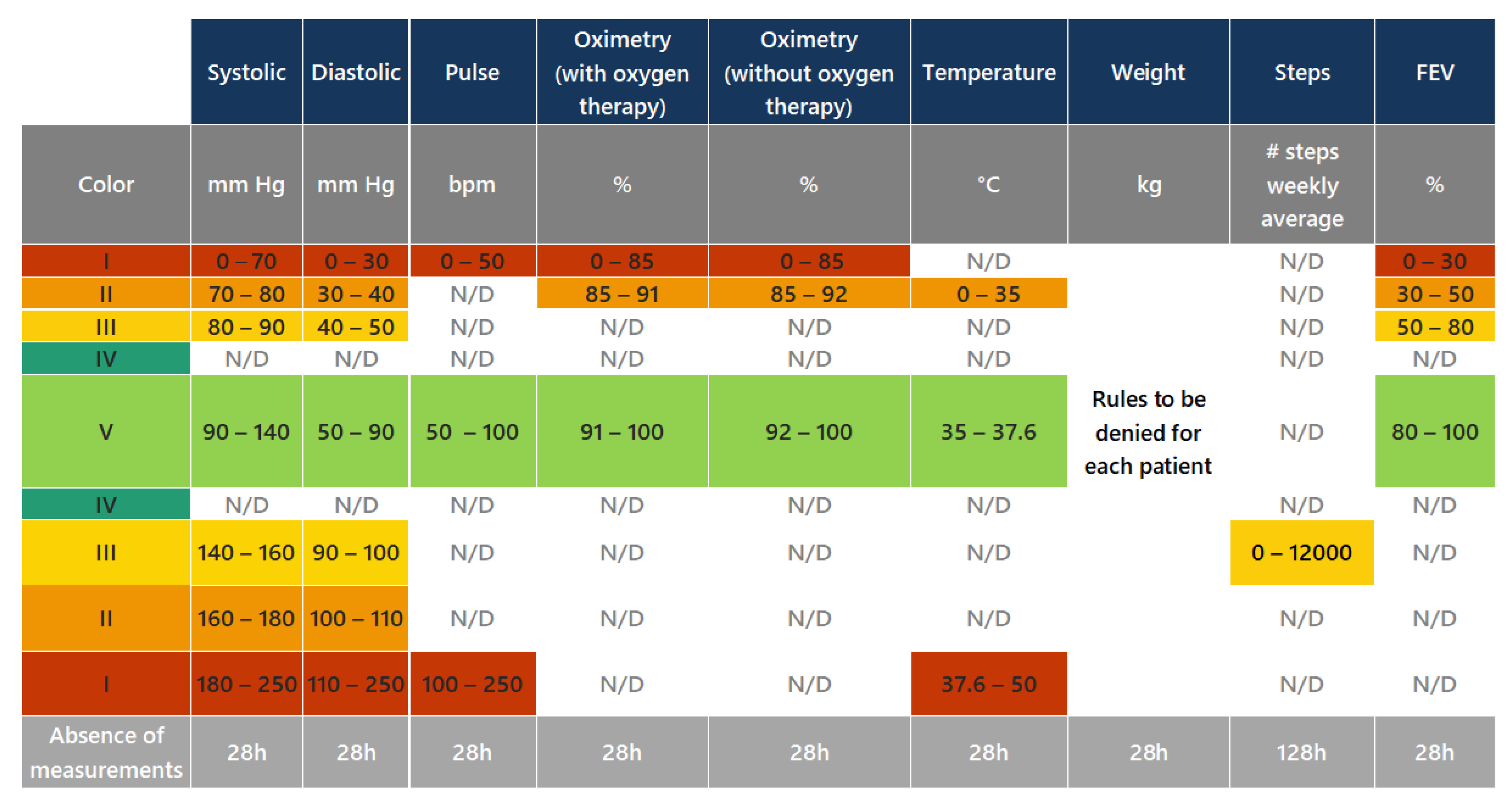
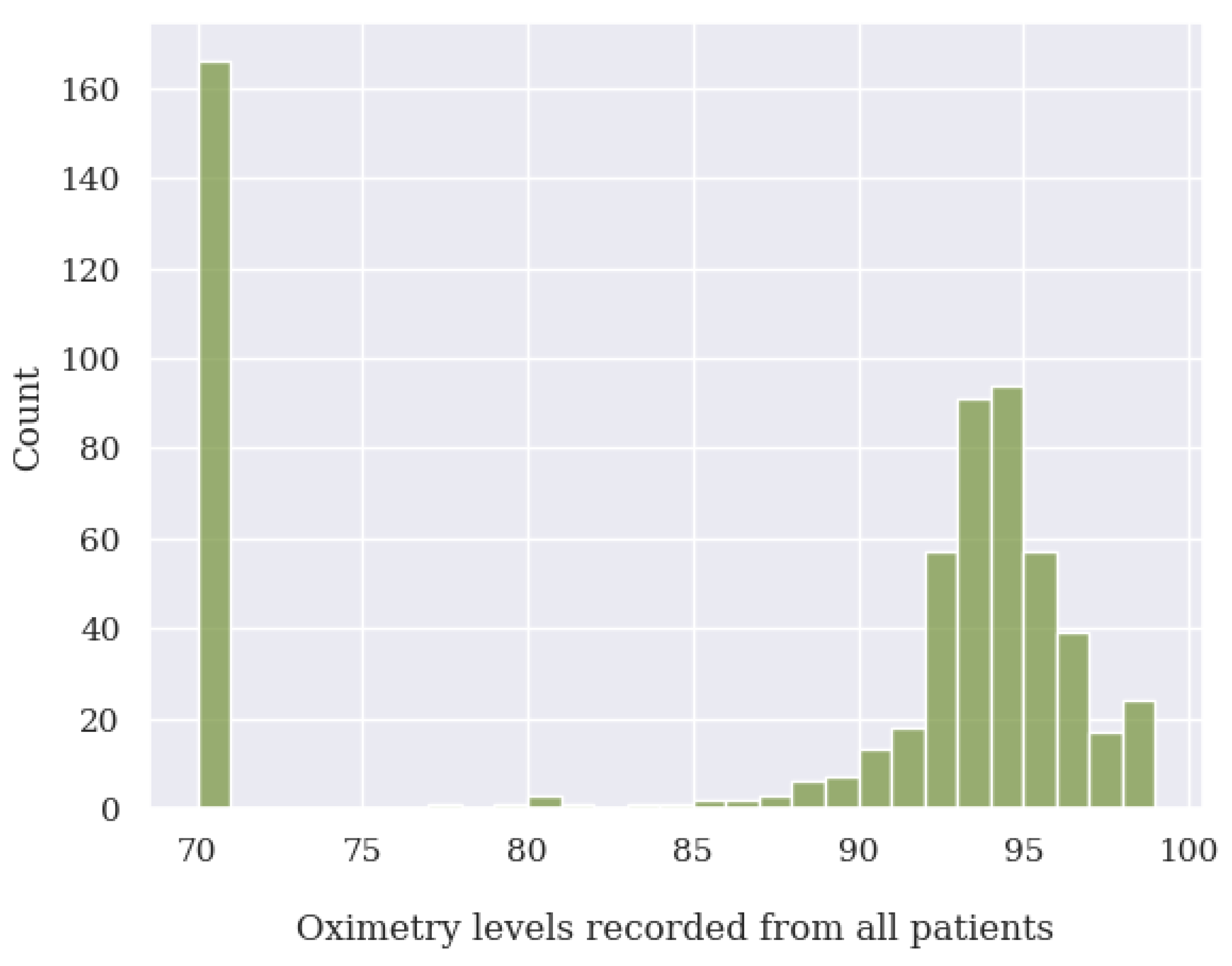
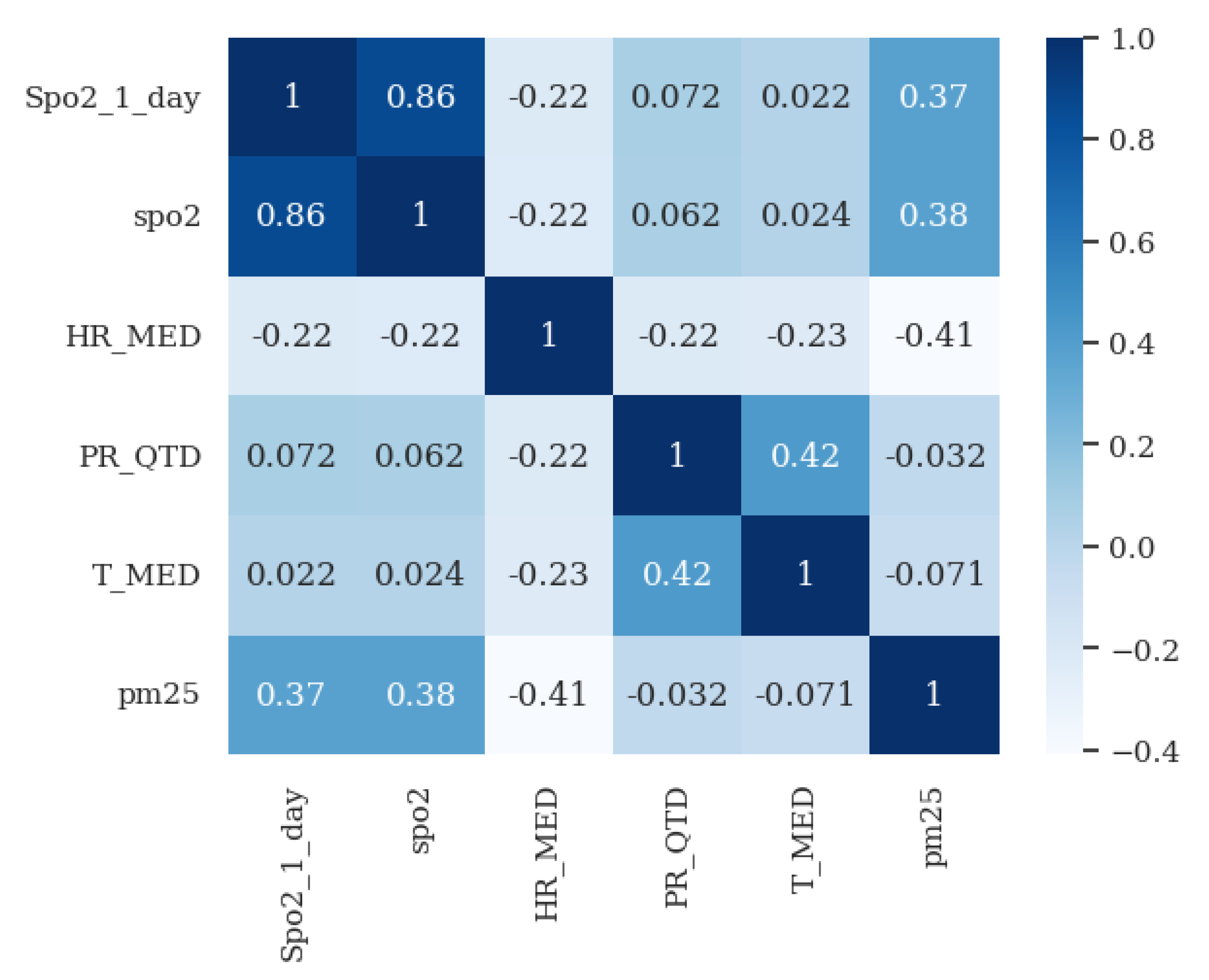
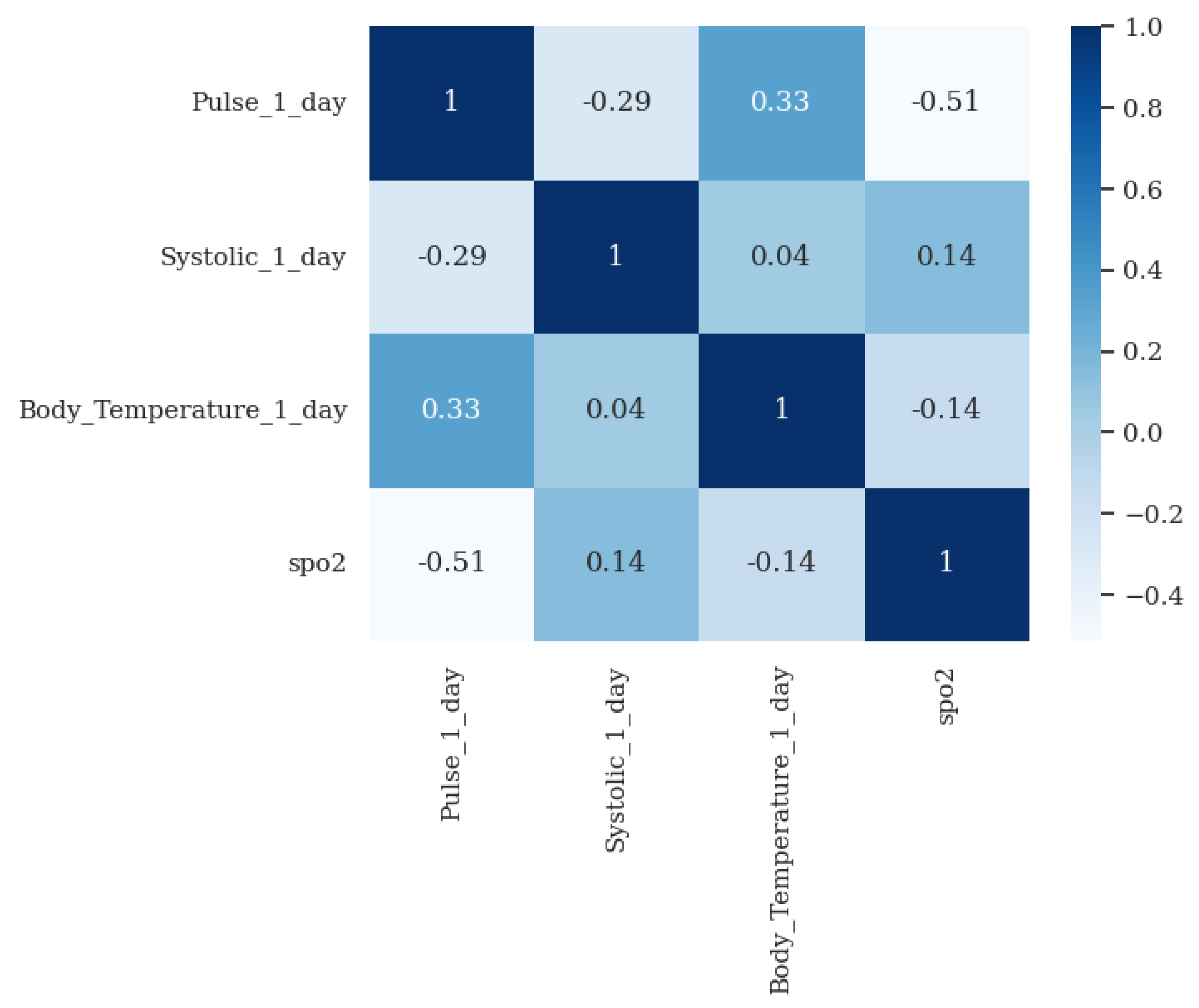
- Validation of clinical rules: This component compares the measurements taken by the patient with a set of business rules defined according to Hope Care guidelines. For example, a measurement of oxygen saturation above 100 or below 20 cannot be correct since a percentage value cannot exceed 100, and a value below 20 corresponds to situations of compromised brain function and even comas. The medical team involved in this research work validated all ranges used to filter the vital signs.
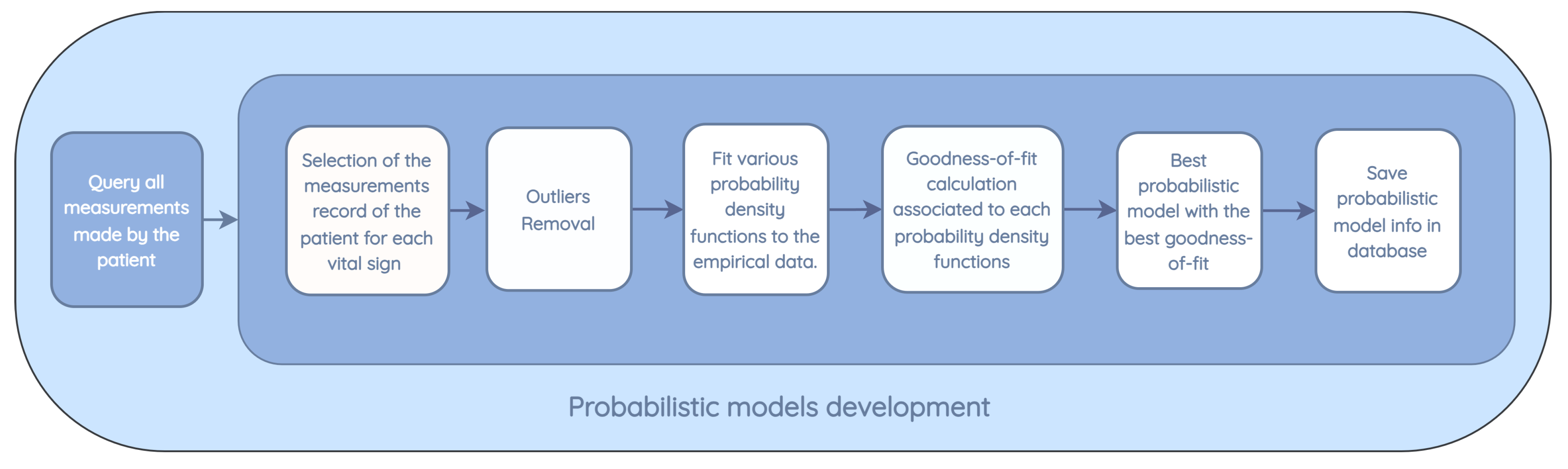
- Patient pattern modeling: The objective of this component is to approximate a probability density function for each metric in the patient’s measurements. These probability density models are then stored in the database, eliminating the need to repeat the function modeling each time a new inference is made. This module runs monthly to create a new probability function that captures the variability of the new measurements entered by the patient.
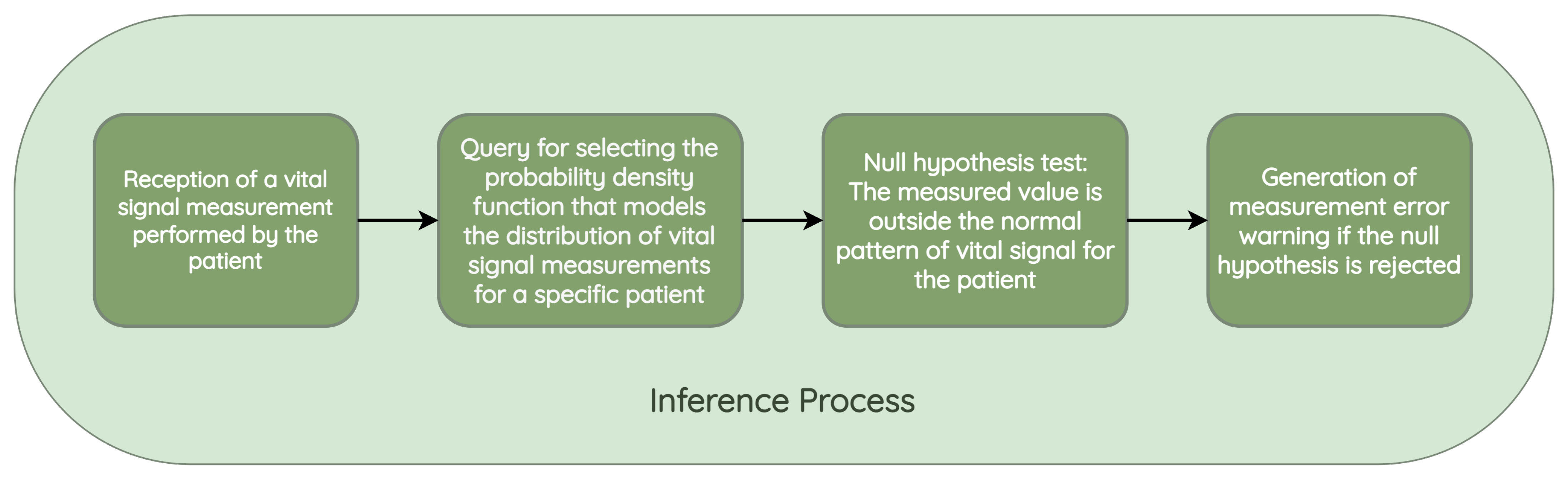
- Validation of atypical measurements based on the patient’s history: This module uses the probability density models stored in the database, which are associated with each patient’s vital signs, to determine whether a newly recorded measurement falls within the normal patterns for that specific patient. As these variations could be due to disease exacerbation, improvements from a new medication, or other factors, need to be validated by a nurse and, if necessary, by the patients themselves, to determine the true cause of the variation.
- Oxygen saturation above 100 or below 20;
- Body temperature below 30 or above 40;
- Systolic blood pressure below 50 or above 350;
- Diastolic blood pressure below 40 or above 200;
- Pulse rate less than or equal to 30, or greater than 250.
- From all the measurements collected for the patient, only the measurements made for specific vital signs in training are used.
- Existing outliers in the database, prior to modeling, are removed. Outliers are removed based on the standard deviation by calculating the standard score (z-score), which corresponds to the number of standard deviations by which a newly recorded value deviates from the mean of the observed measurements. If the z-score is greater than 3, which corresponds to a value that is three times the standard deviation away from the mean of the data, the value is not used in the modeling.
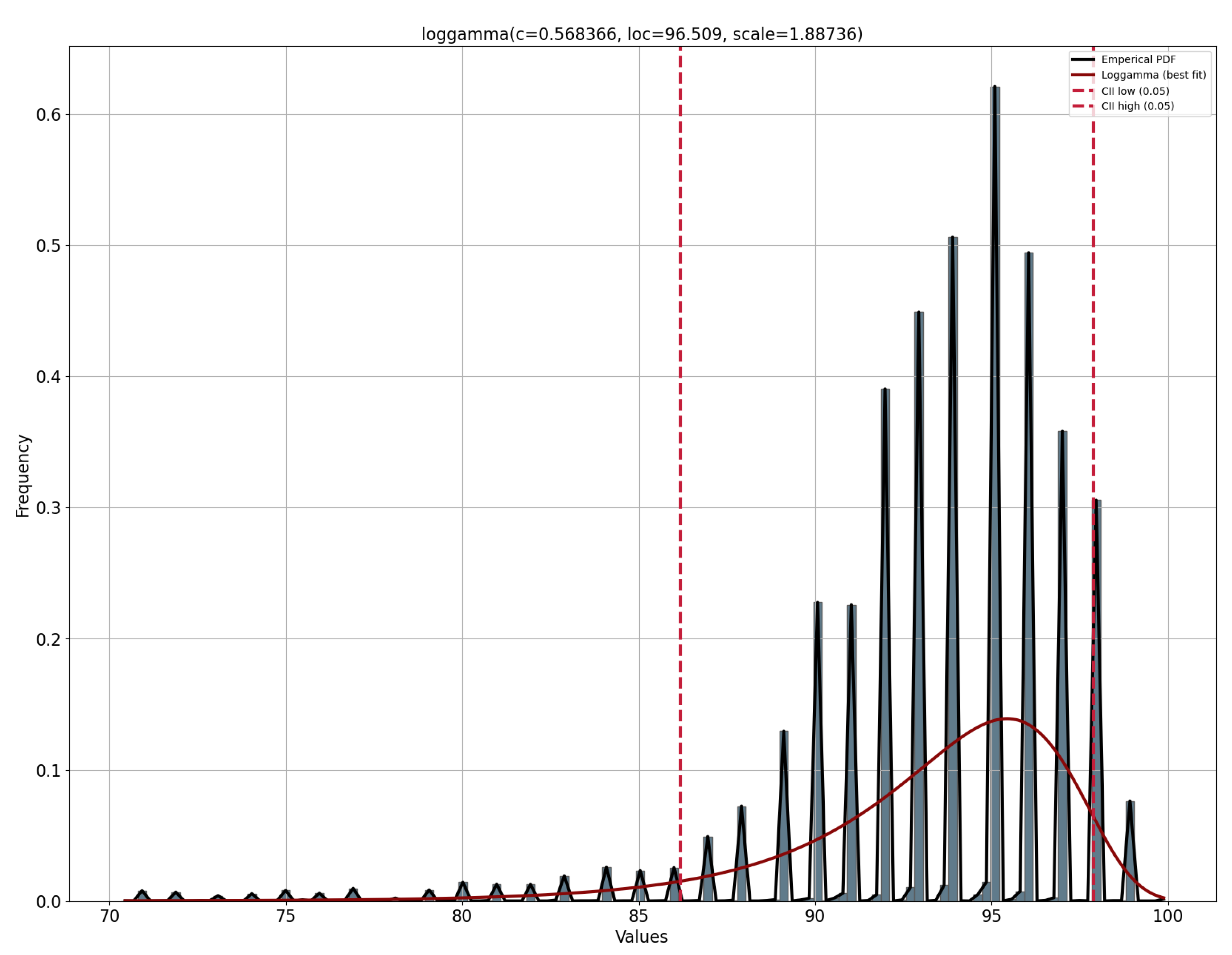
- The following distributions are tested: normal, exponential, Pareto, double Weibull, t, generalized extreme value distribution, gamma, lognormal, beta, and uniform. For each distribution, the density and weights of the histogram are computed. Subsequently, an estimation of the function parameters is performed based on the data. The maximum likelihood estimation (MLE) is used to identify the values that best fit the data.
- The goodness-of-fit is calculated with a test of the sum of squares of the residuals for each distribution found.
- The model with the best goodness-of-fit, which implies a lower value in the sum of squares of the residuals, is stored for the vital signs of the patient under study.
3.4. Basal Value Monitoring
3.4.1. Basal Value Monitoring Module Architecture
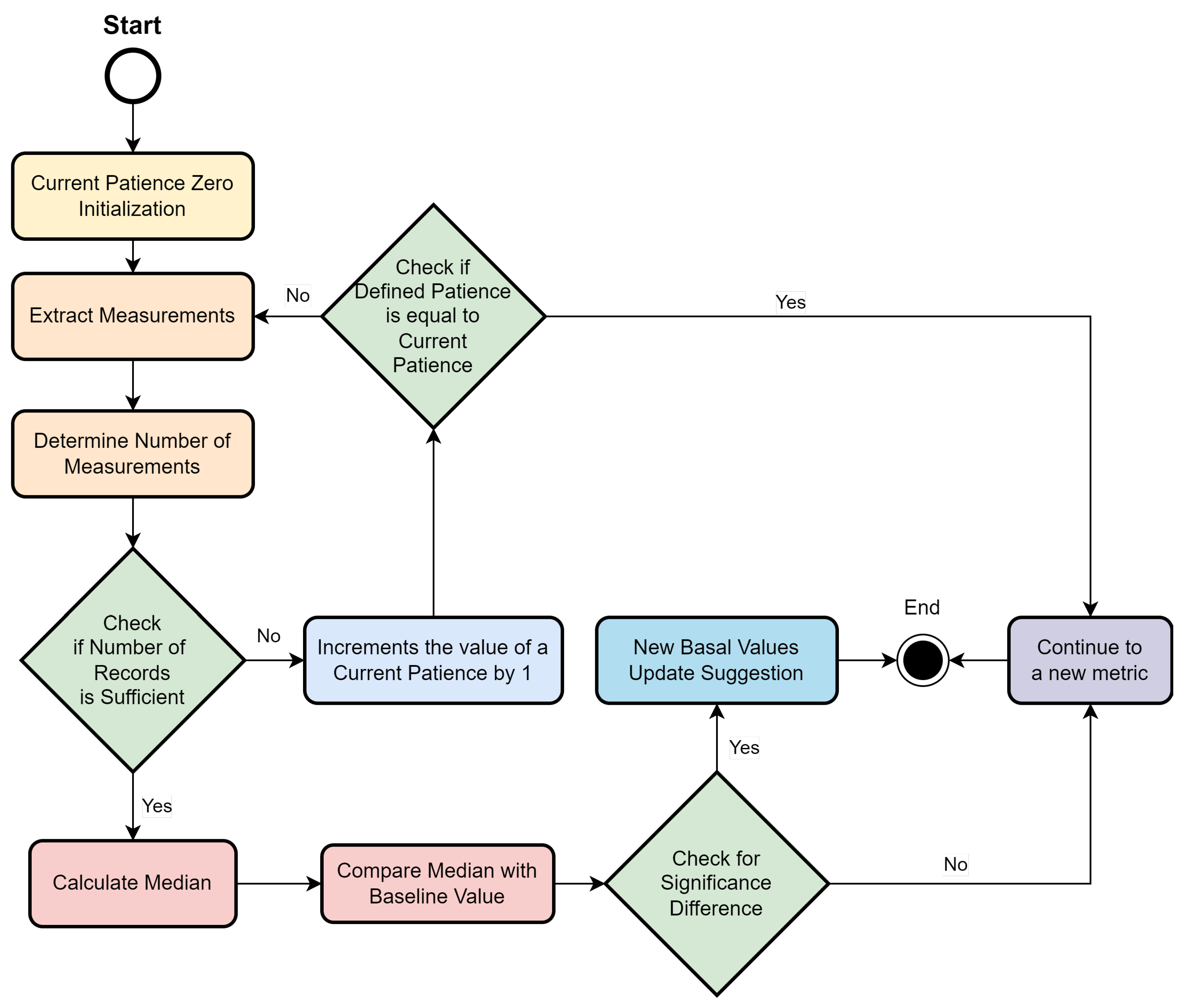
- Number of months considered: This indicates the past time window that is analyzed for the baseline calculation. The default value is 3 months, which indicates that when this module runs, the measurements taken from the last 3 months are extracted for the baseline calculation. This value can be configured by rules in the system.
- Minimum number of records: This corresponds to the minimum number of measurements taken by the patient, so that the calculated baseline information is considered reliable. If the patient does not have a satisfactory number of measurements in the time horizon under study, the module will not provide recommendations. For example, a patient with only five SpO2 measurements over 3 months will not be considered for updating the baseline value. This value is configurable by a rule, and value 50 is used by default in the system.
- Patience: In case the patient does not present enough measurements of a certain parameter in the defined time horizon, the system expands the time horizon of the search to include more months of history until it finds an acceptable amount of records. For example, with a patience of 3 months and a minimum of 50 required measurements, if the patient only has 30 measurements, an additional month will be incorporated into the analysis, and the module will be rerun using the past four months, reducing the patience counter by 1. In case patience reaches zero, and the minimum value of measurements defined is not reached, the system will not provide any recommendation for the given parameter due to the lack of consistency in the measurements. The default value for patience, which can be configurable by a rule, is 3.
3.4.2. Basal Value Monitoring Module Implementation
- 1.
- A flag representing the current patience is initialized to zero.
- 2.
- The measurements are related to the period of months corresponding to the last X months from the date of execution of the module, where X is the sum between the system input “number of months to consider” and the current patience value.
- 3.
- The number of measurements performed by the patient is calculated.
- (a)
- In case the number of measurements is not sufficient, the current patience is incremented by 1.
- (i)
- If the current patience value is equal to the user-defined patience value, no recommendation is displayed, and the cycle continues to the next measurement in the list.
- (ii)
- If the current patience value is less than the set patience value, the system summarizes the run from step 2.
- (b)
- In case the measurements are sufficient, the system summarizes the run in step 4.
- 4.
- The median of the patient’s measured values of a given vital sign is calculated.
- 5.
- The median value is compared with the baseline value recorded in the clinical protocol.
- (a)
- If the values are very different, a recommendation is made to update the baseline value to reflect the new median value recorded in the time interval under consideration. This recommendation should be evaluated by a medical professional.
- (b)
- If the values are similar, the baseline value is not adjusted, and the system summarizes in step 1, with a new iteration of a new metric under evaluation.
- 6.
- The cycle ends when all metrics in the list have been processed.
3.5. Vital Signs Prediction Module
3.5.1. Predictive Model Development
Data Treatment
- For oxygen saturation (SpO2), any values below or equal to 70% and above 100%. Since we have detected many measurements at exactly 70%, we suspect these are measurement errors;
- For body temperature, all values below 30 C and above 40 C;
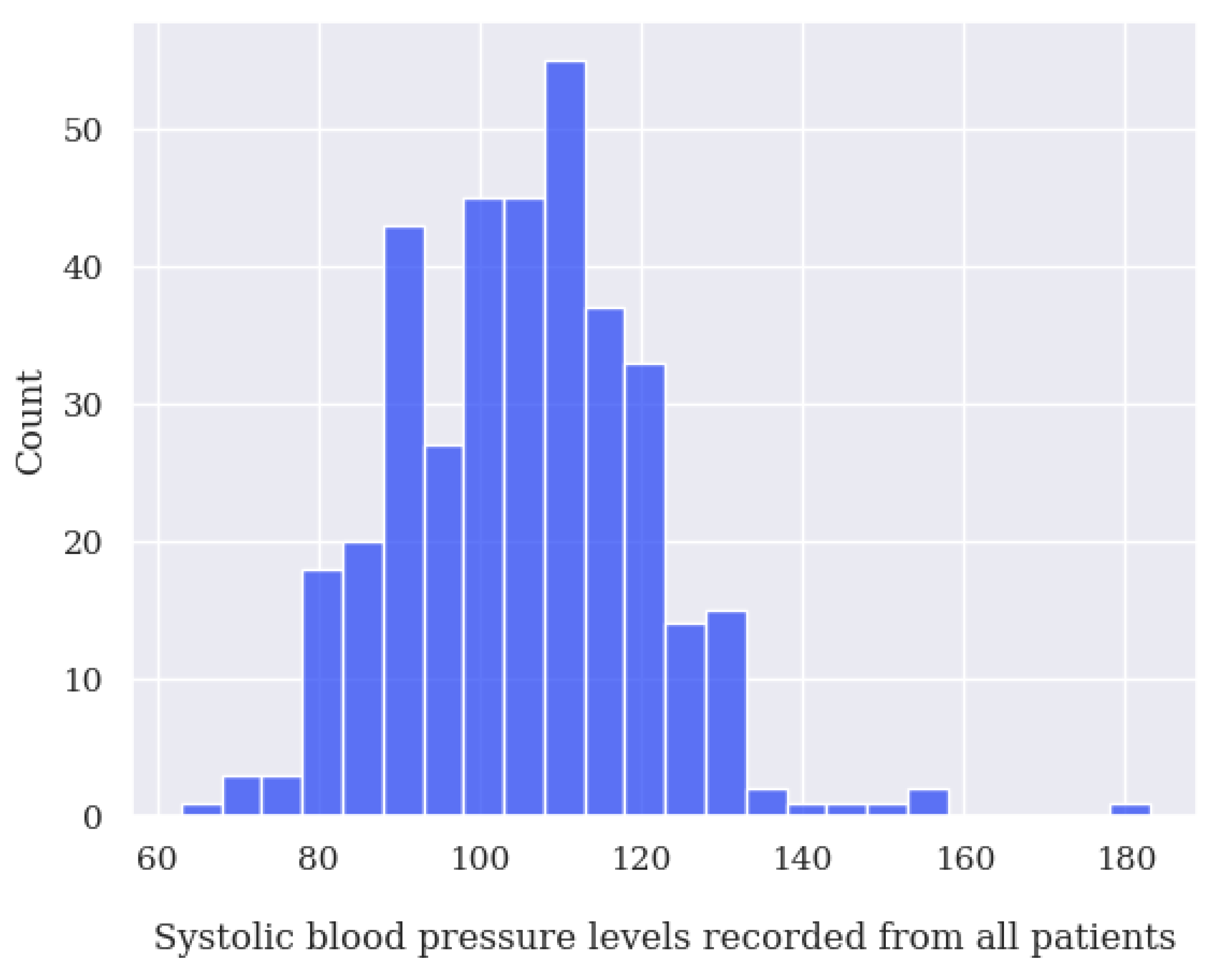
- For systolic blood pressure (SBP), any values below 50 mmHg and above 350 mmHg;
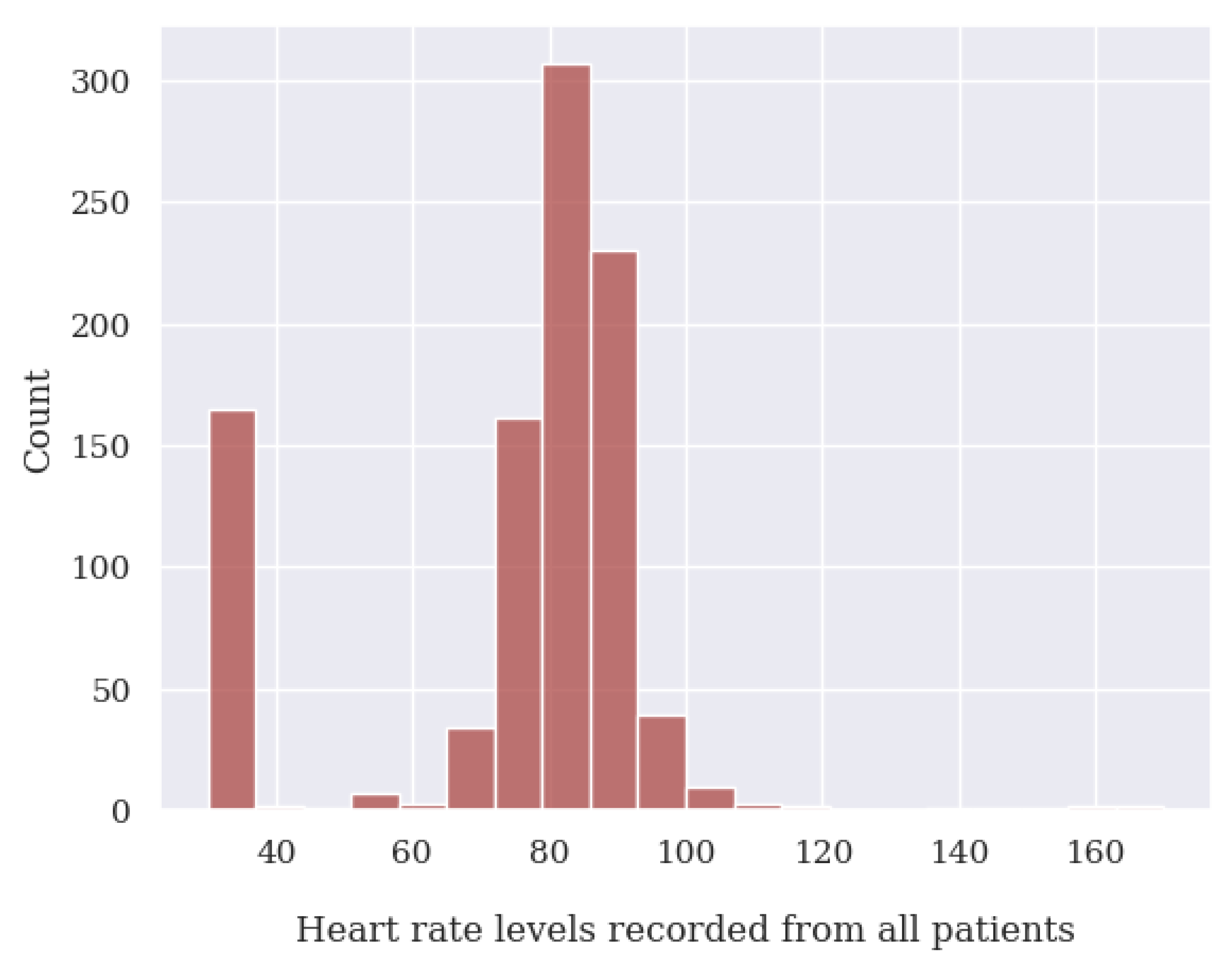
- For heart rate (HR), any values below 39 BPM and above 250 BPM.
- For diastolic blood pressure (DBP), any values below 40 mmHg or above 200 mmHg.
Modeling and Evaluation
3.5.2. Production
3.6. Early Warning Score Calculation Module
4. Demonstration and Evaluation
| Listing 1. Structure of the JSON file provided to HCAlert for patient risk information on 25 April. |
 |
| Listing 2. Suggested new basal values for patient 300 to the HCAlert platform. |
 |
| Listing 3. Structure of the JSON file provided to HCAlert for patient risk information on 25 October. |
 |
System Evaluation
5. Conclusions
5.1. Work Conclusions
5.2. Limitations
5.3. Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Available online: www.who.int/data/global-health-estimates (accessed on 1 August 2023).
- Portuguese Society of Pneumology. Available online: https://www.sppneumologia.pt/saudepublica/dpoc/ (accessed on 1 August 2023).
- Portuguese Lung Foundation. Observatório Nacional Doenças Respiratórias 2022. Available online: https://ondr2022.fundacaoportuguesadopulmao.org/ (accessed on 1 August 2023).
- Mogo, S.; Cachorro, V.E.; de Frutos, A.M. Morphological, chemical and optical absorbing characterization of aerosols in the urban atmosphere of Valladolid. Atmos. Chem. Phys. 2005, 5, 2739–2748. [Google Scholar] [CrossRef]
- Arranz, M.C.; Moreno, M.F.M.; Medina, A.A.; Capitán, M.A.; Vaquer, F.C.; Gómez, A.A. Health impact assessment of air pollution in Valladolid, Spain. BMJ Open 2014, 4, e005999. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H. Effect of Global Climate Change-Related Factors on COPD Morbidity. Tanaffos 2017, 16, S24. [Google Scholar] [PubMed]
- World Health Organization(WHO). Review of Evidence on Health Aspects of Air Pollution: REVIHAAP Project: Technical Report. Available online: https://apps.who.int/iris/handle/10665/341712 (accessed on 1 August 2021).
- World Health Organization(WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 1 August 2023).
- Gregório, J.; Reis, L.; Peyroteo, M.; Maia, M.; da Silva, M.M.; Lapão, L.V. The role of Design Science Research Methodology in developing pharmacy eHealth services. Res. Soc. Adm. Pharm. 2021, 17, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Peffers, K.; Tuunanen, T.; Rothenberger, M.A.; Chatterjee, S. A design science research methodology for information systems research. J. Manag. Inf. Syst. 2007, 24, 45–77. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Pare, G.; Jaana, M.; Sicotte, C. Systematic Review of Home Telemonitoring for Chronic Diseases: The Evidence Base. J. Am. Med. Inform. Assoc. 2007, 14, 269–277. [Google Scholar] [CrossRef]
- Jang, S.; Kim, Y.; Cho, W.K. A Systematic Review and Meta-Analysis of Telemonitoring Interventions on Severe COPD Exacerbations. Int. J. Environ. Res. Public Health 2021, 18, 6757. [Google Scholar] [CrossRef]
- Kruse, C.; Pesek, B.; Anderson, M.; Brennan, K.; Comfort, H. Telemonitoring to Manage Chronic Obstructive Pulmonary Disease: Systematic Literature Review. JMIR Med. Inform. 2019, 7, e11496. [Google Scholar] [CrossRef]
- Achelrod, D.; Schreyögg, J.; Stargardt, T. Health-economic evaluation of home telemonitoring for COPD in Germany: Evidence from a large population-based cohort. Eur. J. Health Econ. 2016, 18, 869–882. [Google Scholar] [CrossRef]
- Hong, N.; Liu, C.; Gao, J.; Han, L.; Chang, F.; Gong, M.; Su, L. State of the Art of Machine Learning–Enabled Clinical Decision Support in Intensive Care Units: Literature Review. JMIR Med. Inform. 2022, 10, e28781. [Google Scholar] [CrossRef]
- Pépin, J.L.; Degano, B.; Tamisier, R.; Viglino, D. Remote Monitoring for Prediction and Management of Acute Exacerbations in Chronic Obstructive Pulmonary Disease (AECOPD). Life 2022, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Exarchos, K.; Aggelopoulou, A.; Oikonomou, A.; Biniskou, T.; Beli, V.; Antoniadou, E.; Kostikas, K. Review of Artificial Intelligence Techniques in Chronic Obstructive Lung Disease. IEEE J. Biomed. Health Inform. 2022, 26, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-W.; Wang, Y.; Sun, Y.; Zhang, Q.; Yan, L.-M.; Wang, Y.-X.; Gao, J.-H.; Yin, Y.; Wang, Q.-Y.; Li, X.-L.; et al. Effectiveness of Telemonitoring for Reducing Exacerbation Occurrence in COPD Patients With Past Exacerbation History: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Morillo, D.; Fernandez-Granero, M.A.; Leon-Jimenez, A. Use of predictive algorithms in-home monitoring of chronic obstructive pulmonary disease and asthma. Chronic Respir. Dis. 2016, 13, 264–283. [Google Scholar] [CrossRef]
- Carlin, C.; Taylor, A.; van Loon, I.; McDowell, G.; Burns, S.; McGinness, P.; Lowe, D.J. Role for artificial intelligence in respiratory diseases—Chronic obstructive pulmonary disease. J. Hosp. Manag. Health Policy 2021, 5, 27. [Google Scholar] [CrossRef]
- Rajeh, A.A.; Hurst, J. Monitoring of Physiological Parameters to Predict Exacerbations of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review. J. Clin. Med. 2016, 5, 108. [Google Scholar] [CrossRef]
- Martín-Lesende, I.; Orruño, E.; Bilbao, A.; Vergara, I.; Cairo, M.C.; Bayón, J.C.; Reviriego, E.; Romo, M.I.; Larrañaga, J.; Asua, J.; et al. Impact of telemonitoring home care patients with heart failure or chronic lung disease from primary care on healthcare resource use (the TELBIL study randomised controlled trial). BMC Health Serv. Res. 2013, 13, 118. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.M.; Kim, S.K.; Yoo, K.H.; Jung, K.S.; Lee, S.H.; Rhee, C.K. Factors associated with chronic obstructive pulmonary disease exacerbation, based on big data analysis. Sci. Rep. 2019, 9, 6679. [Google Scholar] [CrossRef]
- Liu, Z.; Alavi, A.; Li, M.; Zhang, X. Self-Supervised Contrastive Learning for Medical Time Series: A Systematic Review. Sensors 2023, 23, 4221. [Google Scholar] [CrossRef]
- Bui, C.; Pham, N.; Vo, A.; Tran, A.; Nguyen, A.; Le, T. Time Series Forecasting for Healthcare Diagnosis and Prognostics with the Focus on Cardiovascular Diseases. In Proceedings of the 6th International Conference on the Development of Biomedical Engineering in Vietnam (BME6), Ho Chi Minh City, Vietname, June 2016; Springer: Singapore, 2017; pp. 809–818. [Google Scholar]
- Kaieski, N.; da Costa, C.A.; da Rosa Righi, R.; Lora, P.S.; Eskofier, B. Application of artificial intelligence methods in vital signs analysis of hospitalized patients: A systematic literature review. Appl. Soft Comput. 2020, 96, 106612. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Yu, Z.; Guo, B. Enabling Timely Medical Intervention by Exploring Health-Related Multivariate Time Series with a Hybrid Attentive Model. Sensors 2022, 22, 6104. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Qu, F.; Nie, P. Ensembles of Gradient Boosting Recurrent Neural Network for Time Series Data Prediction. IEEE Access 2021. [Google Scholar] [CrossRef]
- da Silva, D.B.; Schmidt, D.; da Costa, C.A.; da Rosa Righi, R.; Eskofier, B. DeepSigns: A predictive model based on Deep Learning for the early detection of patient health deterioration. Expert Syst. Appl. 2021, 165, 113905. [Google Scholar] [CrossRef]
- Haselbeck, F.; Killinger, J.; Menrad, K.; Hannus, T.; Grimm, D.G. Machine Learning Outperforms Classical Forecasting on Horticultural Sales Predictions. Mach. Learn. Appl. 2022, 7, 100239. [Google Scholar] [CrossRef]
- Chacón, H.; Koppisetti, V.; Hardage, D.; Choo, K.K.R.; Rad, P. Forecasting call center arrivals using temporal memory networks and gradient boosting algorithm. Expert Syst. Appl. 2023, 224, 119983. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: https://goldcopd.org/2023-gold-report-2/ (accessed on 1 August 2023).
- Draxler, R.; Hess, G. Hybrid Single-Particle Lagrangian Integrated Trajectories (HY-SPLIT): Version 4.0-Description of the Hysplit_4 Modeling System; NOAA Technical Memorandum ERL ARL-224; NOAA: Silver Spring, MD, USA, 2010; Volume 12. [Google Scholar]
- Rolph, G.; Stein, A.; Stunder, B. Real-time Environmental Applications and Display sYstem: READY. Environ. Model. Softw. 2017, 95, 210–228. [Google Scholar] [CrossRef]
- Chandra, R.; Goyal, S.; Gupta, R. Evaluation of deep learning models for multi-step ahead time series prediction. IEEE Access 2021, 9, 83105–83123. [Google Scholar] [CrossRef]
- Prat, N.; Comyn-Wattiau, I.; Akoka, J. Artifact Evaluation in Information Systems Design-Science Research—A Holistic View. 2014. Available online: https://aisel.aisnet.org/pacis2014/23/ (accessed on 1 August 2023).







| Subsection | Query |
|---|---|
| In-Home Healthcare for COPD |
(“Healthcare Management Systems” AND “Real-time Detection”) |
| E-Healthcare supported by Predictive analytics | (“Healthcare Management Systems” AND “Early Detection” AND (“Artificial Intelligence” AND “Machine Learning”)) |
| Factors related with COPD deterioration | (“Early Detection” AND “Vital Signs” AND “COPD”) |
| Machine Learning for for Early Identification of a Deterioration | (“Early Detection” AND “Vital Signs” AND “Machine Learning”) |
| Eligibility Criteria | |
|---|---|
| Inclusion Criteria | Exclusion Criteria |
| Written in English or Portuguese | Not written in English or Portuguese |
| Publication date after/during 2010 | Publication date before 2010 |
| Requirements | Module |
|---|---|
| The predictive service should collect environmental data, such as air quality, seasonal infection incidences, and weather conditions | Vital Signs Prediction |
| The predictive service should correlate parameters and detect patterns | |
| The predictive service should reevaluate the weighting of each parameter, depending on the context (e.g., patient, clinical history, etc.) | |
| The collected data should undergo anonymization (if applicable), normalization, and data fusion | |
| The predictive service should consider the early warning score to generate alerts | Communication Manager |
| The predictive service should consider the alert classification to detect false positives | |
| The predictive service should advise the user to take a new measurement and launch inquiries to validate if it is a false positive | Biometric Sign Error Detection |
| The predictive system should apply the early warning score to the clinical protocol and suggest changes to the protocol based on the basal value | Early Warning Calculation |
| The predictive service should calculate the early warning score (define the correlation weighting of each parameter in the EWS calculation) | |
| The predictive system should recommend a reassessment of the basal value | Basal Value Monitoring |
| The predictive system should take into account changes made to the clinical protocol by the clinical team | |
| The predictive system should analyze the threshold for advising changes to the applied clinical protocol for the patient |
| idRawMeasurement | Measurement Identifier |
|---|---|
| createdOn | Measurement creation date |
| clientID | Identification of the patient to whom the measurement belongs |
| Latitude | Latitude of the patient |
| Longitude | Longitude of the patient |
| ProviderMNameStandard | Standard name of the type of measurement |
| Value | Measurement value |
| Unit | Units of measurement (in the dataset are available %, C, bpm, count, mmHg, NA, null, and percent) |
| idWeatherMeasurement | Measurement Identifier |
|---|---|
| Station ID | Station identifier |
| Latitude | Latitude of the station |
| Longitude | Longitude of the station |
| Year | Year of the collected measurement |
| Month | Month of the collected measurement |
| Day | Day of the collected measurement |
| T_MED | Value of the daily mean temperature in Celsius |
| HR_MED | Value of the daily mean relative humidity in percent |
| idParticlesMeasurement | Measurement Identifier |
|---|---|
| Location | Location of the station |
| Latitude | Latitude of the station |
| Longitude | Longitude of the station |
| Date | Date of the collected measurement |
| PM10 | Value of PM10 |
| PM2_5 | Value of PM2.5 |
| Model | SpO2 | Heart Rate | Systolic Blood Pressure | Body Temperature |
|---|---|---|---|---|
| ARIMA | 2.080718 | 7.089329 | 9.783878 | 0.247163 |
| XGBoost | 0.817778 | 0.96435 | 2.407083 | 0.302518 |
| LightGBM | 0.064668 | 0.380769 | 2.170715 | 0.058705 |
| GRU | 0.083168 | 0.110159 | 0.130179 | 0.131379 |
| LSTM | 0.092241 | 0.573169 | 0.135822 | 0.137075 |
| BILSTM | 0.084948 | 0.113384 | 0.132097 | 0.130094 |
| Vital Sign Predicted | Type | RMSE |
|---|---|---|
| SpO2 | LightGBM | 0.064668 |
| Heart Rate | GRU | 0.110159 |
| Systolic Blood Pressure | GRU | 0.130179 |
| Body Temperature | LightGBM | 0.058705 |
| Description | 0 Points | 1 Point | 2 Points | 3 Points | |
|---|---|---|---|---|---|
| SpO2 | Difference between the predicted value for the day and the value from the previous day | <3% | 3–5% | 6–7% | >7% |
| Heart Rate | BPM Value | 46–100 | 101–110 | 111–115 | >115 or <46 |
| Systolic Blood Pressure | Percentage difference between the predicted value for the day and the baseline value | <20% | ≥20% | ≥23% | ≥25% |
| Body Temperature | Temperature value in Celsius | <37.5 | 37.5–37.9 | 38–38.4 | >38.5 |
| Date (yy-mm-dd) | Heart Rate (BPM) | Body Temperature (C) | SpO2 (%) | Systolic Blood Pressure (mmHg) | T MED (C) | HR MED (%) | PR QTD (mm) | pm25 (Count) |
|---|---|---|---|---|---|---|---|---|
| 2022-04-20 | 60.0 | 37.1 | 96.0 | 92.0 | 9.68 | 51.30 | 0.11 | 0.82 |
| 2022-04-21 | 61.0 | 36.2 | 95.0 | 95.0 | 9.60 | 63.25 | 1.86 | 1.66 |
| 2022-04-22 | 63.0 | 36.0 | 95.0 | 93.0 | 7.53 | 82.97 | 23.25 | 0.93 |
| 2022-04-23 | 59.0 | 36.5 | 96.0 | 96.0 | 8.95 | 69.24 | 1.91 | 0.58 |
| 2022-04-24 | 65.0 | 36.2 | 96.0 | 100.0 | 10.79 | 67.82 | 0.29 | 1.14 |
| 2022-04-25 | 57.0 | 35.9 | 96.0 | 102.0 | 12.35 | 65.43 | 0.01 | 2.63 |
| Date (yy-mm-dd) | SpO2 (%) | Heart Rate (BPM) | Systolic Blood Pressure (mmHg) | Body Temperature (Celsius) |
|---|---|---|---|---|
| 2022-04-26 | 95.028053 | 63.863962 | 98.327346 | 36.244274 |
| 2022-04-27 | 94.801013 | 64.027884 | 98.783749 | 36.162657 |
| 2022-04-28 | 94.948091 | 64.413307 | 99.589877 | 36.218256 |
| 2022-04-29 | 95.127560 | 64.438053 | 99.516291 | 36.246443 |
| 2022-04-30 | 95.054558 | 64.429125 | 99.496265 | 36.196343 |
| Date (yy-mm-dd) | SpO2 (%) | Heart Rate | Systolic Blood Pressure | Body Temperature |
|---|---|---|---|---|
| 2022-04-26 | 0 | 1 | 0 | 0 |
| 2022-04-27 | 0 | 1 | 0 | 0 |
| 2022-04-28 | 0 | 1 | 0 | 0 |
| 2022-04-29 | 0 | 1 | 0 | 0 |
| 2022-04-30 | 0 | 1 | 0 | 0 |
| Dataset Used to Train the Model | Model | Parameter | Value (RMSE) |
|---|---|---|---|
| 304 | BILSTM | Spo2 | 0.285014 |
| 181 | GRU | Heart Rate | 1.520008 |
| 184 | BILSTM | Systolic Blood Pressure | 1.904305 |
| 181 | GRU | Body Temperature | 0.250580 |
| Date (yy-mm-dd) | Heart Rate (BPM) | Body Temperature (C) | SpO2 (%) | Systolic Blood Pressure (mmHg) | T MED (C) | HR MED (%) | PR QTD (mm) | pm25 (Count) |
|---|---|---|---|---|---|---|---|---|
| 2022-10-20 | 68.0 | 35.40 | 96.0 | 96.0 | 14.47 | 82.26 | 10.73 | 1.42 |
| 2022-10-21 | 68.0 | 35.60 | 96.0 | 96.0 | 15.05 | 79.13 | 3.94 | 1.94 |
| 2022-10-22 | 74.0 | 35.80 | 96.0 | 96.0 | 14.91 | 74.00 | 18.72 | 1.20 |
| 2022-10-23 | 70.0 | 35.90 | 95.0 | 94.0 | 14.15 | 67.11 | 5.45 | 2.91 |
| 2022-10-24 | 72.0 | 35.80 | 97.0 | 93.0 | 14.32 | 72.58 | 1.47 | 1.93 |
| 2022-10-25 | 76.0 | 35.00 | 95.0 | 98.0 | 16.13 | 64.89 | 7.87 | 1.94 |
| Date (yy-mm-dd) | SpO2 (%) | Heart Rate (BPM) | Systolic Blood Pressure (mmHg) | Body Temperature (C) |
|---|---|---|---|---|
| 2022-10-26 | 96.386055 | 70.779388 | 95.078346 | 35.292265 |
| 2022-10-27 | 96.228622 | 72.117355 | 94.664948 | 35.597720 |
| 2022-10-28 | 96.208916 | 72.186485 | 94.973228 | 35.796912 |
| 2022-10-29 | 96.297836 | 73.253487 | 95.260201 | 35.886715 |
| 2022-10-30 | 96.020462 | 72.828354 | 96.042572 | 35.796912 |
| 2022-10-31 | 96.320145 | 71.559845 | 95.059273 | 35.292265 |
| Date (yy-mm-dd) | SpO2 (%) | Heart Rate (BPM) | Systolic Blood Pressure (mmHg) | Body Temperature (C) |
|---|---|---|---|---|
| 2022-10-26 | 0 | 1 | 0 | 0 |
| 2022-10-27 | 0 | 1 | 0 | 0 |
| 2022-10-28 | 0 | 1 | 0 | 0 |
| 2022-10-29 | 0 | 1 | 0 | 0 |
| 2022-10-30 | 0 | 1 | 0 | 0 |
| 2022-10-31 | 0 | 1 | 0 | 0 |
| Criteria | Questions | Objective Statement | Eval 1 | Eval 2 |
|---|---|---|---|---|
| Clinical impact on patient treatment | Indicates the importance of an smart clinical decision support system capable of provide 5-day early warning scores for monitoring patients with COPD. | Importance of the intelligent clinical decision support system for monitoring patients with COPD. | 5 | 5 |
| Patients Life Quality Impact | Indicates the impact of a smart clinical decision support system, providing a 5-day early warning score on the quality of life of a patient with COPD. | Impact of a clinical intelligence decision support system on the quality of life of a patient with COPD. | 5 | 5 |
| Utility | Indicates the usefulness of a system for healthcare professionals; generates information whenever there are changes in patients’ baseline values. | Usefulness of a intelligent clinical decision support system that notifies about patient baseline value modifications. | 4 | 5 |
| Indicates the importance of a system that provides short-time horizon (in minutes) early warning scores for the clinical follow-up of patients with COPD. | Importance of an intelligent clinical decision support system on the clinical follow-up of patients with COPD. | 5 | 5 | |
| Indicates the usefulness of a real-time alert system for healthcare professionals whenever an abnormal measurement occurs for a specific patient. | Usefulness of an intelligent clinical decision support system that notifies about abnormal measurement detections. | 5 | 5 | |
| Consistency with the organization | Indicates the relevance of involving healthcare professionals in defining clinical intervals for abnormal measurements. | Clinical validation on the definition of intervals for abnormal measurements. | 5 | 5 |
| Indicates the relevance of involving healthcare professionals in defining the formula for calculating the basal value. | Clinical validation on the definition of the basal value calculation formula. | 4 | 5 | |
| Indicates the relevance of involving healthcare professionals in selecting environmental and clinical parameters (e.g., vital signs) that most influence the clinical progression of patients with COPD. | Clinical validation on the selection of environmental and biometric signs that most influence the clinical progression of patients with COPD. | 5 | 5 | |
| Integration with clinical protocols | Indicates the relevance of the adopted early warning score matrix for clinical decision-making and adjustment of therapeutic protocols for patients. | Relevance of the adoption of the early warning score matrix for the clinical decision-making and adjustment of therapeutic protocols for patients. | 5 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, J.; Antunes, N.; Rosa, J.; Ferreira, J.C.; Mogo, S.; Pereira, M. Intelligent Clinical Decision Support System for Managing COPD Patients. J. Pers. Med. 2023, 13, 1359. https://doi.org/10.3390/jpm13091359
Pereira J, Antunes N, Rosa J, Ferreira JC, Mogo S, Pereira M. Intelligent Clinical Decision Support System for Managing COPD Patients. Journal of Personalized Medicine. 2023; 13(9):1359. https://doi.org/10.3390/jpm13091359
Chicago/Turabian StylePereira, José, Nuno Antunes, Joana Rosa, João C. Ferreira, Sandra Mogo, and Manuel Pereira. 2023. "Intelligent Clinical Decision Support System for Managing COPD Patients" Journal of Personalized Medicine 13, no. 9: 1359. https://doi.org/10.3390/jpm13091359
APA StylePereira, J., Antunes, N., Rosa, J., Ferreira, J. C., Mogo, S., & Pereira, M. (2023). Intelligent Clinical Decision Support System for Managing COPD Patients. Journal of Personalized Medicine, 13(9), 1359. https://doi.org/10.3390/jpm13091359








